Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by sandip mondal.
Hydrochar, a carbonaceous material, is derived through the process of hydrothermal carbonization (HTC) applied to biomass feedstocks.
- activated hydrochars
- pharmaceutical contaminant removal
- water contamination
- sustainable remediation
1. Introduction
Water contamination is a global environmental crisis that affects over 2 billion people worldwide [1]. This pervasive issue is primarily driven by pollutants stemming from various sources, such as sewage and leachate laden with pathogens, giving rise to profound concerns regarding public health and environmental well-being. Exposure to contaminants in water sources poses a severe threat, leading to waterborne diseases and long-term health issues [2]. Among the diverse array of water pollutants, pharmaceutical contaminants have emerged as a major concern, encompassing prescription and over-the-counter medications, personal care products, hormones, antibiotics, cytostatic drugs, antipyretics and analgesics, beta blockers, psychotropic medications, nonsteroidal anti-inflammatory drugs (NSAIDs), X-ray contrast media, herbicides, pesticides, and veterinary medications [3,4,5][3][4][5]. These pharmaceutical contaminants find their way into aquatic environments through various pathways, including human and animal wastewater, agricultural runoff, and industrial effluents [6].
What sets pharmaceutical contaminants apart is their exceptional persistence and mobility in aquatic ecosystems. They traverse substantial distances, affecting surface water, groundwater, and even potable water supplies, necessitating urgent measures to control and mitigate their presence. Effective disposal, advanced wastewater treatment, and rigorous monitoring are essential to tackle this multifaceted issue.
The escalated usage of pharmaceuticals, coupled with advances in detection methods and increased awareness of the associated risks, underlines the gravity of pharmaceutical contaminants as a distinct challenge. These contaminants are introduced into water bodies via numerous routes, leading to their prolonged presence and substantial threats to both public health and the environment. As such, effective disposal measures, advanced wastewater treatment, and rigorous monitoring are indispensable in addressing this complex problem.
The impact of pharmaceutical contaminants is far-reaching, affecting aquatic ecosystems and human populations alike. They disrupt aquatic life, causing population declines and accumulating in organisms, which can result in biomagnification and altered growth and development [7,8,9][7][8][9]. Furthermore, the presence of antibiotic-resistant bacteria in aquatic environments contributes to antibiotic resistance, while toxicological effects lead to increased mortality rates and decreased biodiversity [10,11][10][11]. Algal blooms triggered by pharmaceutical contaminants further disrupt aquatic ecosystems and produce harmful toxins. For humans, the risks include drinking water contamination, chronic exposure, direct health effects, and potential drug interactions [12,13][12][13]. The concept of “One Health” acknowledges the interconnectedness of human, animal, and environmental health, underscoring the necessity of water treatment and monitoring to mitigate these risks [14].
Efforts to address this multifaceted issue are centered on improving wastewater treatment, reducing pharmaceutical waste, and implementing regulatory measures. Pharmaceutical contaminant remediation plays a pivotal role in ensuring the safety and quality of pharmaceutical products. A diverse range of techniques, such as filtration, chromatography, distillation, crystallization, extraction, adsorption, membrane filtration, biodegradation, advanced oxidation processes, chemical precipitation, enzyme-based remediation, complexation, chelation, pH adjustment, advanced data analysis, and quality by design, are employed [15,16][15][16]. Each technique serves a unique purpose, from eliminating particulate pollutants through filtration to separating impurities by size using chromatography. The removal of volatile impurities is achieved through distillation, while crystallization isolates impurities from the target compound. Adsorption relies on specific adsorbents, whereas membrane filtration utilizes characteristics such as size, charge, and molecular weight to filter out impurities. Biodegradation addresses organic pollutants, while advanced oxidation processes generate reactive radicals for degradation [17]. Chemical precipitation leads to the formation of insoluble precipitates containing contaminants, while enzyme-based remediation catalyzes breakdown or modification. Adjusting the pH can prevent the precipitation of pollutants. Typically, a combination of these techniques is employed to ensure effective contaminant remediation within the pharmaceutical industry.
