Noscapine, a substance first noted for its antitussive and anti-stroke qualities, has drawn significant interest for its potential as a cancer treatment
[10][13]. Its exceptional efficacy as an antineoplastic agent against a wide variety of malignancies has been revealed by the research. Phase I/II clinical trials are now being conducted, which represents a substantial advancement towards its potential medical use
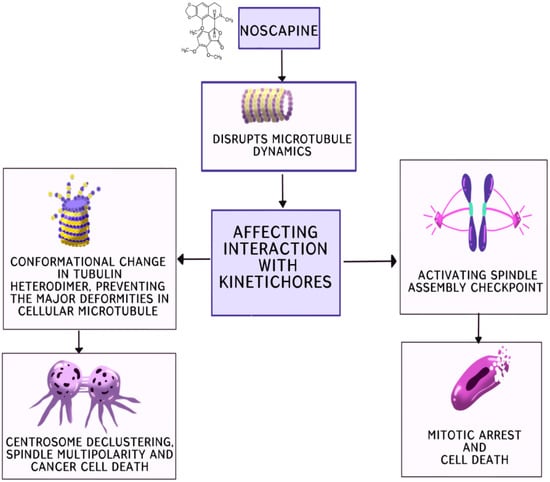
[11][24]. Noscapine and its analogues, derived by modifying specific sites of the parent compound, show promise in combating cancer. They function as potent centrosome de-clustering agents, disrupting the microtubule dynamics. Cancer cells with amplified centrosomes cluster them to survive mitosis, but these drugs prevent this clustering, leading to spindle multipolarity, cell cycle arrest and, ultimately, cell death. Noscapine and its analogues induce alterations in the microtubule dynamics, affecting their interaction with kinetochores during mitosis, activating the checkpoint of the spindle assembly and leading to mitotic arrest and eventual cell death (
Figure 2). Additionally, they alter the tubulin heterodimer configuration, acting as tubulin-stabilising agents. Bromonoscapine and reduced bromonoscapine induce centrosome amplification and, along with this, result in centrosome de-clustering, spindle multipolarity and cancer cell death, making these compounds potential cancer-selective chemotherapeutics due to their specificity to cancer-related anomalies like supernumerary centrosomes. Furthermore, noscapine alters the dynamics of microtubule assembly by stoichiometrically binding to them without significantly promoting or inhibiting microtubule polymerisation. It extends the duration of microtubules in an attenuated or paused state. By binding to tubulin, noscapine changes its conformation, arresting the cell at mitosis without causing major deformities in the cellular microtubules. This alteration eliminates kinetochore tension, leading to chromosome congression failure and the loss of tension across sister kinetochores, subsequently activating the spindle checkpoint.
Figure 2.
Noscapinoids: potential cluster bombs including centrosome de-clustering.
Numerous cancer cells, including drug-resistant varieties, exhibit cell growth inhibition when treated with noscapine, but healthy cells are left unaffected. This exceptional selectivity opens the door to a promising new approach to targeted cancer therapy. Furthermore, noscapine is a different possibility in the battle against cancer due to its effect on microtubules, which differs greatly from that of other drugs
[10][12][13][11,13,21].
It is interesting to note in
Figure 2 that noscapine causes metaphase arrest and death in dividing cells, highlighting its potential as an anticancer drug. By altering the kinetics of microtubule assembly, it affects cell division and achieves its goal
[14][25]. Noscapine can lessen the tension between kinetochores, but it does not change the tubulin polymer/monomer ratio, according to the research. As a result, the spindle checkpoint is triggered, stopping the continuation of the mitotic cycle
[15][26].
Furthermore, tubulin undergoes a conformational shift upon the stoichiometric binding of noscapine, which alters the assembly of the microtubules without hampering the total microtubule mass. The activation of the spindle assembly checkpoint as a result of this modification to the assembly dynamics causes mitotic arrest and eventually apoptosis, highlighting its anticancer potential
[11][15][24,26]. Noscapine is a viable possibility for cancer therapy due to its notable low toxicity and lack of immune system suppression.
