Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Wojciech Czyzewski.
Traumatic Brain Injury (TBI) represents a significant health concern, necessitating advanced therapeutic interventions. Following injury, astrocytes exhibit reactive transformations, differentiating into pro-inflammatory (A1) and neuroprotective (A2) phenotypes.
- astroglia
- astrocytes
- traumatic brain injury
1. Introduction
Astrocytes, characterized by their heterogeneity, play a multifaceted role essential to cerebral function. These cells are instrumental in maintaining ion homeostasis, modulating the clearance of neurotransmitters, and regulating cerebral blood flow and water dynamics. Additionally, they are pivotal in the upkeep of synaptic structures and significantly contribute to the integrity of the blood-brain barrier [1,2][1][2]. Traumatic brain injury (TBI) is defined as brain damage caused by external mechanical force and it is the leading cause of death and disability especially in children and young adults [3,4,5][3][4][5]. It concerns approximately 64–74 million people annually [6]. In the United States alone, approximately 1.7 million people experience TBI, and over 5.3 million suffer from a trauma-related disability [7] reaching a 3% mortality rate [8]. This results in costs of roughly 56–221 billion US $ which are spent on diagnosis, treatment, and rehabilitation of patients with TBI each year [9]. The global incidence of TBI from any cause and all severity is estimated at 939 per 100,000 people. Brain injuries are traditionally classified by the Glasgow coma scale (GCS) according to their severity into mild (13–15 GCS points ), moderate (12–9 GCS points), and severe (<9 GCS points) [10,11][10][11]. The first group includes the least serious injuries and accounts for 80–90% of the total. They are usually caused by blunt, non-penetrating head trauma. Symptoms are usually transient and not very specific. They may include headache, mild cognitive symptoms, memory problems, nausea, and vomiting [4,6][4][6]. Moderate and severe TBI are associated with greater force of the trauma and their effect are often visible in imaging tests. The most severe of the groups is also associated with a very high mortality rate, both in the acute phase and later, reaching 35–75% [5,12,13][5][12][13]. However, despite its much lower prevalence, it is moderate and severe TBI that pose a serious problem for medicine. It is estimated that they cause approximately 90% of total TBI medical costs [14]. Many efforts have been implemented to improve the prognosis of patients as much as possible; however, no specific neuroprotective measures have been identified that can affect the mortality of patients after TBI on a broad scale [15]. Current guidelines for the management of trauma patients are primarily aimed at reducing secondary brain damage caused by a cascade of physiological processes that are the body’s response to injury. For this reason, it is so important to properly understand these relationships. As studies have shown, astrocytes play a key role in this process through the mechanism of reactive astrogliosis. According to preceding studies, restoration and regeneration following brain trauma is as complex as it is challenging due to various aspects [16]. Numerous cellular groups and biochemical compounds participate in this process, among which astrocytes are a key component. During the acute phase, injury results not only from the direct impact of external force upon the body but also from the reactive responses of astrocytes and microglia cells. These cells undergo alterations in their transcriptional and morphological profiles, leading to the initiation of both pro-inflammatory and anti-inflammatory processes. These physiological adaptations are designed to facilitate neuronal regeneration and the clearance of damaged tissue [17,18][17][18]. Upon exposure to specific stimuli, frequently originating from central nervous system (CNS) injuries or pathologies, astrocytes undergo a significant transformation into a reactive state. This process, termed “reactive astrogliosis”, is characterized by substantial changes in gene expression, cellular morphology, and functional dynamics [19]. Multiple theoretical frameworks have been proposed to elucidate the mechanisms underlying astrocyte responses to cerebral injury. Confronted with brain trauma, astroglial cells exhibit notable alterations in both functionality and phenotypic characteristics [20,21,22][20][21][22]. Although this reactive state of astrocytes can be conducive to healing and recovery, exemplified by functions such as stabilizing the blood-brain barrier (BBB), facilitating neurogenesis, and secreting neurotrophic compounds, it also has the potential to produce adverse effects. A significant consequence is the formation of a glial scar, traditionally regarded as an impediment to CNS repair. This scar, emerging from the collective response of reactive astrocytes, is a primary factor exacerbating neuronal regeneration. The fibrotic scar tissue not only presents a physical and chemical barrier but also disrupts the process of synaptogenesis [23,24][23][24].
2. Role of Astroglia in Brain Injury
2.1. Blood Brain Barrier
One of the influences of astrocytes on the homeostasis of the CNS is their effect on the blood-brain barrier. Astrogliosis acts protectively on surrounding neural tissue through the maintenance of the blood-brain barrier (BBB), repair of damaged tissues, production of neuroprotective compounds, and mediation of inflammation [22,27,28][22][25][26]. This process is possible because of the communication with endothelial cells [29,30,31][27][28][29]. Astrocytes also oversee the functioning of the BBB through specialized astrocytic extensions known as “endfeet” that are equipped with the potassium channel Kir 4.1 and Aquaporin-4, which play a crucial role in regulating ion and water balance, contributing to BBB integrity [32][30]. In the event of insult to the brain, astrocyte-derived factors possess a key role in recovery and disruption of the blood-brain barrier. The astrocyte-derived factors that influence vascular permeability encompass vascular endothelial growth factors, glutamate, matrix metalloproteinases, nitric oxide, and endothelin-1. These elements heighten the permeability of the blood-brain barrier, ultimately leading to its disruption.2.2. Immunological Response
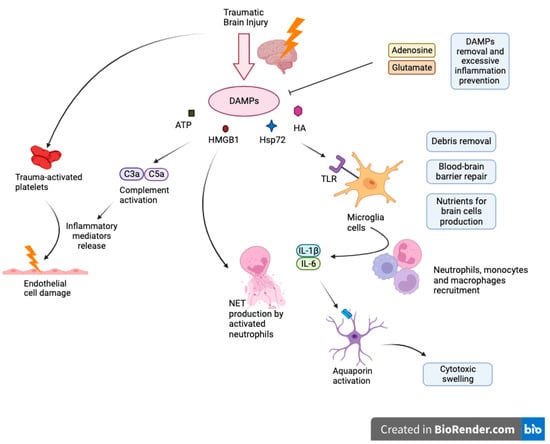
Activated astrocytes are important regulators of immune response. In response to damage, astroglial cells release neurotrophic factors that act to protect the brain [33][31]. For example, brain-derived neurotrophic factor (BDNF) secreted by astroglial cells has been shown to combat drug-related neurotoxic effects and neuronal degeneration associated with normal aging. Suboptimal levels of BDNF have been associated with neuronal death [34,35][32][33]. Another neuroprotective contribution is through the release of 17β-estradiol (E2) compounds from activated astrocytes [36,37][34][35]. E2 is important in mediating inflammatory responses and in reducing microglial activation in the brain [38,39][36][37]. Overactivation of microglia can lead to a feedback loop which induces more reactive astrogliosis and further inflammatory damage as a result [40,41,42][38][39][40]. Also, astrocytes function as an inhibitor of neuroinflammation through various physiological pathways such as TGF-β signaling or estrogen receptor signaling pathway [43,44][41][42]. Even though astroglia can downregulate inflammatory responses, its activation can also increase inflammation. Studies have found that activated astrocytes are associated with higher amounts of inflammatory markers [45,46][43][44]. Cytokines released from astrocytes also promote inflammation through microglial cells after traumatic brain injury [47,48][45][46]. Glial cell necrosis mitigates cell’s functionality what triggers the release and subsequent accumulation of molecules known as Damage-Associated Molecular Patterns (DAMPs), such as intracellular ions, nucleic acids, high mobility group box 1 protein (HMGB1), heat shock protein 72 (Hsp72), HA and ATP. These DAMPs activate immune receptors, notably Toll-Like Receptors (TLR), Receptors for Advanced Glycation End Products (RAGE) or purinergic receptors on myeloid cells encompassing macrophages, glial cells, dendritic cells, and astrocytes which cause their activation as well as inflammasome assembly (NALP1) that advocates generation of cytokines as IL-18 or IL-1Β [53][47]. Upon activation, resident microglia undertake cleanup of cellular debris, restore the integrity of the damaged blood-brain barrier, and aim to deliver essential nutrients requisite for neuronal cells. However, microglia exhibit a high degree of flexibility, and their role in a non-infectious immune response is determined by their activation status, the extent of the damage, interplay with adjacent cells, and the makeup of the immune cells that have infiltrated the area. Further release of proinflammatory cytokines such as IL-1β and IL-6 facilitates the recruitment of peripheral immune cells into the site of injury. When neutrophils traverse the BBB, they exacerbate leukocyte activation, proinflammatory cytokines levels, and incite brain tissue swelling Figure 1.
Figure 1.
Cascade of neuroinflammation in Traumatic Brain Injury: DAMPs, glial activation, and systemic consequences.