2. The Emergence of Hydrochar as a Sustainable Adsorbent
Hydrochar, a carbonaceous material, is derived through the process of hydrothermal carbonization (HTC) applied to biomass feedstocks [22][18]. HTC is a thermochemical procedure that converts biomass into a carbon-rich substance under conditions of 180–250 °C and 2–20 MPa pressure in the presence of water [23][19]. Hydrochar possesses a suite of attributes that render it a promising and sustainable adsorbent for the removal of various water and wastewater pollutants. These attributes encompass a high surface area, a porous structure, a diverse array of surface functional groups, hydrophobic characteristics, and chemical and mechanical stability. Notably, hydrochar has demonstrated efficacy in the removal of a wide array of pollutants from water and wastewater, including heavy metals, organic contaminants, pharmaceutical residues, dyes, and nutrients [24,25][20][21]. Hydrochar generally exhibits non-toxicity and hydrophobicity. The characteristics of hydrochar, including surface chemistry, porosity, particle size, and specific surface area, are contingent upon the temperature and reaction duration applied during hydrothermal carbonization (HTC). The hydrochar surface is typically endowed with numerous oxygen-containing functional groups that manifest favorable adsorption affinities toward both polar and non-polar functional groups, thereby resulting in elevated adsorption capacity. It is noteworthy, however, that such advantageous properties may be compromised during gas-phase activation aimed at augmenting specific surface area. Consequently, judicious selection of processing conditions is imperative to preserve the desired attributes of hydrochar [26][22]. The hydrochar synthesis process occurs in an aqueous environment, typically employing a stainless steel autoclave loaded with biomass and a specified quantity of water (typically within the range of 1:3 to 1:10 ratios of biomass to water). In comparison to biochar, hydrochar exhibits a slightly acidic nature attributed to a higher presence of oxygenated functional groups. Pyrolysis-induced loss of carboxyl and hydroxyl groups renders biochar alkaline, with alkalinity influenced by inorganic and metal compounds like Ca and Mg. Hydrothermal carbonization (HTC) results in the removal of some inorganic components in the aqueous medium, contributing to the acidic pH of hydrochar [27][23]. Due to the lower temperature of the HTC process, carbon conversion is reduced compared to pyrolysis, yielding higher atomic ratios of H/C and O/C in hydrochar. Consequently, hydrochar demonstrates elevated atomic ratios of hydrogen to carbon and oxygen to carbon in contrast to biochar [23][19]. The increased hydrogen content in hydrochar, known for its involvement in polar interactions, may enhance its adsorption capacity for pharmaceutical compounds exhibiting polar or hydrogen-bonding functionalities. Additionally, the oxygen-containing functional groups in hydrochar can engage in various chemical interactions, including hydrogen bonding and Lewis acid–base interactions, potentially influencing the adsorption of pharmaceutical compounds with oxygen-binding sites or those susceptible to