Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Clarissa Amanda Seidler and Version 2 by Jessie Wu.
Allergens are substances that cause abnormal immune responses and can originate from various sources. IgE-mediated allergies are one of the most common and severe types of allergies, affecting more than 20% of the population in Western countries. Allergens can be subdivided into a limited number of families based on their structure, but this does not necessarily indicate the origin or the route of administration of the allergen, nor is the molecular basis of allergenicity clearly understood.
- allergens
- IgE
- cross-reactivity
- pH-dependence
- linear epitope
1. Structural Characterization and Cross-Reactivities—What Makes a Protein an Allergen?
Allergens do not have a single “allergen-specific fold”, but rather exhibit various folds and tertiary structures that contribute to their allergenic activity. Typically, allergens are relatively small proteins, ranging in size from 5 to 100 kDa [1]. These sizes may be subject to artefacts due to multimer formation: some protein allergens form dimers, trimers or other large multimers in their natural state. The most prominent examples are the major peanut allergens Ara h 1 and Ara h 3, which naturally form trimers of bicupins and hexamers of bicupins, respectively. Based on the characterization by SDS-PAGE, these multimers are often monomerized, which can lead to artefacts in determining allergen size [2].
Attempts have been made to group allergens into families. AllFam is a database that aims to classify allergens into families based on common structural and functional properties [3]. The classification is supported by the definitions of Pfam. Currently, 959 out of 1042 known allergens are classified into a total of 151 allergen families, of which only 23 are populated with at least 10 members (https://www.meduniwien.ac.at/allfam/browse.php, accessed on 17 December 2023) [4][5]. Prominent examples of such families include the pathogenesis-related (PR) protein class 10, which contains the major birch-pollen allergen Bet v 1, profilins, which include the ragweed pollen allergen Amb a 8, or the Group 5/6 grass pollen allergens, with the timothy grass pollen allergen Phl p 6 as a member [6][7][8]. Proteins within the same family are known to frequently cause cross-reactions. However, structural similarity does not necessarily correlate with a common immune response. There are proteins that are structurally similar to known allergens but do not trigger allergic reactions.
Cross-reactivity can occur when specific IgE antibodies are able to bind not only to the original epitope but also to structural elements that have high similarity to other allergens. Importantly, not the entire polypeptide chain of the allergen is involved in antigen binding since mainly the surface residues are fundamental for antibody recognition.
The concept of cross-reactivity describes the relationship between more than one allergen to an IgE antibody. Sensitization occurs when the immune system first encounters an allergenic substance, leading to the production of allergen-specific antibodies. If these antibodies can also recognize a secondary allergen through cross-reactive epitopes and link the protein to mast cells, cross-reactivity may occur. An allergen very often has homologous proteins originating from very distinct sources, and also the route of exposure may not be the same for the primary and the secondary cross-reactive allergen. It is very common for allergic patients to have a pollen allergy and to be cross-reactively allergic to certain foods. For instance, patients may be allergic to inhaled birch pollen as well as to apples when consumed orally [9][10]. In addition, recent studies show that the airway may be an alternative sensitization pathway for food allergies, which is consistent with the multiple pathway theory mentioned earlier [11]. This condition is also referred to as oral allergy syndrome (OAS) or pollen-food syndrome. It is triggered by cross-reactivity due to structural similarities. For instance, birch pollen-apple allergy is caused by PR10 family allergens that have a highly conserved structural fold. Another crucial factor in OAS is the impact of food processing. Some allergens are sensitive to physical processes such as heating during the cooking process. These processes can affect the structure and conformational epitopes of protein allergens, which in turn affects their allergenicity. For example, patients who are allergic to eating raw apples may no longer be affected after cooking. Identifying the conformational epitopes involved in OAS, and the modifications of such post-processing, could be crucial in understanding OAS and developing strategies to prevent allergic reactions in patients [12]. One further significant consideration of food processing is the formation of neo-allergens, which are new allergenic compounds formed through the Maillard reaction when different food ingredients interact during cooking. Heating processes can accelerate this reaction, which can alter allergen epitopes through glycation reactions [13].