2.3. Role in Synthesis and Function of Synapses
In addition to providing support to neuronal cells, astrocytes also exist as basic components of the neuronal circuit known as the tripartite synapse and are essential to the structural integrity of synaptic transmission in the brain [57,58,59,60][48][49][50][51]. Tripartite synapses refer to the integrative communication between the presynaptic neurons, postsynaptic neurons, and the surrounding glial cells [61,62][52][53]. In the pathogenesis of altered consciousness or neurodegenerative diseases, astrocytic interactions within tripartite synapses seem to play a role in mood and behavior alterations and the predisposition to neurological diseases [63,64,65][54][55][56].
Astroglia serves a morphological and functional role in these synapses [57,66][48][57]. As part of the structural components of the tripartite synapse, astrocytes act as a controller for the active metabolic milieu surrounding neurons [60,67,68,69][51][58][59][60]. The metabolic milieu in the CNS is important in the formation, maintenance, and function of synapses. Perisynaptic astroglia regulate the CNS milieu through various mechanisms and pathways such as altering extracellular ions, regulating pH, managing waste, and assisting in the exchange of signaling molecules [70,71,72][61][62][63].
Studies have found that under circumstances where astroglia function is compromised, the chemical environment can become neurotoxic and damaging to the surrounding neuronal tissue, thereby negatively affecting synaptic transmission [40,73][38][64].
In conjunction with synaptogenesis, astroglial cells can also control synaptic plasticity [94,95][65][66]. Astrocytes release neuromediators that induce an increase in the formation of excitatory synapses which are associated with the expression of synaptic plasticity [96][67]. The induction of synaptic plasticity plays a role in memory formation [97,98][68][69]. Contrarily, astrocytes also have the potential to inhibit neural plasticity [99][70].
Astrocytes are also essential for neurovascular coupling (NVC), which coordinates communication between neurons and blood vessels in the brain. These versatile cells contribute significantly to proper blood vessel density and branching during development, as well as in conditions like ischemic stroke, traumatic spine injury, and by analogy TBI. In response to neurotransmitter release, like glutamate and ATP, astrocytes elevate Ca2+ levels, releasing vasoactive molecules onto blood vessels to drive NVC [102][71].
Astrocytes also dynamically regulate cerebral vessel diameter through Ca2+-dependent mechanisms, releasing substances such as EETs (epoxyeicosatrienoic acids) and PGE2 to dilate vessels or 20-HETE (20-hydroxytetraenoic acid) to constrict them. Additionally, they help maintain ion balance by absorbing excess extracellular K+ during neuronal activity, which triggers Ca2+ increases, activates BK channels, and releases K+ onto vascular mural cells.
Astroglia also plays a role in synapse maturation. Several astrocytic-derived genes are associated with proteins that participate in the maturation of synapses and neuronal circuits [104][72].
2.4. Scar Tissue Formation
Reactive astrocytes play a crucial role in isolating brain injuries by forming glial scar tissue around the injury site [114,115,116][73][74][75]. While sealing off injuries provides a protective mechanism, reactive astrogliosis can also hinder brain recovery by blocking axon re-growth and adopting neurotoxic behaviors [117,118,119][76][77][78]. The formation of dense glial scars creates both physical and chemical barriers that obstruct axonal regeneration, potentially leading to further neuronal death [120,121,122,123][79][80][81][82]. In the wake of neural tissue damage, the immediate response involves inflammation and cell death [124,125,126][83][84][85]. This inflammation then drives the formation of scar tissue through cell proliferation, aiming to replace the damaged tissue [127][86]. The neural tissue has two mechanisms in which scarring can occur. One of these scarring systems is modulated through fibroblasts [23,128,129][23][87][88]. The other scarring is facilitated by reactive astrocytes in conjunction with reactive microglial cells and glial precursor cells [130][89]. Astrocytes play an integral part in the support network surrounding neural tissues such as glutamate reuptake, maintenance of the blood-brain barrier, and homeostasis [16,131,132][16][90][91]. Studies have found the expression of both the P2X and P2Y receptors on the cell surface of astrocytes [143,144][92][93]. In the CNS, ATP acts as a neurotransmitter and participates in neuromodulation. Similar to the actions of ATP in other parts of the body, ATP released after neural tissue damage stimulates the P2X and P2Y cell surface receptors that are present in astrocytes in the CNS [145,146][94][95]. The extent of neural damage is correlated to the presence of extracellular nucleotides in CSF [147][96]. These ATP-activated purinergic receptors are shown to be involved in astrogliosis or reactive astrocyte formation and subsequently the formation of scar tissue [148][97]. There are two types of reactive astrocytes that are important in the formation of neural scar tissues: A1 and A2 [149][98]. A1 astrocytes stimulate the death of neurons and oligodendrocytes whereas A2 astrocytes are considered neurotropic and neuroregenerative [118,150][77][99]. Both A1/A2 astrocytes are stimulated by inflammatory signals released by microglia in response to insults [151,152][100][101]. The various types of astrocytes may explain both the inhibitory and protective role of astrocytes during the recovery process after injury [153,154][102][103]. In its inhibitory role, astrocytes have been associated with inhibition of axonal regeneration [163,164][104][105]. Reactive astrocytes produce chondroitin sulfate proteoglycans (CSPG) [165][106]. After an injury to neural tissue, the expression of CSPG is upregulated and greatly increased [166,167][107][108]. Studies have shown that CSPG contributes to the failure in neural tissue regeneration by inhibiting neurite growth [168,169,170][109][110][111]. Therefore, the release of CSPG acts as an inhibitory factor to the migration of cells and axons [171,172,173][112][113][114]. For normal glial scar formation to occur, intermediate filaments such as glial fibrillary acidic protein (GFAP) and vimentin are needed [175,176][115][116]. In the immediate response to neural tissue injury, GFAP is released by reactive astrocytes. Therefore, GFAP can be utilized as a marker for the presence of astrocytes [177,178][117][118]. At the site of insult, the amount of GFAP is related to the amount of reactive astroglial cells [179,180][119][120]. GFAP is an important intermediate filament that allows astrocytes to become hypertrophic through the synthesis of cytoskeletal structures and elongation of processes [181,182][121][122]. Research findings indicate that the absence of insulin receptors (IRs) in astrocytes, specifically in GFAP-expressing astrocytes, results in reduced expression of the vascular endothelial growth factor pathway. IRs in these astrocytes play a crucial role in brain glucose regulation and responses to glucose. They facilitate the entry of circulating insulin into the brain, impacting glucose uptake, brain perfusion, mitochondrial function, and brain vascularization. The presence of IRs in astroglial end-feet, particularly in GFAP-expressing astrocytes, supports their role in neurovascular coupling and angiogenic signaling. Altered angiogenic signaling in mice lacking astrocytic IRs, specifically in GFAP-expressing astrocytes, may result from changes in mitochondrial function. Several cytokines such as IL-10, IL-6, and TNF-ɑ that are produced in response to neural damage may play a regulatory role in the proliferation of astrocytes and thus, the formation of glial scar tissue [185,186,187][123][124][125]. Another factor that may be important in the activation of astrocytes following injury is the TGF-β and Smad 2/3 signaling pathway [188,189][126][127]. Increased expressions of GFAP are associated with the Smad 2/3 signaling pathway which also affects the activation of TGF-β signaling [190,191][128][129]. Smad signaling pathway has also shown a reduction in the amount of immune cells and astrocytes present after injury, aiding in faster wound closure and reduction in cell proliferation [192][130]. During the healing process, damaged scar tissue formed by the reactive astrocytes that have undergone hypertrophy and morphological changes become scar-forming astrocytes [195][131]. The recruitment of scar-forming astrocytes forms a protective border around inflamed tissue, separating it from healthy neural tissue, and aiding in the recovery process [196,197][132][133]. The protective border formed by astrocytes aids in blood-brain barrier repair through the release of ECM components [198][134]. Scar borders are shown to have been formed from newly proliferated astrocytes that have extended processes [199,200][135][136]. These elongated processes have been shown to exhibit overlapping morphology creating a mesh-like network [201][137]. Astrogliosis and its protective effect are mediated by a transcription factor, STAT3 [202,203][138][139]. STAT3 also plays a role in the maturation of glial scars [204][140] which occurs within weeks after injury [205][141].2.5. Neural Stem Cells and Astrocyte Generation