such interactions. Hydrochar’s selectivity for pharmaceutical compounds over other organics in waters and wastewaters can be attributed to its porous structure, chemical functional groups, surface chemistry, electrostatic interactions, aromaticity, and the potential for specific affinity. The porous nature of hydrochar provides an effective medium for adsorption, while its surface features and chemical composition may favor interactions with pharmaceutical molecules. The presence of aromatic structures in hydrochar aligns with the aromatic rings often found in pharmaceutical compounds. Additionally, tailored modifications to the hydrochar surface can enhance its selectivity for pharmaceuticals. These combined factors contribute to the effectiveness of hydrochar as a selective adsorbent for pharmaceutical compounds in water treatment applications. The sustainability of hydrochar as an adsorbent arises from its ability to be derived from a variety of biomass feedstocks, such as agricultural residues, forestry byproducts, and municipal solid waste [28,29][24][25]. Furthermore, the production of hydrochar can be integrated into other bioenergy processes like anaerobic digestion and biodiesel production [30][26]. Importantly, hydrochar can be regenerated and reused multiple times, reducing the demand for new adsorbent materials. The emergence of hydrochar as a sustainable adsorbent represents a promising development in the realm of water and wastewater treatment and stands as a cost-effective and environmentally friendly alternative to traditional adsorbent materials, including activated carbon. Noteworthy examples of hydrochar’s use as a sustainable adsorbent in water and wastewater treatment include its effectiveness in arsenic removal from groundwater [31][27], elimination of organic pollutants (e.g., pesticides and herbicides) from agricultural runoff, the extraction of pharmaceutical contaminants from wastewater [26][22], removal of dyes from industrial wastewater [32][28], and nutrient retention (e.g., phosphorus and nitrogen) from wastewater. Hydrochar, while still a relatively recent innovation, holds significant potential for revolutionizing the sustainable treatment of water and wastewater. Its emergence as a sustainable adsorbent represents a substantial advancement in the fields of environmental science and water treatment [28][24]. Hydrochar, originating from the hydrothermal carbonization of organic feedstocks like agricultural residues, sewage sludge, and organic waste, brings several salient advantages to the forefront: the inherently sustainable nature of hydrochar production, wherein it employs organic waste materials that would otherwise be discarded or landfilled, consequently reducing waste while adding value to these materials; the ability to derive hydrochar from a wide spectrum of renewable feedstocks, rendering it versatile and adaptable to diverse regional contexts and less reliant on fossil-based adsorbents; and the carbon-sequestering attributes of the production process, contributing to climate change mitigation by converting organic carbon into a stable, long-lasting form [33][29]. The tunable properties of hydrochar, such as surface area and functional