Noscapine may revolutionise cancer treatment since more studies are being undertaken to see how effective it is against different forms of cancer
[16][27].
2. Non-Small Cell Lung Cancer
Noscapine, notably in the form of noscapine HCL [Nos], has emerged as a promising anticancer drug in the field of treating non-small cell lung cancer [NSCLC]. Its promise in both in vitro and in vivo conditions has been highlighted by extensive research
[11][24]. H460 cells were exposed to different concentrations of Nos in a thorough investigation, proving how powerfully it can limit cell proliferation. Nos impressively induced apoptosis, a vital component in the fight against cancer. Using Nos to treat xenografted H460 tumours in female athymic Nu/nu mice resulted in significantly smaller tumor sizes, further demonstrating its effectiveness
[17][18][28,29].
The
res
earchtudy’s most significant finding was that the modulation of important proteins, such as PARP, Bax and caspase-3, was crucial in the Nos-induced inhibition of tumour growth. Notably, Nos and gemcitabine showed an increased apoptotic impact, suggesting a potential synergistic strategy against NSCLC
[19][30]. It is significant that the work goes into the molecular elements, illuminating Nos’s role in triggering apoptosis via the mitochondrial pathways. This discovery holds great promise for the creation of NSCLC medicines that work
[20][31].
Noscapine has drawn interest due to the subpar clinical outcomes of the available therapies for NSCLC. Its promise as a strong chemotherapeutic drug has been proven by vast research conducted both in vitro and in vivo. Notably, noscapine exhibits synergistic effects when combined with other medications like gemcitabine and cisplatin, possibly altering the treatment options for people with non-small cell lung cancer
[19][20][21][30,31,32].
3. Glioblastoma
Due to the tough blood–brain barrier, glioblastoma, a particularly difficult form of cancer, presents considerable treatment challenges. Glioblastoma patients commonly have a dismal outlook because the existing chemotherapeutics frequently fail to overcome this barrier. Noscapine, however, shows promise as a treatment option because it can cross the blood–brain barrier and stop the proliferation of glioblastoma cells. Although frequent dosing is required due to its short plasma half-life, Madan et al. have investigated novel formulations to prolong its absorption into the body
[17][28].
Notably, noscapine does not cause much toxicity in vital regions like the dorsal root ganglia or different organs, suggesting that it has the potential to be used as a non-neurotoxic medication. Furthermore, it does not result in peripheral neuropathy, a problem with some microtubule-targeting drugs. To assess its impact on intracranial pressure, however, further investigation is required
[18][19][29,30].
Noscapine shines as a possible ally in the field of glioblastoma treatment. It has the ability to suppress the development of TMZ-resistant glioma cells and work in concert with present chemotherapeutics to boost their efficacy. Noscapine is advantageous for people receiving radiation therapy because it also increases radiation sensitivity. Noscapine has potential as a non-neurotoxic treatment option for glioblastoma, but more research is necessary
[20][21][22][23][31,32,33,34].
4. Thymic Carcinoma
Though extremely uncommon and categorised as orphan illnesses, thymic tumours have a noteworthy relationship with myasthenia gravis, affecting roughly 10–15% of those who have the condition. Thymoma is a cancer that presents serious challenges for patients
[7][24][7,35].
Noscapine appears to be a promising therapy option, which is encouraging. In mice with E.G7-OVA thymoma cells, a study showed that noscapine administration, either intraperitoneally or intragastrically, resulted in a decrease in tumour size
[14][24][25,35]. In addition to this encouraging outcome, DNA fragmentation was seen within 8 h of noscapine treatment, and there was a 50% rise in apoptotic bodies within 24 h
[11][24][25][24,35,36].