Radial glial cells (RGCs), which maintain self-renewal and differentiation characteristics and are responsible for the successive creation of neurons, astrocytes, and oligodendrocytes, are generated from neuroepithelial cells of the neural tube throughout the formation of the human CNS. RGCs start directly differentiating into astrocytes or beginning to produce glia-restricted intermediate progenitor cells that can differentiate into astrocytes or oligodendrocyte precursor cells at about 12 post-conceptional weeks in humans [212][142]. As for the spinal cord, astrocytes are produced in all of its domains following neurogenesis. They migrate laterally along the trajectories of their progenitors following a glial switch and do not migrate tangentially from their domains of origin. A bHLH gene is expressed only in the ventral p2 domain of stem cell leukemia (SCL) and suppresses Olig2 and oligodendrocyte production. The p1, p2, and p3 domains are distinguished by the combinatorial expression of homeodomain proteins Pax6 and Nkx6.1, which are guided by neuronal migration factors such as Slit1 and Reelin in the white matter of the spinal cord. These findings reinforce the notion of region-specific astrocyte subtypes that upon generation stick to coded pathways [213][143]. Adult neural stem cells are located in the dentate gyrus, in the subgranular zone, and lateral ventricles in the subventricular zone, generating new neurons throughout our life [214][144]. There are distinct differences between these two zones; the dentate gyrus-based neurons stay in their original place becoming interneurons of the olfactory bulb [215][145], while their LV-generated counterparts’ fate leads them to superficial granule cells and peri-glomerular cells [26][146]. Mechanically, neural stem cells (NSCs) tend to start their proliferation as adherent cells, forming neurospheres in vitro [216][147]. The Sonic hedgehog morphogene-dependent cells that reside in the ventricular zone of the amygdala-hippocampal area during late gestation contribute to the formation of the SGZ in the perinatal stages and are an interesting point for further exploration [218][148]. Signaling pathways and dynamic transcription factor expression trigger transformation in NSCs resulting in gliogenesis [91][149]. The components involved in these conversions are mentioned: bone morphogenic protein (BMP) families, the leukemia inhibitory factor/ciliary neurotrophic factor (LIF/CNTF), and the Notch pathway [91][149]. Neurons formed from neural stem cells are undergoing a “gliogenic switch” so that NSCs achieve the ability to generate glial cells—predominantly astrocytes and oligodendrocytes. In turn, the NPCs (neural progenitor cells) convert into glial precursor cells (glioblasts), which through a series of divisions and migrating along radial glia processes, transform into astrocytes. In the last phase of NPCs differentiation by disconnecting radial glia from VZ is formed unipolar transitional radial glia (tRG), which develop into protoplasmic and fibrous astrocytes in the cortex [91][149]. Steps taken that were necessary for such an outcome were to induce differentiation of hPSC by blocking SMAD signaling. Afterward, the aforementioned SHH and WNT signaling pathways were inhibited using cyclopamine and DKK1, forming proper neural progenitors, expanded in the presence of BDNF, EGF, and bFGF, ready for the glial switch [217][150]. This model, although efficient, is still unable to provide us with an astrocyte population indistinguishable from the one native to the human CNS, mainly because of the environmental impression on the final stages of astrocyte development [220][151]. Leventoux et al. created a generation model of a highly homogenous astrocyte population from induced pluripotent stem cells (iPSCs). In this study, an iPSC cell line was derived from a 36-year-old woman. Subsequently, the bone morphogenetic protein inhibitor Dorsomorphin, the TGFβ inhibitor SB431542 and the GSK3β inhibitor CHIR99021 were used, thus iPSCs were unaltered into mesodermal and non-neuronal ectodermal lineages and began the transition into glial and neuronal lines.3. Differences in Localization, Age, and the Type of Injury
Astrocytes are essential cells in the human CNS, and they are categorized into four main types: protoplasmic, interlaminar, varicose projection, and fibrous [224][152]. Of significant note, protoplasmic astrocytes are distributed in the grey matter of cortical layers II–VI. They uniquely organize themselves into distinct domains, likely granting them the capability to modulate and synchronize blood flow with synaptic activity [225][153]. Interlaminar astrocytes, exclusive to primates, project within the cerebral cortex [226,227][154][155]. Fibrous astroglia, located near white matter tracts, might interact with neurovasculature [228,229][156][157]. Varicose projection astrocytes span specific cortical layers and might play roles in primate cognition [224,230][152][158]. Regional differences in the glial response were also noted in the case of a cerebral stab wound and a transcranial injury of the spinal cord. Localization of reactive astrocytes that display changed morphology depends on the proximity to the insult. Astrocytes response does not differ only in terms of their location in the CNS. Studies conducted in rodents have also shown differences in astrocytes depending on the type of injury. The heterogeneous population of reactive astrocytes in the glial scar is most likely due to the ability of NG2 glial cells to differentiate into astrocytes in damaged brain tissue. NG2 glial cells, integral to the adult mammalian CNS, make up 5–8% of its cells and are pivotal in producing myelinating oligodendrocytes and astrocytes. Positioned near neuronal bodies, they receive synaptic inputs and play a role in modulating the neuronal network. Their activity, prominent in early life, diminishes with age, linking them to certain neurodegenerative conditions. They are essential for maintaining a healthy neural environment, with their dysfunction leading to neuronal impairments [241][159]. Moreover, in a recent study, Hasel et al. effectively illustrated the diversity of reactive astrocytes in the brains of mouse models induced with LPS [244][160]. However, differences in type, degree, and location of injury, in vitro conditions, and used animal species make accurate typing of reactive astrocytes more complicated [149][98]. Numerous cytokines are involved in the changes, both at the local and systemic level, which, in the event of an excessive inflammatory response, cause an autoreactive response against nerve cells. This chronic body response begins when the failure occurs and can last for many weeks [248][161]. Early-stage glial scar formation is a favorable phenomenon as it isolates the site of injury and potentially dangerous molecules from healthy tissue. Therefore, it is considered to be the body’s protective mechanism. During the acute phase, the most important cells of the immune system are neutrophils, macrophages, microglia, and T-lymphocytes.4. Astrocytes as a Therapeutic Option in TBI
In the field of neuroscience, it is constantly difficult to find therapeutic options for numerous disease entities. For example, there is still a significant challenge in assisting individuals with CNS injuries. There are various approaches to searching for therapeutic methods for people with disabilities. These methods encompass different mechanisms and stages of the condition. Several of them include: gene therapy, pharmacotherapy, neuroprotection, physical intervention, bioengineering, neurorehabilitation, or cell therapy that directly affects cells (including the astrocytes) [250,251,252,253][162][163][164][165]. The activity of astrocytes in the pathogenesis of traumatic spinal cord and brain injuries is being used as a basis for developing healing methods [149,254][98][166]. It has been demonstrated that after injury, astrocytes undergo activation into reactive astrocytes among which can be distinguished two subpopulations: A1 and A2. The A1 population is responsible for pro-inflammatory and neurotoxic mechanisms, whereas A2 acts in an anti-inflammatory and neuroprotective role [145,149,255][94][98][167]. In the secondary phase, there is an inflammatory process taking place. It involves many cells, both of brain cells and originating from the peripheral immune system, among which, notable are: microglial cells, astrocytes, macrophages, leukocytes, monocytes, and neutrophils. Their actions lead to neuronal damage, consequently resulting in neurodegeneration of brain tissue. Similar to spinal cord injuries, gaining a better understanding of the mechanisms during the second phase of brain injury could become a promising focal point for future TBI therapies [22,257][22][168]. One such method involves utilizing endocannabinoids present in astrocytes and the associated processes. Endocannabinoids are molecules produced by the body that act as neurotransmitters and exhibit significant anti-inflammatory effects. One of them is 2-arachidonoylglycerol. Furthermore, another research group carried out trials with the use of 17β-estradiol. The studied group consisted of mice after brain injury. This inquiry proved that 17β-estradiol may have a meaningful role in processes enabling the resumption of proper functioning of the brain. It reduces the expression of neurotoxic astrocyte-activating factors e.g., NF-κ, and also inhibits the release of pro-inflammatory cytokines produced by astrocytes. The clinical effect of these processes is the improvement of neurological functions in mice after TBI [39][37]. The mechanism of this process has remained unknown until now. It was decided to investigate it more thoroughly. The focus was on the role of GJA1-20K/Cx43 in interactions between astrocytes and neurons. The experiment was conducted on mice. Cortical brain neurons were cultured from C57BL/6 rodent fetuses. Their damage was artificially induced using nitrogen-oxygen mixed gas to mimic natural conditions. Subsequently, a transwell astrocyte-neuron co-culture system was established to replicate the interaction between these cells. It has been demonstrated that overexpression of GJA1-20K enhances the expression of functional Cx43 in astrocytes, facilitating the transfer of mitochondria from astrocytes to neurons. It is speculated that this process may contribute to the formation of protective functions that astrocytes perform towards neurons [23]. Also investigated was how exogenous GJA1-20k affects the apoptosis and mitochondrial function of neurons after TBI.5. Conclusions
Traumatic Brain Injury persists as a formidable challenge in contemporary medicine, particularly among younger demographics. The absence of specific neuroprotective strategies that significantly affect patient mortality necessitates current interventions focused on mitigating secondary cerebral damage initiated by a cascade of physiological responses to the injury. In this context, astrocytes play a pivotal role through the mechanism of reactive astrogliosis. Nonetheless, research indicates their bidirectional role in CNS injury response. Astrocytes critically influence the function and structural integrity of the blood-brain barrier, affecting its regeneration, ionic equilibrium, and vascular permeability. This is achieved through their interactions with endothelial cells and the secretion of specific cytokines. Additionally, they are integral in maintaining synaptic transmission integrity by modulating the metabolic environment (regulating pH, neurotransmitter exchange, ion metabolism), and secreting synaptogenic factors like thrombospondin, hevin, neuroligin, and transforming growth factor beta (TGF-β). There is also evidence of astrocytes influencing synaptic maturation and functionality, as well as producing neuroprotective factors such as Brain-Derived Neurotrophic Factor (BDNF) and 17β-estradiol (E2).References
- Dityatev, A.; Wilson, M.R.; Rusakov, D.; Kruyer, A. Astrocyte Heterogeneity in Regulation of Synaptic Activity. Cells 2022, 11, 3135.