The cross-reactivity discussed earlier pertains to IgE interactions. When a T-cell is reactive to more than one peptide-MHC ligand, stimulation of the T-cells is possible, which can further induce the production of IgE antibodies. This is known as T-cell cross-reactivity. In both cases of cross-reactivity, sequence and structural homology play important roles, as does physiochemical stability [10].
To distinguish allergenic proteins from their non-allergenic counterparts, several hypotheses have been proposed. Two factors may come into consideration to play a role in determining the allergenicity: the abundance and the stability of a particular protein. Abundance is related to the probability of contact, thereby modulating the likelihood of allergenic sensitization, while stability correlates with the ability to not be degraded too early in the gastric system or even in the endosome [14]. This second property is particularly crucial for allergens that are ingested orally, such as food allergens, but also for other routes of administration.
To assess allergenicity, a series of tools have been developed. These tools either focus on the sequence similarity to known allergens, on the recognition of known motifs of allergens, or on the biophysical properties of surface patches or residues [15]. The latter ones are mostly machine learning methods, which are based on molecular descriptors. The sequences of a potential allergen are characterized by the biophysical properties of the individual residues, such as size, hydrophobicity, abundance, or the ability to form certain secondary structures. These properties are then transformed into binary fingerprints, resulting in comparable coefficients.
Protein allergens are complex molecules. Their conformational and structural arrangements play a central role in determining allergenicity and immunogenicity, i.e., whether a protein is recognized as foreign. The exposure of epitopes is influenced by conformational rearrangements and flexibility, which in turn are properties that co-determine allergenicity. Conformational rearrangements can affect the susceptibility of proteases responsible for the digestion and processing of allergens, thus directly affecting the immune response. This also applies to non-protein allergens, where the triggering substance is often a small peptide or molecule. In most cases, the drug is too small to be presented directly on the surface of antigen-presenting cells, so complexation with carrier molecules or polypeptides is critical, as small molecules are sometimes recognized only in the form of a drug–protein conjugate [16].
For the cross-linkage between the receptor on the surface of mast cells or basophils and the antibody, an allergen must possess at least two epitopes [1]. Identifying these epitopes is a crucial step in allergen research. In the past, technical limitations restricted the identification of epitopes to those of contiguous nature, which consist of a contiguous stretch of amino acids. Investigating amino acids involved in binding in a folded protein, where residues at large distances may be in close proximity due to folding events, is experimentally much more challenging. However, allergens have well-defined and conserved structures, and the epitopes recognized by antibodies are, in most cases, of a conformational nature and therefore non-contiguous [17]. Conformational epitopes are more likely to be destroyed during allergen uptake and processing, but they seem to appear more frequently due to the folded and three-dimensional structures of protein allergens. Identifying and characterizing conformational epitopes is essential for understanding protein–protein interactions and structural determinants that cause cross-reactions and allergic immune responses [18]. Additionally, the allergenicity of an allergen is determined by its fold stability, which is affected by pH dependence and endosomal degradation. The fold stability is inherently affected by pH dependence, which, in turn, affects endosomal degradation. Furthermore, the digestion by proteases is dependent on the variability of a conformational ensemble of the allergen itself, with a higher variability of the probability of unfolding, and therewith the chances of a protease to digest the allergen are enhanced. In previous works, this has been shown to correlate in consequence with thermal stability [19][20].