groups, during its production enable optimization for specific contaminant adsorption. Moreover, the environmental impact of hydrochar production in terms of energy consumption and emissions tends to be lower than some traditional adsorbents. The broad effectiveness of hydrochar in adsorbing a wide variety of contaminants, including heavy metals, organic pollutants, and pharmaceuticals, makes it suitable for diverse applications in water treatment, soil remediation, and beyond. The regenerability and reusability of hydrochar further enhance its economic and environmental efficiency. Ongoing research continues to refine synthesis methods and enhance the adsorption properties of hydrochar, opening up new possibilities for its application. The rise of hydrochar as a sustainable adsorbent aligns with global objectives of waste reduction, climate change mitigation, and ensuring access to clean water resources. Its potential to contribute to environmental sustainability and remediation makes it a promising material for addressing challenges related to water and soil contamination. Hydrochars exhibit several unique properties that render them suitable for a multitude of applications. These properties include a typical surface area ranging from 100 to 500 m2/g, which makes them effective adsorbents for a broad spectrum of pollutants. Their porous structure allows for the adsorption of a wide range of contaminants, spanning both organic and inorganic compounds. Hydrochars feature various surface functional groups, such as hydroxyl, carboxyl, and carbonyl groups, which can be tailored to suit specific applications. Their inherent hydrophobicity, repelling water, proves advantageous in applications where water contact is undesirable, such as water filtration and soil improvement [34][30]. Furthermore, hydrochars demonstrate both chemical and mechanical stability, rendering them durable and reusable materials [23][19]. The properties of hydrochars can be influenced by several factors, including the type of biomass feedstock utilized in their production, with woody biomass yielding hydrochars with higher surface area and porosity compared to non-woody-biomass-derived hydrochars [35][31]. Additionally, the HTC process parameters, such as temperature, pressure, and reaction time, can impact the properties of hydrochars; for instance, higher temperatures during production tend to yield hydrochars with lower surface area and porosity than those produced at lower temperatures [36][32]. In sum, hydrochars hold promise as materials with diverse potential applications due to their unique attributes, encompassing high surface area, porous structure, diverse surface functional groups, hydrophobicity, and chemical and mechanical stability. These characteristics make them amenable to applications in water and wastewater treatment, soil improvement, energy storage, and the development of value-added products. Figure 1 provides an illustration of the preparation of waste biomass hydrochar and its various potential applications.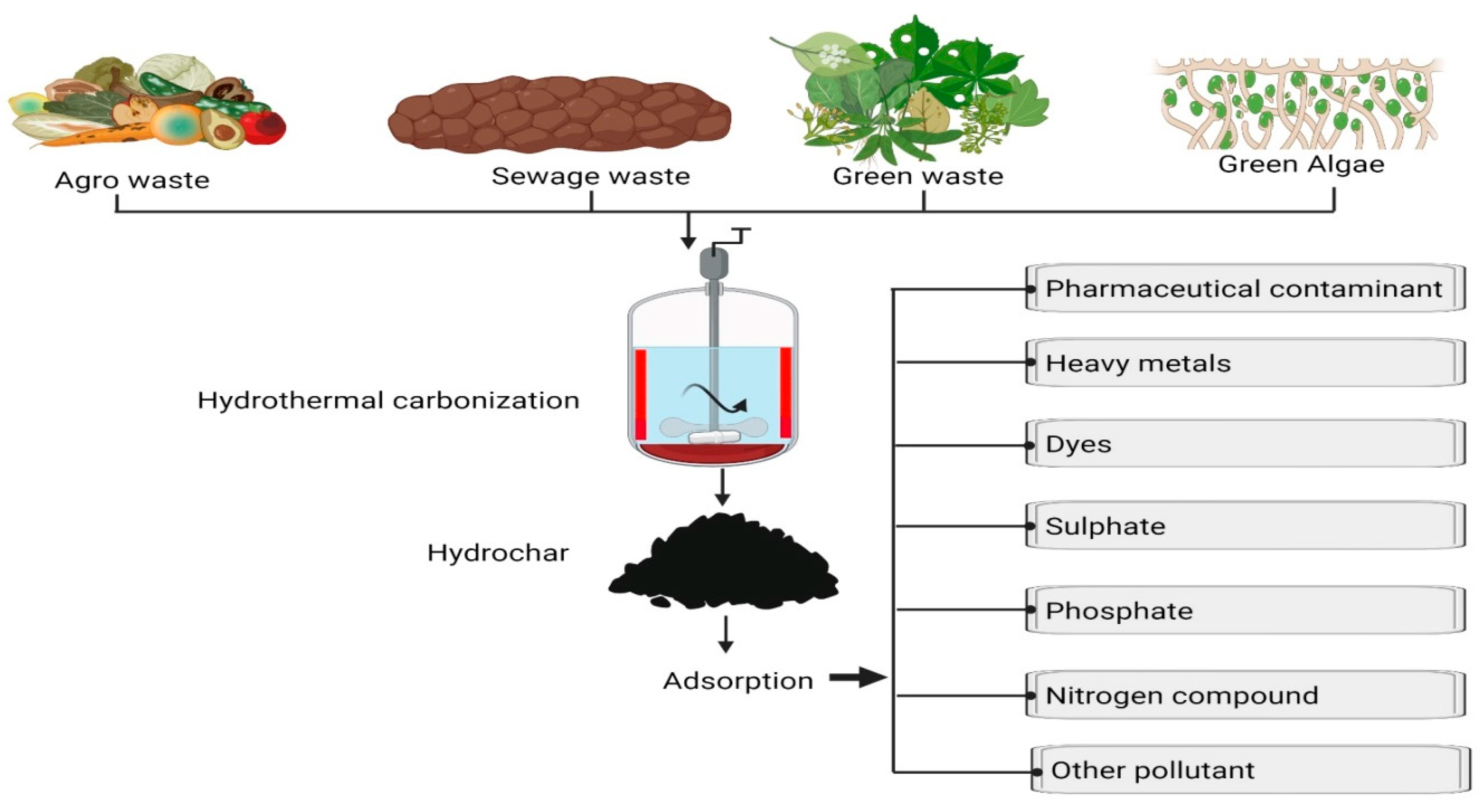
Figure 1. Waste biomass hydrochar production—preparation techniques and multifaceted applications for sustainable resource utilization.