- Boghdadi, A.G.; Teo, L.; Bourne, J.A. The Neuroprotective Role of Reactive Astrocytes after Central Nervous System Injury. J. Neurotrauma 2020, 37, 681–691.
- Quaglio, G.; Gallucci, M.; Brand, H.; Dawood, A.; Cobello, F. Traumatic Brain Injury: A Priority for Public Health Policy. Lancet Neurol. 2017, 16, 951–952.
- Blennow, K.; Brody, D.L.; Kochanek, P.M.; Levin, H.; McKee, A.; Ribbers, G.M.; Yaffe, K.; Zetterberg, H. Traumatic Brain Injuries. Nat. Rev. Dis. Primers 2016, 2, 16084.
- Maas, A.I.R.; Menon, D.K.; David Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic Brain Injury: Integrated Approaches to Improve Prevention, Clinical Care, and Research. Lancet Neurol. 2017, 16, 987–1048.
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the Global Incidence of Traumatic Brain Injury. J. Neurosurg. 2018, 130, 1080–1097.
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The Epidemiology and Impact of Traumatic Brain Injury: A Brief Overview. J. Head Trauma Rehabil. 2006, 21, 375–378.
- The Epidemiology and Impact of Traumatic Brain Injury: A Bri…: The Journal of Head Trauma Rehabilitation. Available online: https://journals.lww.com/headtraumarehab/abstract/2006/09000/the_epidemiology_and_impact_of_traumatic_brain.1.aspx (accessed on 10 January 2024).
- Oberholzer, M.; Müri, R.M. Neurorehabilitation of Traumatic Brain Injury (TBI): A Clinical Review. Med. Sci. 2019, 7, 47.
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A Practical Scale. Lancet 1974, 304, 81–84.
- Rimel, R.W.; Jane, J.A.; Edlich, R.F. An Injury Severity Scale for Comprehensive Management of Central Nervous System Trauma. J. Am. Coll. Emerg. Physicians 1979, 8, 64–67.
- Salas, C.E.; Casassus, M.; Rowlands, L.; Pimm, S.; Flanagan, D.A.J. “Relating through Sameness”: A Qualitative Study of Friendship and Social Isolation in Chronic Traumatic Brain Injury. Neuropsychol. Rehabil. 2018, 28, 1161–1178.
- Corral, L.; Ventura, J.L.; Herrero, J.I.; Monfort, J.L.; Juncadella, M.; Gabarrós, A.; Bartolomé, C.; Javierre, C.; García-Huete, L. Improvement in GOS and GOSE Scores 6 and 12 Months after Severe Traumatic Brain Injury. Brain Inj. 2007, 21, 1225–1231.
- Seifert, J. Incidence and Economic Burden of Injuries in the United States. J. Epidemiol. Community Health 2007, 61, 926.
- Stocchetti, N.; Taccone, F.S.; Citerio, G.; Pepe, P.E.; Roux, P.D.; Oddo, M.; Polderman, K.H.; Stevens, R.D.; Barsan, W.; Maas, A.I.R.; et al. Neuroprotection in Acute Brain Injury: An up-to-Date Review. Crit. Care 2015, 19, 186.
- Kim, Y.; Park, J.; Choi, Y.K. The Role of Astrocytes in the Central Nervous System Focused on BK Channel and Heme Oxygenase Metabolites: A Review. Antioxidants 2019, 8, 121.
- Maegele, M.; Schöchl, H.; Menovsky, T.; Maréchal, H.; Marklund, N.; Buki, A.; Stanworth, S. Coagulopathy and Haemorrhagic Progression in Traumatic Brain Injury: Advances in Mechanisms, Diagnosis, and Management. Lancet Neurol. 2017, 16, 630–647.
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The Contribution of Astrocytes and Microglia to Traumatic Brain Injury. Br. J. Pharmacol. 2016, 173, 692–702.
- Tran, A.P.; Warren, P.M.; Silver, J. New Insights into Glial Scar Formation after Spinal Cord Injury. Cell Tissue Res. 2021, 387, 319–336.
- Clark, D.P.Q.; Perreau, V.M.; Shultz, S.R.; Brady, R.D.; Lei, E.; Dixit, S.; Taylor, J.M.; Beart, P.M.; Boon, W.C. Inflammation in Traumatic Brain Injury: Roles for Toxic A1 Astrocytes and Microglial–Astrocytic Crosstalk. Neurochem. Res. 2019, 44, 1410–1424.
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325.
- Zhou, Y.; Shao, A.; Yao, Y.; Tu, S.; Deng, Y.; Zhang, J. Dual Roles of Astrocytes in Plasticity and Reconstruction after Traumatic Brain Injury. Cell Commun. Signal. 2020, 18, 62.
- Ayazi, M.; Zivkovic, S.; Hammel, G.; Stefanovic, B.; Ren, Y. Fibrotic Scar in CNS Injuries: From the Cellular Origins of Fibroblasts to the Molecular Processes of Fibrotic Scar Formation. Cells 2022, 11, 2371.
- Wang, H.; Song, G.; Chuang, H.; Chiu, C.; Abdelmaksoud, A.; Ye, Y.; Zhao, L. Portrait of Glial Scar in Neurological Diseases. Int. J. Immunopathol. Pharmacol. 2018, 31, 2058738418801406.
- Linnerbauer, M.; Rothhammer, V. Protective Functions of Reactive Astrocytes Following Central Nervous System Insult. Front Immunol. 2020, 11, 573256.
- Pekny, M.; Wilhelmsson, U.; Tatlisumak, T.; Pekna, M. Astrocyte Activation and Reactive Gliosis-A New Target in Stroke? Neurosci. Lett. 2019, 689, 45–55.
- Heithoff, B.P.; George, K.K.; Phares, A.N.; Zuidhoek, I.A.; Munoz-Ballester, C.; Robel, S. Astrocytes Are Necessary for Blood–Brain Barrier Maintenance in the Adult Mouse Brain. Glia 2021, 69, 436–472.
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69.
- Kriaučiūnaitė, K.; Kaušylė, A.; Pajarskienė, J.; Tunaitis, V.; Lim, D.; Verkhratsky, A.; Pivoriūnas, A. Immortalised Hippocampal Astrocytes from 3xTG-AD Mice Fail to Support BBB Integrity In Vitro: Role of Extracellular Vesicles in Glial-Endothelial Communication. Cell. Mol. Neurobiol. 2021, 41, 551–562.
- Kinboshi, M.; Ikeda, A.; Ohno, Y. Role of Astrocytic Inwardly Rectifying Potassium (Kir) 4.1 Channels in Epileptogenesis. Front. Neurol. 2020, 11, 626658.
- Michinaga, S.; Koyama, Y. Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage. Int. J. Mol. Sci. 2019, 20, 571.
- Liu, F.; Wu, J.; Gong, Y.; Wang, P.; Zhu, L.; Tong, L.; Chen, X.; Ling, Y.; Huang, C. Harmine Produces Antidepressant-like Effects via Restoration of Astrocytic Functions. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 258–267.
- Molinari, C.; Morsanuto, V.; Ruga, S.; Notte, F.; Farghali, M.; Galla, R.; Uberti, F. The Role of BDNF on Aging-Modulation Markers. Brain Sci. 2020, 10, 285.
- Martin-Jiménez, C.; Gaitán-Vaca, D.M.; Areiza, N.; Echeverria, V.; Ashraf, G.M.; González, J.; Sahebkar, A.; Garcia-Segura, L.M.; Barreto, G.E. Astrocytes Mediate Protective Actions of Estrogenic Compounds after Traumatic Brain Injury. Neuroendocrinology 2019, 108, 142–160.
- Wang, J.; Sareddy, G.R.; Lu, Y.; Pratap, U.P.; Tang, F.; Greene, K.M.; Meyre, P.L.; Tekmal, R.R.; Vadlamudi, R.K.; Brann, D.W. Astrocyte-Derived Estrogen Regulates Reactive Astrogliosis and Is Neuroprotective Following Ischemic Brain Injury. J. Neurosci. 2020, 40, 9751–9771.
