Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Leszek Kotula and Version 2 by Sirius Huang.
Triple negative breast cancer (TNBC) comprises 10–20% of diagnosed breast cancers. TNBCs are devoid of common biomarkers such as an estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Research is being conducted to determine the androgen receptor’s (AR) role in TNBC and determine its ability to be utilized as an effective drug target in the absence of the commonly targeted receptors.
- AR
- TNBC
- CDK4/6
- CYP17 lyase
- PI3K/AKT
- ER
- PR
- HER2
- DHT
1. Introduction
Breast cancer is one of the most common cancers in women, yet metastatic breast cancer proves to be the least curable. Specifically, TNBC, devoid of commonly targeted receptors, is an aggressive type that is difficult to treat. Additionally, breast-cancer-related deaths are largely related to metastasis [1]. The current treatment management protocol for TNBC is dependent on the programmed death-ligand 1 (PD-L1) and BReast CAncer gene 1/2 (BRCA1/2) status. If patients are PD-L1-positive, then a combination therapy of pembrolizumab plus chemotherapy is initiated [2]. If patients are PD-L1-negative, but exhibit germline BRCA1/2 mutations, olaparib therapy is initiated [3]. To treat those TNBC cases that are PD-L1- and BRCA1/2-negative, it is urgent to uncover more information regarding the related receptors and proteins that can act as drug targets, one such receptor being AR and its associated signaling pathways. AR’s specific role in breast cancer is currently widely unknown and its association to signaling pathways such as CDK4/6, CYP17 lyase, and PI3K can be a huge development in the treatment of TNBC.
Given CDK4/6’s potent activity in breast cancer cells, researchers aimed to inhibit this activity in conjunction with AR inhibitors [4]. Some studies are combining CDK4/6 inhibitors with AR modulators, the logic behind this being that when ER is positive, AR activation seems to develop tumor suppression activity [5]. If these treatments are showing success in other cancer cell lines, it is imperative to test them on TNBC cell lines as well.
CYP17 lyase inhibitors have been studied extensively in the context of prostate cancer. Many studies have sought its effects specifically in castration-resistant prostate cancer to improve overall survival [6][7][8][6,7,8]. New trials are aiming to apply this mechanistic effect on breast cancer patients, specifically using drugs that inhibit androgens via the CYP17 lyase pathway [9][10][9,10].
Similarly, most AR-targeted drugs are approved in the context of prostate cancer, but are still being studied extensively in the context of breast cancer [11]. A combination with PI3K inhibition seemed to catalyze the effects and is another important pathway to research in the ongoing search for effective drugs for TNBC patients [12]. The mechanisms behind these combination drug targets are constantly being further studied to improve treatment. Without this knowledge, the metastasis of breast cancer, in TNBC specifically, will continue to cause death among patients enduring this disease.
2. TNBC Subtype of Breast Cancer
TNBC is the deadliest subtype of breast cancer comprising 10–20% of all and characterized by its metastatic phenotype [13]. The majority of TNBC tumors are basal-like, with a reported 60–90% overlap between the subgroups, but the terms TNBC and basal-like are not interchangeable. Basal-like tumors are characterized by genes present in normal breast myoepithelial cells such as cytokeratins CK 5, CK17, P-cadherin, nestin, caveolin 1–2, CD44, and EGFR and have an increased incidence of p53 and BRCA1 mutations, which results in high genomic instability, tumor aggressiveness, and poor prognosis [14]. Basal-like tumors appear at an early age, having a large tumor size and high histological grade. They also have a high mitotic index and the pattern of metastatic relapse is aggressive, which mainly occurs in visceral organs such as the lungs, central nervous system, and lymph nodes.
The androgen receptor is a novel target in TNBC. “Luminar AR” (LR) is a subtype of TNBC with a better prognosis but resistance to neoadjuvant chemotherapy. Seminal studies by Lehman et al., 2011, defined four molecular subtypes of TNBC based on their transcriptional profiles: two basal subtypes (BL1 and BL2), a mesenchymal (M) subtype lacking immune cells, and a luminal androgen receptor subtype (LAR) that is enriched in AR expression and follows its transcription program [15].