2.1. Production Methods for Hydrochars
Hydrochars are generated through the hydrothermal carbonization (HTC) process, which entails subjecting organic feedstocks to controlled conditions involving elevated temperature and pressure while in the presence of water [36,37][32][33]. The specific methodologies employed in hydrochar production may vary contingent on the chosen feedstock and the targeted properties of the resultant hydrochar. The primary steps encompassed in hydrochar production are as follows:2.1.1. Feedstock Selection
The initial phase involves the selection of an appropriate feedstock. Organic materials such as agricultural residues (e.g., crop residues, wood), sewage sludge, algae, or organic waste are commonly employed. The selected feedstock is often subjected to pre-processing measures to enhance uniformity and its suitability for hydrothermal carbonization. This pre-processing can encompass actions such as shredding, drying, or size reduction to yield a more homogenous material.2.1.2. Hydrothermal Reactor Utilization
The prepared feedstock is loaded into a specialized hydrothermal reactor, designed to endure elevated temperatures and pressures. The reactor is meticulously sealed to prevent gas escape. Water is introduced into the reactor to create a saturated or supercritical water environment. The temperature is then elevated, typically within the range of 180–250 °C, with a simultaneous increase in pressure, often ranging from 10 to 50 bar. The feedstock is subjected to hydrothermal treatment for a designated duration, usually spanning from several hours to a day or more. This process involves the rupture of chemical bonds within the organic materials, the polymerization of carbon compounds, and the eventual formation of hydrochar.2.1.3. Cooling and Depressurization
Subsequent to the hydrothermal treatment, the reactor is meticulously cooled and depressurized, facilitating the safe removal of the formed hydrochar [37,38][33][34].2.1.4. Collection and Post-Treatment
The resultant hydrochar, manifesting as a solid carbonaceous product, is gathered and separated from the aqueous phase. It may subsequently undergo a washing process to eliminate water-soluble impurities and is often subjected to drying to reduce moisture content. Depending on the aspired properties and the intended applications of the hydrochar, post-treatment processes may be administered. These may encompass activation, physical or chemical modification, and further drying. The resultant hydrochar is frequently characterized to evaluate properties such as surface area, porosity, and functional group composition, which serve to determine its suitability for specific applications.2.1.5. Versatility and Tailoring
The production of hydrochars is highly versatile, and the operational conditions can be customized to achieve desired properties and performance characteristics. As such, the process parameters may fluctuate contingent on factors such as the type of feedstock, temperature, pressure, and treatment duration. This adaptability makes hydrothermal carbonization a valuable technology for the conversion of organic waste materials into a diverse array of sustainable and valuable products [38][34].2.2. Activation Techniques for Enhancing Adsorption Properties
Activation techniques play a pivotal role in augmenting the adsorption properties of materials, including hydrochars, through the augmentation of their surface area, pore volume, and reactivity. These techniques are employed to optimize the adsorption capacity of hydrochars for particular contaminants. The following elucidates several common activation methods: Physical activation entails subjecting hydrochars to elevated temperatures in an oxygen-depleted environment, a process that expels volatile compounds and augments porosity. This results in activated hydrochars endowed with enhanced adsorption properties [25][21]. Chemical activation methods involve treating hydrochars with potent acids (e.g., phosphoric acid) or bases (e.g., potassium hydroxide). This treatment fosters the creation of pores and augments surface area, thus amplifying the hydrochars’ adsorption capacity. Subsequently, the activated hydrochars are meticulously washed to eliminate residual chemicals [39][35]. Chemical modification encompasses the introduction of functional groups (e.g., amino or thiol groups) through chemical processes, which enhances the hydrochar’s affinity for specific contaminants and improves adsorption efficiency [40][36]. The application of microwave irradiation to hydrochars promotes the release of volatile compounds, heightens porosity, and augments adsorption properties due to localized and rapid heating effects [41][37]. Steam activation involves the introduction of steam to the hydrochar under controlled conditions. This procedure engenders additional porosity and ameliorates adsorption properties [42][38]. Electrochemical methods are utilized to create activated hydrochars by applying an electric current or potential. This application induces the formation of pores and surface functional groups, thereby enhancing adsorption capabilities [43][39]. In situ activation occurs within the hydrothermal carbonization process through adjustments in reaction conditions, such as temperature, duration, and pressure. These modifications are tailored to the properties of the resulting hydrochars, aligning them with specific adsorption applications. Certain research endeavors employ a combination of multiple activation techniques to maximize adsorption properties. For instance, a hydrochar may undergo chemical activation followed by pyrolysis or steam activation [42][38]. The integration of other materials, such as nanoparticles or metal oxides, into hydrochars enhances their adsorption properties for specific contaminants. This process, referred to as composite formation, may also involve doping with substances like nitrogen or sulfur [44][40]. Activation techniques are instrumental in customizing hydrochars to meet the precise requirements of adsorption applications, whether these pertain to the removal of heavy metals, organic pollutants, or pharmaceuticals from water and wastewater. The selection of the most suitable activation method hinges on the target contaminants, feedstock characteristics, and intended properties of the hydrochar. Table 1 provides a comparative analysis of the physical properties of activated hydrochar in relation to other commonly used adsorbents.Table 1.