- Guo, H.; Yang, J.; Liu, M.; Wang, L.; Hou, W.; Zhang, L.; Ma, Y. Selective Activation of Estrogen Receptor β Alleviates Cerebral Ischemia Neuroinflammatory Injury. Brain Res. 2020, 1726, 146536.
- Wang, J.; Hou, Y.; Zhang, L.; Liu, M.; Zhao, J.; Zhang, Z.; Ma, Y.; Hou, W. Estrogen Attenuates Traumatic Brain Injury by Inhibiting the Activation of Microglia and Astrocyte-Mediated Neuroinflammatory Responses. Mol. Neurobiol. 2021, 58, 1052–1061.
- Liu, L.R.; Liu, J.C.; Bao, J.S.; Bai, Q.Q.; Wang, G.Q. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front. Immunol. 2020, 11, 1024.
- McAlpine, C.S.; Park, J.; Griciuc, A.; Kim, E.; Choi, S.H.; Iwamoto, Y.; Kiss, M.G.; Christie, K.A.; Vinegoni, C.; Poller, W.C.; et al. Astrocytic Interleukin-3 Programs Microglia and Limits Alzheimer’s Disease. Nature 2021, 595, 701–706.
- Sano, F.; Shigetomi, E.; Shinozaki, Y.; Tsuzukiyama, H.; Saito, K.; Mikoshiba, K.; Horiuchi, H.; Cheung, D.L.; Nabekura, J.; Sugita, K.; et al. Reactive Astrocyte-Driven Epileptogenesis Is Induced by Microglia Initially Activated Following Status Epilepticus. JCI Insight 2021, 6, e135391.
- Kwon, H.S.; Koh, S.H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42.
- Long, X.; Yao, X.; Jiang, Q.; Yang, Y.; He, X.; Tian, W.; Zhao, K.; Zhang, H. Astrocyte-Derived Exosomes Enriched with MiR-873a-5p Inhibit Neuroinflammation via Microglia Phenotype Modulation after Traumatic Brain Injury. J. Neuroinflammation 2020, 17, 89.
- Ghaemi, A.; Alizadeh, L.; Babaei, S.; Jafarian, M.; Khaleghi Ghadiri, M.; Meuth, S.G.; Kovac, S.; Gorji, A. Astrocyte-Mediated Inflammation in Cortical Spreading Depression. Cephalalgia 2018, 38, 626–638.
- Lavi, E.; Cong, L. Type I Astrocytes and Microglia Induce a Cytokine Response in an Encephalitic Murine Coronavirus Infection. Exp. Mol. Pathol. 2020, 115, 104474.
- Kim, S.; Son, Y. Astrocytes Stimulate Microglial Proliferation and M2 Polarization In Vitro through Crosstalk between Astrocytes and Microglia. Int. J. Mol. Sci. 2021, 22, 8800.
- Xue, J.; Zhang, Y.; Zhang, J.; Zhu, Z.; Lv, Q.; Su, J. Astrocyte-Derived CCL7 Promotes Microglia-Mediated Inflammation Following Traumatic Brain Injury. Int. Immunopharmacol. 2021, 99, 107975.
- Balança, B.; Desmurs, L.; Grelier, J.; Perret-Liaudet, A.; Lukaszewicz, A.C. DAMPs and RAGE Pathophysiology at the Acute Phase of Brain Injury: An Overview. Int. J. Mol. Sci. 2021, 22, 2439.
- Arizono, M.; Nägerl, U.V. Deciphering the Functional Nano-Anatomy of the Tripartite Synapse Using Stimulated Emission Depletion Microscopy. Glia 2022, 70, 607–618.
- Heller, J.P.; Rusakov, D.A. The Nanoworld of the Tripartite Synapse: Insights from Super-Resolution Microscopy. Front. Cell. Neurosci. 2017, 11, 374.
- Lalo, U.; Koh, W.; Lee, C.J.; Pankratov, Y. The Tripartite Glutamatergic Synapse. Neuropharmacology 2021, 199, 108758.
- Semyanov, A.; Verkhratsky, A. Astrocytic Processes: From Tripartite Synapses to the Active Milieu. Trends Neurosci. 2021, 44, 781–792.
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, Neurons, Synapses: A Tripartite View on Cortical Circuit Development. Neural. Dev. 2018, 13, 7.
- Miterauer, B.; Baer, W. Disorders of Human Consciousness in the Tri-Partite Synapses. Med. Hypotheses 2020, 136, 109523.
- Liu, C.Y.; Yang, Y.; Ju, W.N.; Wang, X.; Zhang, H.L. Emerging Roles of Astrocytes in Neuro-Vascular Unit and the Tripartite Synapse with Emphasis on Reactive Gliosis in the Context of Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 193.
- Nagai, J.; Rajbhandari, A.K.; Gangwani, M.R.; Hachisuka, A.; Coppola, G.; Masmanidis, S.C.; Fanselow, M.S.; Khakh, B.S. Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell 2019, 177, 1280–1292.e20.
- Zhou, B.; Zuo, Y.X.; Jiang, R.T. Astrocyte Morphology: Diversity, Plasticity, and Role in Neurological Diseases. CNS Neurosci. Ther. 2019, 25, 665–673.
- Papouin, T.; Dunphy, J.; Tolman, M.; Foley, J.C.; Haydon, P.G. Astrocytic Control of Synaptic Function. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160154.
- Marina, N.; Turovsky, E.; Christie, I.N.; Hosford, P.S.; Hadjihambi, A.; Korsak, A.; Ang, R.; Mastitskaya, S.; Sheikhbahaei, S.; Theparambil, S.M.; et al. Brain Metabolic Sensing and Metabolic Signaling at the Level of an Astrocyte. Glia 2018, 66, 1185–1199.
- Verharen, J.P.H.; de Jong, J.W.; Lammel, S. Dopaminergic Control over the Tripartite Synapse. Neuron 2020, 105, 954–956.
- Zhou, Y.D. Glial Regulation of Energy Metabolism. Adv. Exp. Med. Biol. 2018, 1090, 105–121.
- Bellot-Saez, A.; Stevenson, R.; Kékesi, O.; Samokhina, E.; Ben-Abu, Y.; Morley, J.W.; Buskila, Y. Neuromodulation of Astrocytic K+ Clearance. Int. J. Mol. Sci. 2021, 22, 2520.
- Oliveira, J.F.; Araque, A. Astrocyte Regulation of Neural Circuit Activity and Network States. Glia 2022, 70, 1455–1466.
- Theparambil, S.M.; Hosford, P.S.; Ruminot, I.; Kopach, O.; Reynolds, J.R.; Sandoval, P.Y.; Rusakov, D.A.; Barros, L.F.; Gourine, A.V. Astrocytes Regulate Brain Extracellular PH via a Neuronal Activity-Dependent Bicarbonate Shuttle. Nat. Commun. 2020, 11, 5073.
- Soto, C.; Pritzkow, S. Protein Misfolding, Aggregation, and Conformational Strains in Neurodegenerative Diseases. Nat. Neurosci. 2018, 21, 1332–1340.
- Blanco-Suarez, E.; Liu, T.F.; Kopelevich, A.; Allen, N.J. Astrocyte-Secreted Chordin-like 1 Drives Synapse Maturation and Limits Plasticity by Increasing Synaptic GluA2 AMPA Receptors. Neuron 2018, 100, 1116–1132.e13.
- Popov, A.; Brazhe, A.; Denisov, P.; Sutyagina, O.; Li, L.; Lazareva, N.; Verkhratsky, A.; Semyanov, A. Astrocyte Dystrophy in Ageing Brain Parallels Impaired Synaptic Plasticity. Aging Cell 2021, 20, e13334.
- Sancho, L.; Contreras, M.; Allen, N.J. Glia as Sculptors of Synaptic Plasticity. Neurosci Res 2021, 167, 17–29.
- Perez-Catalan, N.A.; Doe, C.Q.; Ackerman, S.D. The Role of Astrocyte-mediated Plasticity in Neural Circuit Development and Function. Neural Dev. 2021, 16, 1.
- Zhou, Z.; Okamoto, K.; Onodera, J.; Hiragi, T.; Andoh, M.; Ikawa, M.; Tanaka, K.F.; Ikegaya, Y.; Koyama, R. Astrocytic CAMP Modulates Memory via Synaptic Plasticity. Proc. Natl. Acad. Sci. USA 2021, 118, e2016584118.
- Baldwin, K.T.; Eroglu, C. Astrocytes “Chordinate” Synapse Maturation and Plasticity. Neuron 2018, 100, 1010–1012.