3. Why Target Androgen Receptor?
The initial results from clinical trials indicated a potential clinical benefit for targeting the AR pathway in breast cancer [12][16][12,16]. Retrospective studies in patients demonstrated that LAR tumors are less responsive to standard chemotherapy than other TNBC tumors, underscoring the need for the identification of novel therapeutic strategies [16][17][16,17].
AR is expressed in normal breast tissue and decreases to 10–50% expression in TNBC [18][19][18,19]. In prostate tumors, many pertinent pathways involved in signaling are regulated by AR activity. AR activity has also been linked to cell cycle promotion, cell metabolism and growth, and other vital cellular processes [20][21][20,21]. Thus, researchers are interested in applying this information to breast cancer studies.
AR can also be utilized as a target and possibly be co-targeted with the other signaling molecules it often interacts with, such as CDK4/6, CYP17 lyase, and the PI3K/AKT signaling pathway, as seen in Figure 1. Some AR expression is beneficial for prognosis, too much expression, however, along with heightened activity, leads to enhanced tumor growth [22]. AR’s effects on tumor growth are outlined by the different subtype being studied and the implicated pathways. In ER (−), HER 2 (+) breast cancer, AR transcriptional activity is promoted which increases tumor growth. In TNBC, a similar effect is observed. In breast cancer tumors with ER expression, AR and ER regulate each other’s transcription, and the ratio between the two receptors determines the outcome [22]. AR works in collaboration with many other proteins and pathways, making it an important biomarker for targeting and co-targeting.
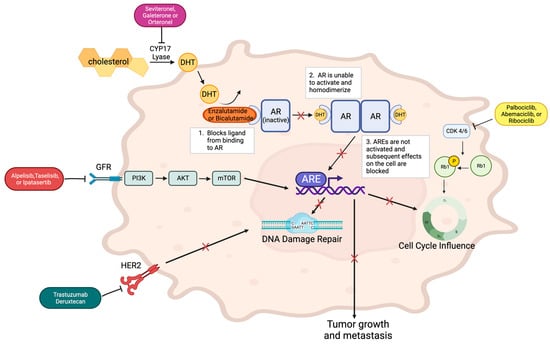
Figure 1. AR is inactive until DHT binds it, allowing for activation via homodimerization. This homodimer binds to androgen response elements (AREs) to contribute to effects on cell cycle, DNA damage repair, and metastasis. The binding action of AR inhibitors, enzalutamide or bicalutamide, blocks the homodimerization of the receptor and its downstream effects. CYP17 lyase inhibitors such as seviteronel, galeterone, or orteronel can augment the effect of AR inhibitors by blocking the CYP17 lyase pathway, which is imperative to forming the DHT that binds and activates AR. PI3K inhibitors can also block expected downstream effects of AR. These inhibitors include alpelisib, taselisib, and ipatasertib. They bind the receptor upstream of the PI3K-AKT-mTOR pathway, which is involved in regulating the growth and proliferation of the cell. CDK4/6 inhibitors such as palbociclib, abemaciclib, and ribociclib block CDK4/6, the kinase responsible for phosphorylating Retinoblastoma (Rb) tumor suppressor gene, which regulates the cell cycle. When this kinase is inhibited, cell cycle regulation is altered, specifically, the checkpoint between the G1 and S phase, amplifying the effects of AR inhibitors on the cell cycle. Trastuzumab deruxtecan inhibits the HER2 receptor, which diminishes its normal impact on DNA damage repair.
AR in the Context of Hormone Dysregulation
AR is activated by androgens such as dihydrotestosterone (DHT), while its inactive form is bound to heat shock proteins [20]. AR captures androgen in the cytoplasm and is subsequently activated to transport to the nucleus and bind to DNA, promoting the transcription of certain genes affecting cell cycle progression such as MYB or CCND1 [23]. The role of AR in females is still not well understood. In prostate cancer, the interaction between AR activity and the signaling pathways it regulates, such as the PI3K and RAS pathways, is well known. AR dysregulation serves as a signal for hormonal disorders such as androgen insensitivity and prostate cancer. For many cases, androgen deprivation therapies have proven successful, as the activation of its signaling promotes tumor growth [20]. In breast cancer, the role is not as unambiguous. For example, in cases where ER is present, AR has exhibited tumor suppressor activity, and selective AR modulators are proving to be more effective treatment options as compared to AR inhibitors [23].