Physical properties of activated hydrochar with other common adsorbents.
| Characteristic | Activated Hydrochar | Activated Carbon | Clay Minerals | References |
|---|---|---|---|---|
| Selectivity for Pharmaceuticals | Customizable with functionalization | Pharmaceutical selectivity varies | Pharmaceutical selectivity varies | [23][19] |
| Sustainability | Eco-friendly, often from organic waste | Source-dependent, non-renewables | Plentiful, naturally sourced, eco-friendly | [45,46][41][42] |
| Adsorption Capacity | High adsorption | Strong adsorption capacity | Very high | [45][41] |
| Tunable Properties | Adjustable via activation methods | Limited tunability; depends on source and process | Limited tunability; properties linked to clay type | [45][41] |
| High Surface Area | High surface area typically observed | High surface area | Less surface area than hydrochar and activated carbon | [23][19] |
| Porous Structure | Porous structure for better adsorption efficiency | Porous structure for efficient adsorption | Porous structure, especially in smectite-type clays | [46,47][42][43] |
| Carbon Sequestration | Carbon sequestration via hydrothermal carbonization | Not applicable | Not applicable | [46][42] |
| Feedstock Diversity | From diverse organic waste | Coal, coconut, wood feedstocks | Various clay mineral types | [47][43] |
| Proven Performance | Efficient for targeted adsorption | Versatile and extensively applied | Removes specific heavy metals | [47][43] |
| Cost | Economical from waste materials | Cost varies with source | Cost-effective due to abundance | [45,46,47][41][42][43] |
| Regeneration | Reusability is common | Regenerable for reuse | Frequently regenerable for reuse | [45,46,47][41][42][43] |
| Application Specificity | Adaptable and targeted adsorption | Highly versatile and effective | Potent against heavy metals | [45,46,47][41][42][43] |
| Customizability | Customizable with varied activation | Customizability limitations | Customizability limitations | [23,45,46,47][19][41][42][43] |
| Environmental Impact | Environmentally friendly | High energy usage | High energy usage | [45,,47][41]46[42][43] |
References
- Berihun, G.; Abebe, M.; Hassen, S.; Gizeyatu, A.; Berhanu, L.; Teshome, D.; Walle, Z.; Desye, B.; Sewunet, B.; Keleb, A. Drinking water contamination potential and associated factors among households with under-five children in rural areas of Dessie Zuria District, Northeast Ethiopia. Front. Public Health 2023, 11, 1199314.
- Pandey, P.K.; Kass, P.H.; Soupir, M.L.; Biswas, S.; Singh, V.P. Contamination of water resources by pathogenic bacteria. AMB Express 2014, 4, 51.
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107 (Suppl. 6), 907–938.
- Daughton, C.G. Pharmaceuticals and Personal Care Products in the Environment: Overarching Issues and Overview; ACS Publications: Washington, DC, USA, 2001.
- Bexfield, L.M.; Toccalino, P.L.; Belitz, K.; Foreman, W.T.; Furlong, E.T. Hormones and pharmaceuticals in groundwater used as a source of drinking water across the United States. Environ. Sci. Technol. 2019, 53, 2950–2960.
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234.
- Zenker, A.; Cicero, M.R.; Prestinaci, F.; Bottoni, P.; Carere, M. Bioaccumulation and biomagnification potential of pharmaceuticals with a focus to the aquatic environment. J. Environ. Manag. 2014, 133, 378–387.