Additional research using methods like the TUNEL assay verified that DNA fragmentation and apoptotic nuclei occurred in both HeLa cells and E.G7-OVA cells. These results highlight the possibility of using noscapine as a thymoma therapy
[14][24][25,35]. As research develops, it provides the groundwork for possible treatment trials, giving people struggling with this uncommon but difficult ailment hope
[16][27].
5. Ovarian Cancer
Standard chemotherapeutics like paclitaxel and cisplatin [CIS] can cause human ovarian cancer cells to develop resistance, which is frequently accompanied by unsettling toxicities and poor solubility. This highlights the need for novel ovarian cancer treatment strategies
[26][27][37,38]. A possible option is noscapine, a non-toxic benzylisoquinoline derivative. Noscapine was found in a study to attach to and suppress the proliferation of ovarian cancer cells while leaving normal cells unaffected. This offers a potential remedy for the problems with drug resistance that are frequently encountered when treating ovarian cancer
[28][29][30][31][32][33][39,40,41,42,43,44].
In addition, noscapine inhibits mitosis in paclitaxel-resistant ovarian cancer cells via interacting differently with tubulin from paclitaxel. This novel mode of action has the potential to overcome beta-tubulin mutation-related resistance
[10][34][35][36][13,45,46,47]. Additionally, noscapine and cisplatin work together synergistically. This not only prevents drug-resistant cells from proliferating but also alters the expression of genes associated with apoptosis, increasing the number of tumour cells that undergo this process
[37][38][39][40][41][48,49,50,51,52]. It is interesting to note that noscapine reverses tumoural resistance, improving the efficacy of doxorubicin and vincristine, two other chemotherapeutics. This multifaceted strategy underlines the potential of noscapine as a useful addition to the toolkit for treating ovarian cancer
[42][43][44][45][53,54,55,56].
To sum up, noscapine appears to be a potential contender in the fight against ovarian cancer, providing fresh hope for patients dealing with drug resistance and the difficulties posed by conventional chemotherapies. Noscapine has the potential to transform the therapeutic approaches to and enhance the outcomes for patients suffering with this severe disease because of its unique modes of action
[7][11][45][46][47][48][7,24,56,57,58,59].
6. Gastric Cancer
Gastric cancer is one of the most prevalent types of cancer globally, especially in the Eastern parts of Asia, Europe and the Andean regions of South America. The alarming 9 million instances reported annually globally highlight the need for more expedient treatment options, particularly those utilising innovative methods. Noscapine has emerged as a promising candidate for the treatment of stomach cancer due to its extensive anticancer characteristics
[7][11][16][7,24,27].
Noscapine’s effect on stomach cancer cells was carefully explored in a study performed by Liu et al. It was conclusively proven by extensively studying four different cell lines that noscapine caused apoptosis, greatly reducing cell viability. Notably, treatment with noscapine resulted in a striking decrease in the number of BGC823 cells that were still alive
[49][50][60,61]. The researchers expanded their investigation by performing studies on mice that had tumour xenografts. Noscapine was given intravenously to each group of these animals at regular intervals. Positively, compared to the control group, this treatment plan led to the development of smaller tumours. Caspases-3 and 9 were also activated, which supported the involvement of these pathways even more
[17][51][28,62].
Noscapine elevated important proteins like Bax and cytochrome c while downregulating Bcl2, according to further study. The Bax/Bcl-2 ratio, a critical sign of apoptosis via the mitochondrial pathways, was significantly raised by this change. Caspases-3 and 9 were again activated, which further suggested the involvement of these pathways
[51][62]. These encouraging preclinical results strongly imply that noscapine therapy has significant potential for patients with gastric cancer, providing some hope in the search for more efficient treatments for this difficult cancer
[52][63].
7. Colon Cancer
The treatment of colorectal cancer is incredibly difficult because it is notorious for being resistant to modern chemotherapy. According to a study, the efficiency of noscapine is hugely dependent on its capability to cause G2/M arrest and apoptosis, and also on the p53/p21 factor and on the susceptibility of cancer cells in the colon [HCT116] to the drug.