- Stackhouse, T.L.; Mishra, A. Neurovascular Coupling in Development and Disease: Focus on Astrocytes. Front. Cell. Dev. Biol. 2021, 9, 702832.
- Farhy-Tselnicker, I.; Boisvert, M.M.; Liu, H.; Dowling, C.; Erikson, G.A.; Blanco-Suarez, E.; Farhy, C.; Shokhirev, M.N.; Ecker, J.R.; Allen, N.J. Activity-Dependent Modulation of Synapse-Regulating Genes in Astrocytes. Elife 2021, 10, e70514.
- D’Ambrosi, N.; Apolloni, S. Fibrotic Scar in Neurodegenerative Diseases. Front. Immunol. 2020, 11, 1394.
- Huang, L.; Nakamura, Y.; Lo, E.H.; Hayakawa, K. Astrocyte Signaling in the Neurovascular Unit After Central Nervous System Injury. Int. J. Mol. Sci. 2019, 20, 282.
- Ponath, G.; Park, C.; Pitt, D. The Role of Astrocytes in Multiple Sclerosis. Front. Immunol. 2018, 9, 217.
- Dong, C.; Wen, S.; Zhao, S.; Sun, S.; Zhao, S.; Dong, W.; Han, P.; Chen, Q.; Gong, T.; Chen, W.; et al. Salidroside Inhibits Reactive Astrogliosis and Glial Scar Formation in Late Cerebral Ischemia via the Akt/GSK-3β Pathway. Neurochem. Res. 2021, 46, 755–769.
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967.
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487.
- Moeendarbary, E.; Weber, I.P.; Sheridan, G.K.; Koser, D.E.; Soleman, S.; Haenzi, B.; Bradbury, E.J.; Fawcett, J.; Franze, K. The Soft Mechanical Signature of Glial Scars in the Central Nervous System. Nat. Commun. 2017, 8, 14787.
- Orlandin, J.R.; Ambrósio, C.E.; Lara, V.M. Glial Scar-Modulation as Therapeutic Tool in Spinal Cord Injury in Animal Models. Acta Cir. Bras. 2017, 32, 168–174.
- Yang, T.; Dai, Y.J.; Chen, G.; Cui, S. Sen Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 78.
- Yoshizaki, S.; Tamaru, T.; Hara, M.; Kijima, K.; Tanaka, M.; Konno, D.J.; Matsumoto, Y.; Nakashima, Y.; Okada, S. Microglial Inflammation after Chronic Spinal Cord Injury Is Enhanced by Reactive Astrocytes via the Fibronectin/Β1 Integrin Pathway. J. Neuroinflammation 2021, 18, 12.
- Molina-Gonzalez, I.; Miron, V.E.; Antel, J.P. Chronic Oligodendrocyte Injury in Central Nervous System Pathologies. Commun. Biol. 2022, 5, 1274.
- Otani, K.; Shichita, T. Cerebral Sterile Inflammation in Neurodegenerative Diseases. Inflamm. Regen. 2020, 40, 28.
- Shi, Z.; Yuan, S.; Shi, L.; Li, J.; Ning, G.; Kong, X.; Feng, S. Programmed Cell Death in Spinal Cord Injury Pathogenesis and Therapy. Cell Prolif. 2021, 54, e12992.
- Manrique-Castano, D.; ElAli, A. Neurovascular Reactivity in Tissue Scarring Following Cerebral Ischemia. In Cerebral Ischemia; Exon Publications: Brisbane, Australia, 2021; pp. 111–130.
- Dias, D.O.; Kim, H.; Holl, D.; Werne Solnestam, B.; Lundeberg, J.; Carlén, M.; Göritz, C.; Frisén, J. Reducing Pericyte-Derived Scarring Promotes Recovery after Spinal Cord Injury. Cell 2018, 173, 153–165.e22.
- Dorrier, C.E.; Aran, D.; Haenelt, E.A.; Sheehy, R.N.; Hoi, K.K.; Pintarić, L.; Chen, Y.; Lizama, C.O.; Cautivo, K.M.; Weiner, G.A.; et al. CNS Fibroblasts Form a Fibrotic Scar in Response to Immune Cell Infiltration. Nat. Neurosci. 2021, 24, 234–244.
- Zhang, Y.; Yang, S.; Liu, C.; Han, X.; Gu, X.; Zhou, S. Deciphering Glial Scar after Spinal Cord Injury. Burn. Trauma 2021, 9, tkab035.
- Bélanger, M.; Magistretti, P.J. The Role of Astroglia in Neuroprotection. Dialogues Clin. Neurosci. 2022, 11, 281–296.
- Liu, K.; Yu, B.; Chen, J.F.; Li, R.X.; Chen, L.; Ren, S.Y.; Wang, F.; Mei, F.; Xiao, L. Dicer Deletion in Astrocytes Inhibits Oligodendroglial Differentiation and Myelination. Neurosci. Bull. 2021, 37, 1135–1146.
- Beltran-Lobo, P.; Reid, M.J.; Jimenez-Sanchez, M.; Verkhratsky, A.; Perez-Nievas, B.G.; Noble, W. Astrocyte Adaptation in Alzheimer’s Disease: A Focus on Astrocytic P2X7R. Essays Biochem. 2022, 67, 119–130.
- Passarella, D.; Ronci, M.; Di Liberto, V.; Zuccarini, M.; Mudò, G.; Porcile, C.; Frinchi, M.; Di Iorio, P.; Ulrich, H.; Russo, C. Bidirectional Control between Cholesterol Shuttle and Purine Signal at the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 8683.
- Liu, J.P.; Liu, S.C.; Hu, S.Q.; Lu, J.F.; Wu, C.L.; Hu, D.X.; Zhang, W.J. ATP Ion Channel P2X Purinergic Receptors in Inflammation Response. Biomed. Pharmacother. 2023, 158, 114205.
- Montilla, A.; Mata, G.P.; Matute, C.; Domercq, M. Contribution of P2X4 Receptors to CNS Function and Pathophysiology. Int. J. Mol. Sci. 2020, 21, 5562.
- Kapaki, E.; Vakrakou, A.G.; Boufidou, F. Novel CSF Biomarkers Tracking Autoimmune Inflammatory and Neurodegenerative Aspects of CNS Diseases. Diagnostics 2022, 13, 73.
- Rodríguez-Giraldo, M.; González-Reyes, R.E.; Ramírez-Guerrero, S.; Bonilla-Trilleras, C.E.; Guardo-Maya, S.; Nava-Mesa, M.O. Astrocytes as a Therapeutic Target in Alzheimer’s Disease-Comprehensive Review and Recent Developments. Int. J. Mol. Sci. 2022, 23, 13630.
- Yu, G.L.; Zhang, Y.; Ning, B. Reactive Astrocytes in Central Nervous System Injury: Subgroup and Potential Therapy. Front. Cell. Neurosci. 2021, 15, 792764.
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Münch, A.E.; Heiman, M.; Barres, B.A. Normal Aging Induces A1-like Astrocyte Reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905.
- Kisucká, A.; Bimbová, K.; Bačová, M.; Gálik, J.; Lukáčová, N. Activation of Neuroprotective Microglia and Astrocytes at the Lesion Site and in the Adjacent Segments Is Crucial for Spontaneous Locomotor Recovery after Spinal Cord Injury. Cells 2021, 10, 1943.
- Patabendige, A.; Singh, A.; Jenkins, S.; Sen, J.; Chen, R. Astrocyte Activation in Neurovascular Damage and Repair Following Ischaemic Stroke. Int. J. Mol. Sci. 2021, 22, 4280.
- Gotoh, M.; Miyamoto, Y.; Ikeshima-Kataoka, H. Astrocytic Neuroimmunological Roles Interacting with Microglial Cells in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 1599.
- Miller, S.J. Astrocyte Heterogeneity in the Adult Central Nervous System. Front. Cell. Neurosci. 2018, 12, 401.
- Andries, L.; Masin, L.; Salinas-Navarro, M.; Zaunz, S.; Claes, M.; Bergmans, S.; Brouwers, V.; Lefevere, E.; Verfaillie, C.; Movahedi, K.; et al. MMP2 Modulates Inflammatory Response during Axonal Regeneration in the Murine Visual System. Cells 2021, 10, 1672.
- Hernaiz-Llorens, M.; Martínez-Mármol, R.; Roselló-Busquets, C.; Soriano, E. One Raft to Guide Them All, and in Axon Regeneration Inhibit Them. Int. J. Mol. Sci. 2021, 22, 5009.
- Akram, R.; Anwar, H.; Javed, M.S.; Rasul, A.; Imran, A.; Malik, S.A.; Raza, C.; Khan, I.U.; Sajid, F.; Iman, T.; et al. Axonal Regeneration: Underlying Molecular Mechanisms and Potential Therapeutic Targets. Biomedicines 2022, 10, 3186.