- Mezzelani, M.; Gorbi, S.; Regoli, F. Pharmaceuticals in the aquatic environments: Evidence of emerged threat and future challenges for marine organisms. Mar. Environ. Res. 2018, 140, 41–60.
- Mondal, S.; Xu, J.; Chen, G.; Huang, S.; Huang, C.; Yin, L.; Ouyang, G. Solid-phase microextraction of antibiotics from fish muscle by using MIL-101(Cr)NH2-polyacrylonitrile fiber and their identification by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2019, 1047, 62–70.
- Mondal, S.; Jiang, J.; Li, Y.; Ouyang, G. Carbon and tin-based polyacrylonitrile hybrid architecture solid phase microextraction fiber for the detection and quantification of antibiotic compounds in aqueous environmental systems. Molecules 2019, 24, 1670.
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795.
- Kock, A.; Glanville, H.; Law, A.; Stanton, T.; Carter, L.; Taylor, J. Emerging challenges of the impacts of pharmaceuticals on aquatic ecosystems: A diatom perspective. Sci. Total Environ. 2023, 878, 162939.
- Xin, X.; Huang, G.; Zhang, B. Review of aquatic toxicity of pharmaceuticals and personal care products to algae. J. Hazard. Mater. 2020, 410, 124619.
- Gebreyes, W.A.; Dupouy-Camet, J.; Newport, M.; Oliveira, C.; Schlesinger, L.S.; Saif, Y.M.; Kariuki, S.; Saif, L.J.; Saville, W.; Wittum, T.; et al. The global one health paradigm: Challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Neglected Trop. Dis. 2014, 8, e3257.
- Imwene, K.; Ngumba, E.; Kairigo, P. Emerging technologies for enhanced removal of residual antibiotics from source-separated urine and wastewaters: A review. J. Environ. Manag. 2022, 322, 116065.
- Mondal, S.; Aikat, K.; Halder, G. Sorptive uptake of Ranitidine hydrochloride by Parthenium hysterophorus based chemically treated N-biochar in static bed continuous flow system. Results Surf. Interfaces 2022, 8, 100071.
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key points of advanced oxidation processes (aops) for wastewater, organic pollutants and pharmaceutical waste treatment: A mini review. Chemengineering 2022, 6, 8.
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247.
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies and Applications. Catalysts 2021, 11, 939.
- Ighalo, J.O.; Rangabhashiyam, S.; Dulta, K.; Umeh, C.T.; Iwuozor, K.O.; Aniagor, C.O.; Eshiemogie, S.O.; Iwuchukwu, F.U.; Igwegbe, C.A. Recent advances in hydrochar application for the adsorptive removal of wastewater pollutants. Chem. Eng. Res. Des. 2022, 184, 419–456.
- Azzaz, A.A.; Khiari, B.; Jellali, S.; Ghimbeu, C.M.; Jeguirim, M. Hydrochars production, characterization and application for wastewater treatment: A review. Renew. Sustain. Energy Rev. 2020, 127, 109882.
- Yaah, V.B.K.; Zbair, M.; de Oliveira, S.B.; Ojala, S. Hydrochar-derived adsorbent for the removal of diclofenac from aqueous solution. Nanotechnol. Environ. Eng. 2021, 6, 1–12.
- Gascó, G.; Paz-Ferreiro, J.; Álvarez, M.; Saa, A.; Méndez, A. Biochars and hydrochars prepared by pyrolysis and hydrothermal carbonisation of pig manure. Waste Manag. 2018, 79, 395–403.
- Shyam, S.; Arun, J.; Gopinath, K.P.; Ribhu, G.; Ashish, M.; Ajay, S. Biomass as source for hydrochar and biochar production to recover phosphates from wastewater: A review on challenges, commercialization, and future perspectives. Chemosphere 2022, 286, 131490.
- Mestre, A.S.; Tyszko, E.; Andrade, M.A.; Galhetas, M.; Freire, C.; Carvalho, A.P. Sustainable activated carbons prepared from a sucrose-derived hydrochar: Remarkable adsorbents for pharmaceutical compounds. RSC Adv. 2015, 5, 19696–19707.
