Listeria monocytogenes is a Gram-positive pathogenic bacterium which can be found in soil or water. Infection with the microorganism can occur after ingestion of contaminated food products. Small and large outbreaks of listeriosis have been described in the past. L. monocytogenes can cause a number of different clinical syndromes, most frequently sepsis, meningitis, and rhombencephalitis, particularly in immunocompromised hosts. L. monocytogenes systemic infections can develop following tissue penetration across the gastrointestinal tract or to hematogenous spread to sterile sites, possibly evolving towards bacteremia. L. monocytogenes only rarely causes bone or joint infections, usually in the context of prosthetic material that can provide a site for bacterial seeding.
- L. monocytogenes
- imaging
- microbiological diagnosis
- surgical approach
- antibiotic treatment
- infections
1. Introduction
| Dairy Products | Fruits and Vegetables | Meat Products | Fish Products |
|---|---|---|---|
| Pasteurized whole milk Chocolate milk Soft cheese (different types) Hard cheese Mexican-style cheese Goat cheese Ice cream Fresh cream |
Coleslaw (cabbage) Lettuce Corn Rice salad Salted mushrooms Sprouts Strawberries Nectarines Apples Cantaloupes Blueberries Stone fruit |
Delicatessen foods (deli meats) Pâté Foie gras Uncooked hot dogs “Rillettes” Pork tongue in aspic Pork pie Beef Turkey franks Jellied pork Cooked ham Ox tongue Undercooked chicken |
Shrimp salad Tuna salad Smoked fish |
2. Imaging Techniques
When suspecting bone or vertebral infections, the use of imaging techniques such as radiography or CT scans could provide valuable information in terms of bone erosions and vertebral bone integrity, mainly in the later stages of the disease; during the early stage of infection, no significant finding is usually detected. Furthermore, spinal stability must be assessed among patients in whom surgical management is being considered. Indeed, vertebral collapse, kyphotic deformity, and loss of normal lordosis can be found in advanced infections. CT also provides guidance for percutaneous aspirations in order to provide specimens for bacteriologic analysis in the presence of a fluid collection. MRI is the gold standard and represents the diagnostic imaging modality of choice. It should be performed among all patients for whom a spinal infection is suspected, unless contraindicated. Unenhanced T1-weighted images usually reveal a hypointense signal at the level of the end plates in the vertebral body and loss of a normal hyperintense fat signal in the vertebral bone marrow. T2-weighted imaging reveals a high signal corresponding to edema in the disk space and occasionally in the bone and paravertebral soft tissues. Gadolinium-enhanced T1-weighted imaging can demonstrate the contrast enhancement of the vertebral body, end plates, the prevertebral and paravertebral soft tissues, and the epidural space. Whenever the MRI is contraindicated or non-diagnostic (e.g., due to the presence of metallic implants causing artifacts), other imaging modalities should be considered. CT myelography provides another way of visualizing the spinal cord and ruling out compression in the setting of suspected cauda equina syndrome. On the contrary, nuclear medicine scans with radionuclide studies offer a high degree of sensitivity in the early stages of the disease. Spinal infections can occasionally be multifocal, so the whole spine should be scanned if an infectious focus is detected.3. Microbiological Diagnosis
The determination of a microbiological diagnosis of L. monocytogenes bone or vertebral infection is challenging, especially in the absence of referred exposures or negative blood tests. In this context, aspiration biopsy or surgical sampling represent the optimal method of providing a valid microbiological diagnosis. As a consequence, empiric antibiotic therapy should be delayed if the patient is hemodynamically stable and has no neurological signs in order to obtain valid samples for cultures; postponing antimicrobial administration can improve the microbiological yield, so it could be preferably deferred in the absence of life-threatening conditions or spinal cord involvement [38][84]. However, the initiation of an antibiotic treatment does not always preclude undertaking a biopsy [39][85]; in those cases where antibiotic treatment has already been started, it has been demonstrated that interrupting and withholding antibiotics for 2 weeks led to a better yield compared to holding for only 3 days pre-biopsy [40][86]. These data can vary according to the pharmacokinetics, dose, duration, and bone penetration of the selected antibiotic. Nevertheless, a short duration of empiric antibiotic exposure does not negatively impact pathogen recovery and therefore is not an absolute contraindication for biopsy [41][87]. Therefore, all these diagnostic and therapeutic issues should be taken into consideration when managing L. monocytogenes vertebral infections.4. Surgical Approach