2. Allergen Proteolytic Susceptibility and pH-Dependency
During sensitization, an allergen undergoes endosomal degradation, which cleaves it into small peptides. These protein fragments are then presented on the cell surface by the major histocompatibility complex II (MHC II), facilitating their recognition by CD4+ T-cells, which then induce an immune response in allergic individuals. The process of protein degradation is depicted in Figure 1A: first, the allergen enters the cell via endocytosis. Proteases cleave the allergen into small fragments, and these fragments are then presented on the cell surface by the MHC II. The naïve CD4+ T-cells recognize and bind the peptides, leading to the differentiation into active T-helper cells. Polarization into TH2-cells results in the release of cytokines that stimulate B-cells to produce IgE antibodies, causing an allergic response [21]. Since the presentation of the fragments directly depends on the ability of proteases to cleave the allergenic proteins, proteolytic susceptibility can be directly correlated to the allergenicity of a protein [22][23]. Also, the density of allergen fragments presented on the surface of antigen-presenting cells (APC) influences the destiny of the T-cell contacting the peptides. High concentrations of antigens promote TH1-cell differentiation, whereas moderate concentrations lead to TH2-cell differentiation [21].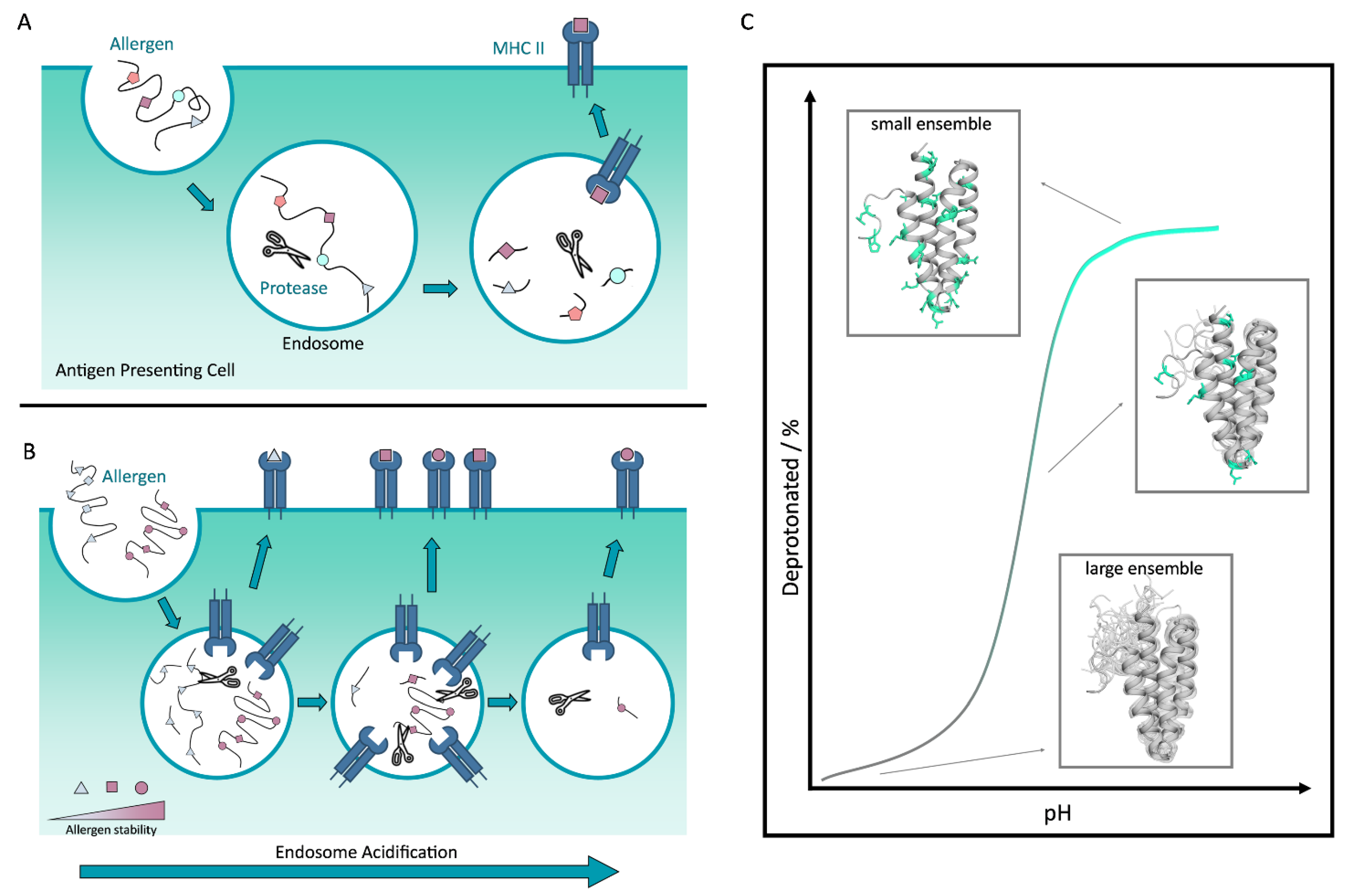
Figure 1. (A) The uptake of the allergen into the endosome of an antigen-presenting cell: the allergen enters the cell via endocytosis. In the endosome, proteases cut the allergen into small peptides, which are then loaded onto the MHC II and presented on the cell surface. (B) The acidification of the endosome in relation to different allergen stabilities. Depending on the stability of the allergen, the proteins are digested earlier or later in the phase of the maturation of the endosome. (C) Schematic representation of the structural behavior of an allergen as a function of varying pH. At low pH, a small fraction of the allergen is deprotonated and the allergen is structurally variable. The conformational ensemble is large. With increasing pH, the conformational ensemble becomes smaller, and a higher fraction of the allergen is deprotonated.