- Francos-Quijorna, I.; Sánchez-Petidier, M.; Burnside, E.R.; Badea, S.R.; Torres-Espin, A.; Marshall, L.; de Winter, F.; Verhaagen, J.; Moreno-Manzano, V.; Bradbury, E.J. Chondroitin Sulfate Proteoglycans Prevent Immune Cell Phenotypic Conversion and Inflammation Resolution via TLR4 in Rodent Models of Spinal Cord Injury. Nat. Commun. 2022, 13, 2933.
- Pan, D.; Li, Y.; Yang, F.; Lv, Z.; Zhu, S.; Shao, Y.; Huang, Y.; Ning, G.; Feng, S. Increasing Toll-like Receptor 2 on Astrocytes Induced by Schwann Cell-Derived Exosomes Promotes Recovery by Inhibiting CSPGs Deposition after Spinal Cord Injury. J. Neuroinflammation 2021, 18, 172.
- Sami, A.; Selzer, M.E.; Li, S. Advances in the Signaling Pathways Downstream of Glial-Scar Axon Growth Inhibitors. Front. Cell. Neurosci. 2020, 14, 174.
- Stern, S.; Hilton, B.J.; Burnside, E.R.; Dupraz, S.; Handley, E.E.; Gonyer, J.M.; Brakebusch, C.; Bradke, F. RhoA Drives Actin Compaction to Restrict Axon Regeneration and Astrocyte Reactivity after CNS Injury. Neuron 2021, 109, 3436–3455.e9.
- Zhang, Q.; Li, Y.; Zhuo, Y. Synaptic or Non-Synaptic? Different Intercellular Interactions with Retinal Ganglion Cells in Optic Nerve Regeneration. Mol. Neurobiol. 2022, 59, 3052–3072.
- Luo, F.; Wang, J.; Zhang, Z.; You, Z.; Bedolla, A.; Okwubido-Williams, F.G.; Huang, L.F.; Silver, J.; Luo, Y. Inhibition of CSPG Receptor PTPσ Promotes Migration of Newly Born Neuroblasts, Axonal Sprouting, and Recovery from Stroke. Cell Rep. 2022, 40, 111137.
- Mencio, C.P.; Hussein, R.K.; Yu, P.; Geller, H.M. The Role of Chondroitin Sulfate Proteoglycans in Nervous System Development. J. Histochem. Cytochem. 2021, 69, 61–80.
- Mukherjee, N.; Nandi, S.; Garg, S.; Ghosh, S.; Ghosh, S.; Samat, R.; Ghosh, S. Targeting Chondroitin Sulfate Proteoglycans: An Emerging Therapeutic Strategy to Treat CNS Injury. ACS Chem. Neurosci. 2020, 11, 231–232.
- Chen, K.Z.; Liu, S.X.; Li, Y.W.; He, T.; Zhao, J.; Wang, T.; Qiu, X.X.; Wu, H.F. Vimentin as a Potential Target for Diverse Nervous System Diseases. Neural Regen. Res. 2023, 18, 969–975.
- Hippert, C.; Graca, A.B.; Basche, M.; Kalargyrou, A.A.; Georgiadis, A.; Ribeiro, J.; Matsuyama, A.; Aghaizu, N.; Bainbridge, J.W.; Smith, A.J.; et al. RNAi-Mediated Suppression of Vimentin or Glial Fibrillary Acidic Protein Prevents the Establishment of Müller Glial Cell Hypertrophy in Progressive Retinal Degeneration. Glia 2021, 69, 2272–2290.
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an Emerging Biomarker in Brain and Spinal Cord Disorders. Nat. Rev. Neurol. 2022, 18, 158–172.
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361.
- Villarreal, A.; Vidos, C.; Monteverde Busso, M.; Cieri, M.B.; Ramos, A.J. Pathological Neuroinflammatory Conversion of Reactive Astrocytes Is Induced by Microglia and Involves Chromatin Remodeling. Front. Pharmacol. 2021, 12, 1448.
- Zhang, Z.; Ma, Z.; Zou, W.; Guo, H.; Liu, M.; Ma, Y.; Zhang, L. The Appropriate Marker for Astrocytes: Comparing the Distribution and Expression of Three Astrocytic Markers in Different Mouse Cerebral Regions. Biomed Res. Int. 2019, 2019, 9605265.
- Lawal, O.; Ulloa Severino, F.P.; Eroglu, C. The Role of Astrocyte Structural Plasticity in Regulating Neural Circuit Function and Behavior. Glia 2022, 70, 1467–1483.
- Zamoner, A.; Pessoa-Pureur, R.; Zamoner, A.; Pessoa-Pureur, R. Intermediate Filaments as a Target of Signaling Mechanisms in Neurotoxicity. In Cytoskeleton-Structure, Dynamics, Function and Disease; Books on Demand: Norderstedt, Germany, 2017.
- Kummer, K.K.; Zeidler, M.; Kalpachidou, T.; Kress, M. Role of IL-6 in the Regulation of Neuronal Development, Survival and Function. Cytokine 2021, 144, 155582.
- Lund, M.C.; Ellman, D.G.; Nissen, M.; Nielsen, P.S.; Nielsen, P.V.; Jørgensen, C.; Andersen, D.C.; Gao, H.; Brambilla, R.; Degn, M.; et al. The Inflammatory Response after Moderate Contusion Spinal Cord Injury: A Time Study. Biology 2022, 11, 939.
- Runge, E.M.; Setter, D.O.; Iyer, A.K.; Regele, E.J.; Kennedy, F.M.; Sanders, V.M.; Jones, K.J. Cellular Sources and Neuroprotective Roles of Interleukin-10 in the Facial Motor Nucleus after Axotomy. Cells 2022, 11, 3167.
- Han, T.; Song, P.; Wu, Z.; Xiang, X.; Liu, Y.; Wang, Y.; Fang, H.; Niu, Y.; Shen, C. MSC Secreted Extracellular Vesicles Carrying TGF-Beta Upregulate Smad 6 Expression and Promote the Regrowth of Neurons in Spinal Cord Injured Rats. Stem Cell Rev. Rep. 2022, 18, 1078–1096.
- Wu, X.; Shen, Q.; Zhang, Z.; Zhang, D.; Gu, Y.; Xing, D. Photoactivation of TGFβ/SMAD Signaling Pathway Ameliorates Adult Hippocampal Neurogenesis in Alzheimer’s Disease Model. Stem Cell Res. Ther. 2021, 12, 345.
- Divolis, G.; Stavropoulos, A.; Manioudaki, M.; Apostolidou, A.; Doulou, A.; Gavriil, A.; Dafnis, I.; Chroni, A.; Mummery, C.; Xilouri, M.; et al. Activation of Both Transforming Growth Factor-β and Bone Morphogenetic Protein Signalling Pathways upon Traumatic Brain Injury Restrains pro-Inflammatory and Boosts Tissue Reparatory Responses of Reactive Astrocytes and Microglia. Brain Commun. 2019, 1, fcz028.
- Vaibhav, K.; Ahluwalia, M.; Gaur, P.; Luo, J. TGF-β as a Key Modulator of Astrocyte Reactivity: Disease Relevance and Therapeutic Implications. Biomedicines 2022, 10, 1206.
- Shen, X.Y.; Gao, Z.K.; Han, Y.; Yuan, M.; Guo, Y.S.; Bi, X. Activation and Role of Astrocytes in Ischemic Stroke. Front. Cell. Neurosci. 2021, 15, 755955.
- Hara, M.; Kobayakawa, K.; Ohkawa, Y.; Kumamaru, H.; Yokota, K.; Saito, T.; Kijima, K.; Yoshizaki, S.; Harimaya, K.; Nakashima, Y.; et al. Interaction of Reactive Astrocytes with Type I Collagen Induces Astrocytic Scar Formation through the Integrin-N-Cadherin Pathway after Spinal Cord Injury. Nat. Med. 2017, 23, 818–828.
- Hassanzadeh, S.; Jalessi, M.; Jameie, S.B.; Khanmohammadi, M.; Bagher, Z.; Namjoo, Z.; Davachi, S.M. More Attention on Glial Cells to Have Better Recovery after Spinal Cord Injury. Biochem. Biophys. Rep. 2021, 25, 100905.
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770.
- Reed, M.J.; Damodarasamy, M.; Banks, W.A. The Extracellular Matrix of the Blood–Brain Barrier: Structural and Functional Roles in Health, Aging, and Alzheimer’s Disease. Tissue Barriers 2019, 7, 1651157.
- Wahane, S.; Sofroniew, M.V. Loss-of-Function Manipulations to Identify Roles of Diverse Glia and Stromal Cells during CNS Scar Formation. Cell Tissue Res. 2022, 387, 337–350.