In the absence of neurological deficits or sepsis, the optimal therapeutic approach comprises medical management with adequate intravenous antibiotics and immobilization of the affected spinal segment. Antibiotic therapy should be started as soon as the microorganism has been isolated in order to achieve sterilization of the infected bone or vertebral disc and prevent the occurrence of a neurological deficit or painful deformity. The duration of antibiotic therapy varies depending on the extent of bone involvement and the status of the patient’s immune system. Neurosurgical intervention should be considered only after taking into account a given patient’s neurological status as well as the extent of bone erosion and the specific vertebral level involved. The principles of surgical treatment include debridement of infected tissue, decompression of neural elements, and the restoration of spinal alignment and/or correction of spinal instability. The presence of neurological deficits is considered to be the most important factor in the decision-making process. Regardless of the duration of the weakness, emergency surgical intervention is offered unless the motor deficit is minimal. Patients for whom non-surgical management is considered should be carefully monitored; this is because early progression with neurological deterioration may occur rapidly. Surgical approaches for spinal infections are usually dictated by the site of compression (ventrally vs. dorsally located lesions) and tailored to the vertebral level involved. The nature of the compressive lesion is also relevant; the liquid collection of pus can be drained, whereas a mass of granulation tissue or retro pulsed bone are better addressed with an open surgical approach. In addition, the optimal surgical approach is selected after consideration of the intrinsic features of each anatomic region of the spine and the likelihood of postoperative instability. The degree of kyphotic deformity, the number of vertebral elements involved, and the bone and posterior tension band integrity can be used to determine the extent of spinal instrumentation required to restore stability. Surgical intervention is also indicated after the failure of medical management or patients with chronic pain, significant deformity, or spine instability in the setting of spinal infection or its sequelae.5. Antibiotic Treatment of Bone and Vertebral L. monocytogenes Infections
Reports of L. monocytogenes bone infections are usually described in patients with predisposing factors, such as diabetes, leukemia, or receipt of long-term corticosteroids or immune-modulant treatments [42][43][44][45][46][88,89,90,91,92]. Usually, native vertebral L. monocytogenes infections have an insidious course, with symptoms, especially back pain, that could be present for over a year, as described in previous reports [47][48][93,94]. In the review by Charlier et al., more than 70% of cases of listeriosis involving bone and joint infections were subacute or chronic at the onset. Furthermore, most of these cases occurred in the hip (60%) and in prosthetic joints [42][88]. In this resviearchw, patients with osteomyelitis caused by L. monocytogenes showed only a mild increased in inflammatory markers compared with those with other forms of bacterial osteomyelitis. Vertebral osteomyelitis represents an even less frequent localization of invasive listeriosis. To date, eight cases [47][48][49][50][51][52][53][54][93,94,95,96,97,98,99,100] have been reported in the literature and most of them had significant risk factors for developing invasive listeriosis (Table 2). Figure 1 showed the MRI evolution of a patient with epidural abscess by L. monocytogenes from diagnosis to complete recovery after both surgical and antibiotic treatment [55][101]. All these patients were treated with ampicillin or amoxicillin or benzyl penicillin; five patients received a treatment combination with aminoglycosides; treatment duration was highly heterogeneous among these reports, ranging from 6 to 28 weeks in accordance with possible delayed clinical responses. Only one report [51][97] used trimetoprim/sulphametoxazole as an oral maintenance treatment; however, this was not combined with amoxicillin in any of the studies. Oral use of amoxicillin was described in two other reports and was administered for a total of 12 and 18 weeks, respectively [52][53][98,99]. The antibiotic treatment should often be associated with surgical intervention in cases of spinal L. monocytogenes infections, especially for those patients experiencing neurological deficit, cord compression, destruction of the vertebrae with instability, large epidural abscesses, or inadequate responses to antimicrobials [56][102].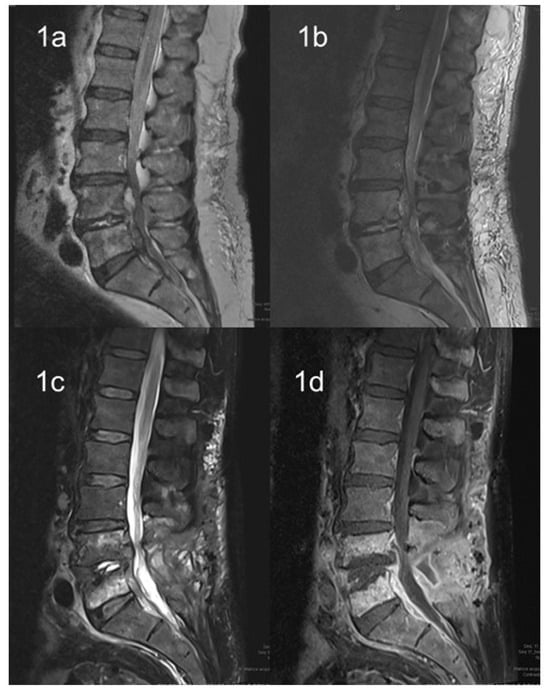
| Author | Age | Gender | Co-Morbidities | Clinical Symptoms | Duration of Symptoms | Antibiotic Treatment and Duration | Surgery |
|---|---|---|---|---|---|---|---|
| Adebolu et al. [47][93] | 60 | M | Polymyalgia rheumatica | Back pain | 12 months | Ampicillin, IV, 6 weeks Gentamicin, IV, 2 weeks |
Yes |
| Khan et al. [48][94] | 69 | M | Prior spinal laminectomy | Back pain | 5 months | Ampicillin, IV * Gentamicin, IV * |
Yes |
| Camp et al. [49][95] | 67 | M | DM, prior lumbar surgery | Back pain | Unknown | Oxacillin, IV * Streptomycin, IV * |
Yes |
| Chirgwin et al. [50][96] | 57 | M | DM, asthma | Fever, back pain | 3 weeks | Ampicillin, IV, 6 weeks Tobramycin, IV, 6 weeks |
Yes |
| Aubin et al. [51][97] | 92 | M | DM, heart failure, hip arthroplasty | Fever | 1 week | Amoxicillin, IV, 6 days Gentamicin, IV, 4 days Trimethoprim-sulfamethoxazole, oral, 12 weeks |
Yes |
| Hasan et al. [52][98] | 63 | M | DM, aortic valve replacement | Fever, back pain | 2 days | Benzyl penicillin, IV, 6 weeks Rifampicin, oral, 4 weeks Amoxicillin, oral, 18 weeks |
Yes |
| Duarte et al. [53][99] | 65 | M | DM | Fever | 5 days | Ampicillin, IV, 2 weeks Amoxicillin, oral, 12 weeks |
Yes |
| Al Ohaly et al. [54][100] | 79 | M | Hypertension, carotid bypass, repair of AAA | Back pain | 3 weeks | Ampicillin, IV, 6 weeks | No |