Table 1. Table listing allergens for which the effect of pH has been shown to alter the protease digestion or conformational epitopes, therewith influencing allergenicity.
| Family | Route of Exposure | Name | Origin | PDB Accession Code | Reference |
|---|---|---|---|---|---|
| Bet v 1 family | Airway | Bet v 1 | European white birch | 4A88 | [22] |
| Airway | Bet v 1 | European white birch | 1FM4 | ||
| Airway | Bet v 1 | European white birch | - | ||
| Profilin | Airway | Amb a 8 | Short ragweed | 5EVE | [7] |
| Airway Airway |
Art v 4 Bet v 2 |
Mugwort European white birch |
5EM0 5NZB |
||
| Group 5/6 grass pollen allergen | Airway | Phl p 6 | Timothy grass | 1NLX | [8] |
References
- Scheurer, S.; Toda, M.; Vieths, S. What makes an allergen? Clin. Exp. Allergy 2015, 45, 1150–1161.
- Mueller, G.A.; Maleki, S.J.; Pedersen, L.C. The molecular basis of peanut allergy. Curr. Allergy Asthma Rep. 2014, 14, 429.
- Radauer, C.; Bublin, M.; Wagner, S.; Mari, A.; Breiteneder, H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J. Allergy Clin. Immunol. 2008, 121, 847–852.e7.
- Schein, C.H.; Negi, S.S.; Braun, W. Still SDAPing Along: 20 Years of the Structural Database of Allergenic Proteins. Front. Allergy 2022, 3, 863172.
- Sonnhammer, E.L.; Eddy, S.R.; Durbin, R. Pfam: A comprehensive database of protein domain families based on seed alignments. Proteins: Struct. Funct. Bioinform. 1997, 28, 405–420.
- Führer, S.; Unterhauser, J.; Zeindl, R.; Eidelpes, R.; Fernández-Quintero, M.L.; Liedl, K.R.; Tollinger, M. The structural flexibility of PR-10 food allergens. Int. J. Mol. Sci. 2022, 23, 8252.
- Hofer, F.; Fischer, A.-L.; Kamenik, A.S.; Waibl, F.; Fernández-Quintero, M.L.; Liedl, K.R. pH-dependent structural diversity of profilin allergens determines thermal stability. Front. Allergy 2022, 3, 1007000.
- Hofer, F.; Dietrich, V.; Kamenik, A.S.; Tollinger, M.; Liedl, K.R. pH-Dependent protonation of the Phl p 6 pollen allergen studied by NMR and cpH-aMD. J. Chem. Theory Comput. 2019, 15, 5716–5726.
- Popescu, F.-D. Cross-reactivity between aeroallergens and food allergens. World J. Methodol. 2015, 5, 31.
- Kamath, S.D.; Bublin, M.; Kitamura, K.; Matsui, T.; Ito, K.; Lopata, A.L. Cross-reactive epitopes and their role in food allergy. J. Allergy Clin. Immunol. 2023, 151, 1178–1190.
- Kulis, M.D.; Smeekens, J.M.; Immormino, R.M.; Moran, T.P. The airway as a route of sensitization to peanut: An update to the dual allergen exposure hypothesis. J. Allergy Clin. Immunol. 2021, 148, 689–693.
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34.
- Gou, J.; Liang, R.; Huang, H.; Ma, X. Maillard reaction induced changes in allergenicity of food. Foods 2022, 11, 530.