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761.
- Chen, H.; Xu, J.; Lin, H.; Zhao, X.; Shang, J.; Liu, Z. Arsenic removal via a novel hydrochar from livestock waste co-activated with thiourea and γ-Fe2O3 nanoparticles. J. Hazard. Mater. 2021, 419, 126457.
- Ferrentino, R.; Ceccato, R.; Marchetti, V.; Andreottola, G.; Fiori, L. Sewage sludge hydrochar: An option for removal of methylene blue from wastewater. Appl. Sci. 2020, 10, 3445.
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598.
- Sivaranjanee, R.; Kumar, P.S.; Rangasamy, G. A recent advancement on hydrothermal carbonization of biomass to produce hydrochar for pollution control. Carbon Lett. 2023, 33, 1909–1933.
- Tag, A.T.; Duman, G.; Yanik, J. Influences of feedstock type and process variables on hydrochar properties. Bioresour. Technol. 2018, 250, 337–344.
- Lima, E.C.; Naushad, M.; Reis, G.S.D.; Dotto, G.L.; Pavan, F.A.; Guleria, A.; Seliem, M.K.; Sher, F. Production of carbon-based adsorbents from lignocellulosic biomass. In Biomass-Derived Materials for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 169–192.
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378.
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578.
- Puccini, M.; Stefanelli, E.; Hiltz, M.; Seggiani, M.; Vitolo, S. Activated carbon from hydrochar produced by hydrothermal carbonization of wastes. Chem. Eng. Trans. 2017, 57, 169–174.
- Liu, L.; Cai, W.; Dang, C.; Han, B.; Chen, Y.; Yi, R.; Fan, J.; Zhou, J.; Wei, J. One-step vapor-phase assisted hydrothermal synthesis of functionalized carbons: Effects of surface groups on their physicochemical properties and adsorption performance for Cr(VI). Appl. Surf. Sci. 2020, 528, 146984.
- Li, Y.; Tsend, N.; Li, T.; Liu, H.; Yang, R.; Gai, X.; Wang, H.; Shan, S. Microwave assisted hydrothermal preparation of rice straw hydrochars for adsorption of organics and heavy metals. Bioresour. Technol. 2019, 273, 136–143.
- Congsomjit, D.; Areeprasert, C. Hydrochar-derived activated carbon from sugar cane bagasse employing hydrothermal carbonization and steam activation for syrup decolorization. Biomass Convers. Biorefinery 2021, 11, 2569–2584.
- Tasca, A.L.; Clematis, D.; Marco, P.; Vitolo, S.; Puccini, M. Herbicide removal from water: Investigating the potential of electrochemistry and hydrochar-based activated carbon. Chem. Eng. Trans. 2019, 74, 841–846.
- Zhang, X.; Wang, Y.; Cai, J.; Wilson, K.; Lee, A.F. Bio/hydrochar sorbents for environmental remediation. Energy Environ. Mater. 2020, 3, 453–468.
- Isemin, R.; Muratova, N.; Kuzmin, S.; Klimov, D.; Kokh-Tatarenko, V.; Mikhalev, A.; Milovanov, O.; Dalibard, A.; Ibitowa, O.A.; Nowotny, M.; et al. Characteristics of Hydrochar and Liquid Products Obtained by Hydrothermal Carbonization and Wet Torrefaction of Poultry Litter in Mixture with Wood Sawdust. Processes 2021, 9, 2082.
- Islam, T.; Sultana, A.I.; Chambers, C.; Saha, S.; Saha, N.; Kirtania, K.; Reza, M.T. Recent Progress on Emerging Applications of Hydrochar. Energies 2022, 15, 9340.
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Othmani, A.; Osagie, C.; Meskini, M.; AlKafaas, S.S.; Malloum, A.; Khanday, W.A.; Jacob, A.O.; et al. Recent advances on sustainable adsorbents for the remediation of noxious pollutants from water and wastewater: A critical review. Arab. J. Chem. 2023, 16, 105303.
More