The maximum sensitivity to noscapine was seen in cells with intact p53, whereas the highest resistance was seen in cells without p53. The crucial function of p53 was demonstrated by the reinstatement of noscapine-induced apoptosis after p53 was introduced into previously deficient cells
[53][54][55][56][64,65,66,67].
It is interesting to note that p21-null cells continued to resist apoptosis in the presence of high p53 levels, demonstrating that p53 is required but insufficient for noscapine-mediated apoptosis and that p21 has a proapoptotic role. These findings suggest that noscapine holds potential as a therapeutic agent for treating colon cancer, particularly in cases where the p53 and p21 expression levels can be modulated
[54][55][56][57][58][65,66,67,68,69].
In addition, another study discovered that noscapine activated the PI3K/mTOR signalling pathway while reducing the PTEN expression in specific colon cancer cells. Furthermore, it was observed that noscapine significantly induced apoptosis in these cells, indicating its potential as an anticancer agent for colon cancer treatment. This is in line with the general consensus that focusing on vital metabolic enzymes can improve drug-induced apoptosis in cancer cells and possibly result in more efficacious treatments. Combining targeted medications with metabolic inhibitors is a viable way to tackle cancer treatment resistance, according to these pooled insights. This may mark a significant development in the fight against cancer, especially for notoriously difficult cases like colorectal cancer
[51][53][58][62,64,69].
8. Breast Cancer
Noscapine has proven to be an impressively effective treatment for breast cancer both in vitro and in vivo. It showed promise in slowing the growth of human and murine breast tumours when administered to animals, mostly by inducing apoptosis. In vivo studies showing a remarkable 80% regression in human breast cancers further supported this promise in hormone-receptor-positive MCF-7 breast cancer cells
[49][60]. The ability of noscapine to kill triple-negative hormone-resistant breast cancer cells is particularly encouraging. It showed a discernible decrease in the tumours caused by MDA-MB-231 xenografts in mice. Even more intriguing is the fact that it showed a synergistic impact when paired with doxorubicin, indicating a potentially advantageous combination for triple-negative breast cancer patients, who currently have limited treatment options
[59][60][70,71].
Innovative noscapine-loaded estrone-conjugated gelatin nanoparticles [Nos-ES-GNs] were developed to address the issue of noscapine’s short biological half-life, poor absorption and limited solubility. These nanoparticles had an IC50 value that was almost 50% lower than the free medication, demonstrating improved efficacy. Additionally, the study showed that estrone-conjugated noscapine-loaded gelatin nanoparticles accumulated more in MCF-7 cells with oestrogen receptor positivity than in MDA-MB-231 cells with oestrogen receptor negativity, indicating the possibility of precision targeting
[60][71].
Additional research, such as that carried outby Chouguleet al., highlighted the dose-dependent antitumour impact of noscapine. Triple-negative breast cancer was treated with oral noscapine [550 mg/kg] and doxorubicin [1.5 mg/kg], which showed a three-fold improvement in antitumour activity. Additionally, research using approximately 36 M of noscapine showed that it inhibits the multiplication of breast cancer cells
[59][70]. NPN [VinPhe-Nos], a noscapine derivative, has become a promising contender for the treatment of invasive malignancies. It significantly reduced the growth of new malignant colonies and effectively stopped the cell cycle during crucial stages. Studies also demonstrated NPN’s capacity to attach to tubulins and alter their tertiary structure, presenting an interesting line of inquiry
[61][62][63][72,73,74].
Noscapine had a negligible effect on microtubules, whereas NPN emerged as a more powerful disruptor, severely harming microtubules and preventing their reconstruction. These results highlight the potential of noscapine and its derivatives to completely alter the way that breast cancer is treated
[50][61].