- Foo, A.C.; Mueller, G.A. Abundance and stability as common properties of allergens. Front. Allergy 2021, 2, 769728.
- Doneva, N.; Doytchinova, I.; Dimitrov, I. Predicting immunogenicity risk in biopharmaceuticals. Symmetry 2021, 13, 388.
- Schnyder, B.; Brockow, K. Pathogenesis of drug allergy–current concepts and recent insights. Clin. Exp. Allergy 2015, 45, 1376–1383.
- Sanchez-Trincado, J.L.; Gomez-Perosanz, M.; Reche, P.A. Fundamentals and methods for T-and B-cell epitope prediction. J. Immunol. Res. 2017, 2017, 2680160.
- Pomés, A.; Mueller, G.A.; Chruszcz, M. Structural aspects of the allergen-antibody interaction. Front. Immunol. 2020, 11, 2067.
- Machado, Y.; Freier, R.; Scheiblhofer, S.; Thalhamer, T.; Mayr, M.; Briza, P.; Grutsch, S.; Ahammer, L.; Fuchs, J.E.; Wallnoefer, H.G. Fold stability during endolysosomal acidification is a key factor for allergenicity and immunogenicity of the major birch pollen allergen. J. Allergy Clin. Immunol. 2016, 137, 1525–1534.
- Kamath, S.D.; Scheiblhofer, S.; Johnson, C.M.; Machado, Y.; McLean, T.; Taki, A.C.; Ramsland, P.A.; Iyer, S.; Joubert, I.; Hofer, H. Effect of structural stability on endolysosomal degradation and T-cell reactivity of major shrimp allergen tropomyosin. Allergy 2020, 75, 2909–2919.
- Freier, R.; Dall, E.; Brandstetter, H. Protease recognition sites in Bet v 1a are cryptic, explaining its slow processing relevant to its allergenicity. Sci. Rep. 2015, 5, 12707.
- Kamenik, A.S.; Hofer, F.; Handle, P.H.; Liedl, K.R. Dynamics rationalize proteolytic susceptibility of the major birch pollen allergen Bet v 1. Front. Mol. Biosci. 2020, 7, 18.
- O’Hehir, R.E.; Prickett, S.R.; Rolland, J.M. T cell epitope peptide therapy for allergic diseases. Curr. Allergy Asthma Rep. 2016, 16, 14.
- Lee, J.-B. Regulation of IgE-mediated food allergy by IL-9 producing mucosal mast cells and type 2 innate lymphoid cells. Immune Netw. 2016, 16, 211–218.
- Beck, L.A.; Leung, D.Y. Allergen sensitization through the skin induces systemic allergic responses. J. Allergy Clin. Immunol. 2000, 106, S258–S263.
- Hu, Y.-B.; Dammer, E.B.; Ren, R.-J.; Wang, G. The endosomal-lysosomal system: From acidification and cargo sorting to neurodegeneration. Transl. Neurodegener. 2015, 4, 18.
- Grutsch, S.; Fuchs, J.E.; Ahammer, L.; Kamenik, A.S.; Liedl, K.R.; Tollinger, M. Conformational flexibility differentiates naturally occurring Bet v 1 isoforms. Int. J. Mol. Sci. 2017, 18, 1192.
- Hofer, F.; Kamenik, A.S.; Fernández-Quintero, M.L.; Kraml, J.; Liedl, K.R. pH-induced local unfolding of the Phl p 6 pollen allergen from cpH-MD. Front. Mol. Biosci. 2021, 7, 603644.
- Egger, M.; Jürets, A.; Wallner, M.; Briza, P.; Ruzek, S.; Hainzl, S.; Pichler, U.; Kitzmüller, C.; Bohle, B.; Huber, C.G. Assessing protein immunogenicity with a dendritic cell line-derived endolysosomal degradome. PLoS ONE 2011, 6, e17278.
- Scheiblhofer, S.; Laimer, J.; Machado, Y.; Weiss, R.; Thalhamer, J. Influence of protein fold stability on immunogenicity and its implications for vaccine design. Expert Rev. Vaccines 2017, 16, 479–489.
More