- Zhou, Z.L.; Xie, H.; Tian, X.B.; Xu, H.L.; Li, W.; Yao, S.; Zhang, H. Microglial Depletion Impairs Glial Scar Formation and Aggravates Inflammation Partly by Inhibiting STAT3 Phosphorylation in Astrocytes after Spinal Cord Injury. Neural Regen. Res. 2023, 18, 1325–1331.
- Verisokin, A.Y.; Verveyko, D.V.; Postnov, D.E.; Brazhe, A.R. Modeling of Astrocyte Networks: Toward Realistic Topology and Dynamics. Front. Cell. Neurosci. 2021, 15, 50.
- Reichenbach, N.; Delekate, A.; Plescher, M.; Schmitt, F.; Krauss, S.; Blank, N.; Halle, A.; Petzold, G.C. Inhibition of Stat3-Mediated Astrogliosis Ameliorates Pathology in an Alzheimer’s Disease Model. EMBO Mol. Med. 2019, 11, e9665.
- Toral-Rios, D.; Patiño-López, G.; Gómez-Lira, G.; Gutiérrez, R.; Becerril-Pérez, F.; Rosales-Córdova, A.; León-Contreras, J.C.; Hernández-Pando, R.; León-Rivera, I.; Soto-Cruz, I.; et al. Activation of STAT3 Regulates Reactive Astrogliosis and Neuronal Death Induced by AβO Neurotoxicity. Int. J. Mol. Sci. 2020, 21, 7458.
- Li, X.; Li, M.; Tian, L.; Chen, J.; Liu, R.; Ning, B. Reactive Astrogliosis: Implications in Spinal Cord Injury Progression and Therapy. Oxid. Med. Cell. Longev. 2020, 2020, 9494352.
- Ren, Y.; Ao, Y.; O’Shea, T.M.; Burda, J.E.; Bernstein, A.M.; Brumm, A.J.; Muthusamy, N.; Ghashghaei, H.T.; Carmichael, S.T.; Cheng, L.; et al. Ependymal Cell Contribution to Scar Formation after Spinal Cord Injury Is Minimal, Local and Dependent on Direct Ependymal Injury. Sci. Rep. 2017, 7, srep41122.
- Arellano, J.I.; Morozov, Y.M.; Micali, N.; Rakic, P. Radial Glial Cells: New Views on Old Questions. Neurochem. Res. 2021, 46, 2512–2524.
- Bayraktar, O.A.; Fuentealba, L.C.; Alvarez-Buylla, A.; Rowitch, D.H. Astrocyte Development and Heterogeneity. Cold Spring Harb. Perspect. Biol. 2015, 7, a020362.
- Ghosh, H.S. Adult Neurogenesis and the Promise of Adult Neural Stem Cells. J. Exp. Neurosci. 2019, 13, 1179069519856876.
- Defteralı, Ç.; Moreno-Estellés, M.; Crespo, C.; Díaz-Guerra, E.; Díaz-Moreno, M.; Vergaño-Vera, E.; Nieto-Estévez, V.; Hurtado-Chong, A.; Consiglio, A.; Mira, H.; et al. Neural Stem Cells in the Adult Olfactory Bulb Core Generate Mature Neurons in Vivo. Stem Cells 2021, 39, 1253–1269.
- Bond, A.M.; Ming, G.L.; Song, H. Ontogeny of Adult Neural Stem Cells in the Mammalian Brain. Curr. Top. Dev. Biol. 2021, 142, 67–98.
- da Silva Siqueira, L.; Majolo, F.; da Silva, A.P.B.; da Costa, J.C.; Marinowic, D.R. Neurospheres: A Potential in Vitro Model for the Study of Central Nervous System Disorders. Mol. Biol. Rep. 2021, 48, 3649–3663.
- Antonelli, F.; Casciati, A.; Pazzaglia, S. Sonic Hedgehog Signaling Controls Dentate Gyrus Patterning and Adult Neurogenesis in the Hippocampus. Neural Regen. Res. 2019, 14, 59–61.
- Griffiths, B.; Bhutani, A.; Stary, C. Adult Neurogenesis from Reprogrammed Astrocytes. Neural Regen. Res. 2020, 15, 973.
- Peteri, U.K.; Pitkonen, J.; Utami, K.H.; Paavola, J.; Roybon, L.; Pouladi, M.A.; Castrén, M.L. Generation of the Human Pluripotent Stem-Cell-Derived Astrocyte Model with Forebrain Identity. Brain Sci. 2021, 11, 209.
- Lanjakornsiripan, D.; Pior, B.J.; Kawaguchi, D.; Furutachi, S.; Tahara, T.; Katsuyama, Y.; Suzuki, Y.; Fukazawa, Y.; Gotoh, Y. Layer-Specific Morphological and Molecular Differences in Neocortical Astrocytes and Their Dependence on Neuronal Layers. Nat. Commun. 2018, 9, 1623.
- Vasile, F.; Dossi, E.; Rouach, N. Human Astrocytes: Structure and Functions in the Healthy Brain. Brain Struct. Funct. 2017, 222, 2017–2029.
- Lee, J.; Kim, S.W.; Kim, K.T. Region-Specific Characteristics of Astrocytes and Microglia: A Possible Involvement in Aging and Diseases. Cells 2022, 11, 1902.
- Padmashri, R.; Ren, B.; Oldham, B.; Jung, Y.; Gough, R.; Dunaevsky, A. Modeling Human-specific Interlaminar Astrocytes in the Mouse Cerebral Cortex. J. Comp. Neurol. 2021, 529, 802.
- Shapson-Coe, A.; Januszewski, M.; Berger, D.R.; Pope, A.; Wu, Y.; Blakely, T.; Schalek, R.L.; Li, P.H.; Wang, S.; Maitin-Shepard, J.; et al. A Connectomic Study of a Petascale Fragment of Human Cerebral Cortex. bioRxiv 2021.
- McNeill, J.; Rudyk, C.; Hildebrand, M.E.; Salmaso, N. Ion Channels and Electrophysiological Properties of Astrocytes: Implications for Emergent Stimulation Technologies. Front. Cell. Neurosci. 2021, 15, 183.
- Prà, I.D.; Armato, U.; Chiarini, A.; Prà, I.D.; Armato, U.; Chiarini, A. Astrocytes’ Role in Alzheimer’s Disease Neurodegeneration. In Astrocyte-Physiology and Pathology; IntechOpen: London, UK, 2018.
- Wei, D.C.; Morrison, E.H. Histology, Astrocytes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Nakano, M.; Tamura, Y.; Yamato, M.; Kume, S.; Eguchi, A.; Takata, K.; Watanabe, Y.; Kataoka, Y. NG2 Glial Cells Regulate Neuroimmunological Responses to Maintain Neuronal Function and Survival. Sci. Rep. 2017, 7, 42041.
- Hasel, P.; Rose, I.V.L.; Sadick, J.S.; Kim, R.D.; Liddelow, S.A. Neuroinflammatory Astrocyte Subtypes in the Mouse Brain. Nat. Neurosci. 2021, 24, 1475–1487.
- Rodrígez-Barrera, R.; Flores-Romero, A.; García-Sánchez, J.; Navarro-Torres, L.K.; Garibay-López, M.; García-Vences, E.; Rodrígez-Barrera, R.; Flores-Romero, A.; García-Sánchez, J.; Navarro-Torres, L.K.; et al. Cytokines in Scar Glial Formation after an Acute and Chronic Spinal Cord Injury. In Cytokines; IntechOpen: London, UK, 2020.
- Khellaf, A.; Khan, D.Z.; Helmy, A. Recent Advances in Traumatic Brain Injury. J. Neurol. 2019, 266, 2878–2889.
- Hastings, N.; Kuan, W.L.; Osborne, A.; Kotter, M.R.N. Therapeutic Potential of Astrocyte Transplantation. Cell Transplant. 2022, 31, 09636897221105499.
- Szymoniuk, M.; Chin, J.H.; Domagalski, Ł.; Biszewski, M.; Jóźwik, K.; Kamieniak, P. Brain Stimulation for Chronic Pain Management: A Narrative Review of Analgesic Mechanisms and Clinical Evidence. Neurosurg. Rev. 2023, 46, 127.
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and Synaptic Integration of Glioma into Neural Circuits. Nature 2019, 573, 539–545.
- Okada, S.; Hara, M.; Kobayakawa, K.; Matsumoto, Y.; Nakashima, Y. Astrocyte Reactivity and Astrogliosis after Spinal Cord Injury. Neurosci. Res. 2018, 126, 39–43.
- Fan, Y.Y.; Huo, J. A1/A2 Astrocytes in Central Nervous System Injuries and Diseases: Angels or Devils? Neurochem. Int. 2021, 148, 105080.
- Loane, D.J. MAnGLed Astrocytes in Traumatic Brain Injury: Astrocytic 2-AG Metabolism as a New Therapeutic Target. Brain 2022, 145, 7–10.
More
 Encyclopedia
Encyclopedia