9. Prostate Cancer
Many people have serious concerns about prostate cancer, especially in light of the fact that, despite improvements in detection and understanding, its metastatic forms are still challenging to treat. Although its early detection has improved, there are currently few viable therapeutic choices for metastatic prostate cancer. Docetaxel is currently used at its highest tolerated dose, but this can have serious adverse effects, including peripheral neuropathy, gastrointestinal toxicity, immunosuppression and myelosuppression. A primary priority is the hunt for a chemotherapeutic drug that is both more efficient and less crippling
[23][64][34,75].
Noscapine exhibited potential in lowering the tumour volume in prostate cancer in a promising research work conducted by Barken et al. Noscapine-treated mice had considerably fewer primary and metastatic tumours than the animals in the control group over the course of two months. Additionally, the group that received noscapine treatment had a greatly decreased rate of metastasis. Comparatively to the control group, where metastasis occurred in 90% of instances, noscapine administered orally each day at a dose of 300 mg/kg led to a marked decrease in metastasis. Given this, it is possible that noscapine can stop or slow the progression of prostate cancer to the lymph nodes
[23][34].
Remarkably, there was no statistically significant difference in lung metastasis between the treatment and control groups. This implies that further research is needed to ascertain whether noscapine can genuinely improve metastasis-free survival in humans.
There is hope for more effective treatments with less disabling side effects thanks to this encouraging breakthrough in the investigation of noscapine’s influence on prostate cancer
[64][75].

 Numerous cancer cells, including drug-resistant varieties, exhibit cell growth inhibition when treated with noscapine, but healthy cells are left unaffected. This exceptional selectivity opens the door to a promising new approach to targeted cancer therapy. Furthermore, noscapine is a different possibility in the battle against cancer due to its effect on microtubules, which differs greatly from that of other drugs [10][12][13][11,13,21].
It is interesting to note in Figure 2 that noscapine causes metaphase arrest and death in dividing cells, highlighting its potential as an anticancer drug. By altering the kinetics of microtubule assembly, it affects cell division and achieves its goal [14][25]. Noscapine can lessen the tension between kinetochores, but it does not change the tubulin polymer/monomer ratio, according to the research. As a result, the spindle checkpoint is triggered, stopping the continuation of the mitotic cycle [15][26].
Furthermore, tubulin undergoes a conformational shift upon the stoichiometric binding of noscapine, which alters the assembly of the microtubules without hampering the total microtubule mass. The activation of the spindle assembly checkpoint as a result of this modification to the assembly dynamics causes mitotic arrest and eventually apoptosis, highlighting its anticancer potential [11][15][24,26]. Noscapine is a viable possibility for cancer therapy due to its notable low toxicity and lack of immune system suppression.
Noscapine may revolutionise cancer treatment since more studies are being undertaken to see how effective it is against different forms of cancer [16][27].
Numerous cancer cells, including drug-resistant varieties, exhibit cell growth inhibition when treated with noscapine, but healthy cells are left unaffected. This exceptional selectivity opens the door to a promising new approach to targeted cancer therapy. Furthermore, noscapine is a different possibility in the battle against cancer due to its effect on microtubules, which differs greatly from that of other drugs [10][12][13][11,13,21].
It is interesting to note in Figure 2 that noscapine causes metaphase arrest and death in dividing cells, highlighting its potential as an anticancer drug. By altering the kinetics of microtubule assembly, it affects cell division and achieves its goal [14][25]. Noscapine can lessen the tension between kinetochores, but it does not change the tubulin polymer/monomer ratio, according to the research. As a result, the spindle checkpoint is triggered, stopping the continuation of the mitotic cycle [15][26].
Furthermore, tubulin undergoes a conformational shift upon the stoichiometric binding of noscapine, which alters the assembly of the microtubules without hampering the total microtubule mass. The activation of the spindle assembly checkpoint as a result of this modification to the assembly dynamics causes mitotic arrest and eventually apoptosis, highlighting its anticancer potential [11][15][24,26]. Noscapine is a viable possibility for cancer therapy due to its notable low toxicity and lack of immune system suppression.
Noscapine may revolutionise cancer treatment since more studies are being undertaken to see how effective it is against different forms of cancer [16][27].

