The venom derived from various sources of snakes represents a vast collection of predominantly protein-based toxins that exhibit a wide range of biological actions, including but not limited to inflammation, pain, cytotoxicity, cardiotoxicity, and neurotoxicity. The venom of a particular snake species is composed of several toxins, while the venoms of around 600 venomous snake species collectively encompass a substantial reservoir of pharmacologically intriguing compounds. Findings have demonstrated the potential application of neurotoxins derived from snake venom in selectively targeting voltage-gated potassium channels (Kv). These neurotoxins include BPTI-Kunitz polypeptides, PLA2 neurotoxins, CRISPs, SVSPs, and various others.
- BPTI-Kunitz polypeptides
- CRISPs
- dendrotoxins
- Kv channels blockers
- PLA2 neurotoxins
- presynaptic neurotoxins
- SVSPs
- snake venom
1. Introduction
2. Voltage-Gated Potassium (Kv) Channels
Potassium channels are the most extensive and heterogeneous group of ion channels. The voltage-gated potassium channels (Kv) are the most significant subfamily among K+ channels. Potassium ions play a crucial role in various physiological processes, as they facilitate the controlled movement of ions along the electrochemical gradient [23]. These processes include the regulation of excitability and the modulation of neuronal action potentials [24[24][25],25], as well as the facilitation of muscular contraction [26,27,28][26][27][28] and the regulation of calcium signaling pathways, among others [29]. These functions have been extensively reviewed by previous studies [14,15][14][15]. The genes responsible for encoding Kv channel α-subunits have been identified and can be categorized into twelve distinct subfamilies [15]. These subfamilies include Kv1 (Shaker) with eight members, Kv2 (Shab) with two members, Kv3 (Shaw) with four members, Kv4 (Shal) having five members, Kv7 (KvLQT) with five members, Kv10 (HERG) with two members, Kv11 (also known as EAG) with three members, and Kv12 (ELK) with three members, as well as the modulatory subfamilies Kv5 (consisting of one member), Kv6 (consisting of four members), Kv8 (consisting of two members), and Kv9 (consisting of three members). This classification system has been established based on the identified genes for Kv channel α-subunits. These channels have been found to be involved in a wide range of neurological, cardiac, and immunological illnesses, making them significant targets for therapeutic interventions [30]. The number of transmembrane domains (TMD) observed in K+ channels has been determined through genetic and structural investigation, revealing the presence of two, four, or six TMD. The majority of Kv channels exhibit a six-TMD arrangement in their functional assembly (see Figure 1A). The regulation of the pore opening is governed by the voltage-sensing domain, which is composed of transmembrane segments S1–S4. This domain, also known as the voltage-sensor domain (VSD), is coupled to the pore domain (PD) through the intracellular loop between segments S4 and S5 [31] (Figure 1B). The latter domain consists of transmembrane proteins S5 and S6, which feature a re-entrant pore loop containing the K+ selectivity motif TVGYG [32]. Following the repolarization phase, the voltage-sensitive domain (VSD) undergoes deactivation, wherein the channel gate is closed, thereby impeding the passage of ions and restoring the VSD to its original resting state. Channels have the ability to be revived, however, if the stimulation caused by depolarization lasts longer than a few milliseconds, inactivation occurs and stops the permeability of potassium ions. Kv channels undergo recovery from the inactivation state exclusively following a short duration at a hyperpolarized potential [33]. C-type inactivation, also known as slow inactivation, is occurring after tens or hundreds of milliseconds from channel activation and is observed in the majority of Kv rectifying channels [34]. Recent research findings provide evidence for a mechanism in which the reorganization of amino acids within the inner cavity and outer vestibule of a channel is accompanied by the redistribution of structural water molecules. This process ultimately results in the collapse of the permeation pathway in C-type inactivation. This mechanism has been extensively discussed and reviewed in Ref. [35]. Several types of Kv channels exhibit rapid inactivation or N-type inactivation, which happens shortly after channel activation. This inactivation is primarily caused by an intracellular blockage by the channel’s intracellular N-terminus, also known as the inactivation particle [36,37][36][37].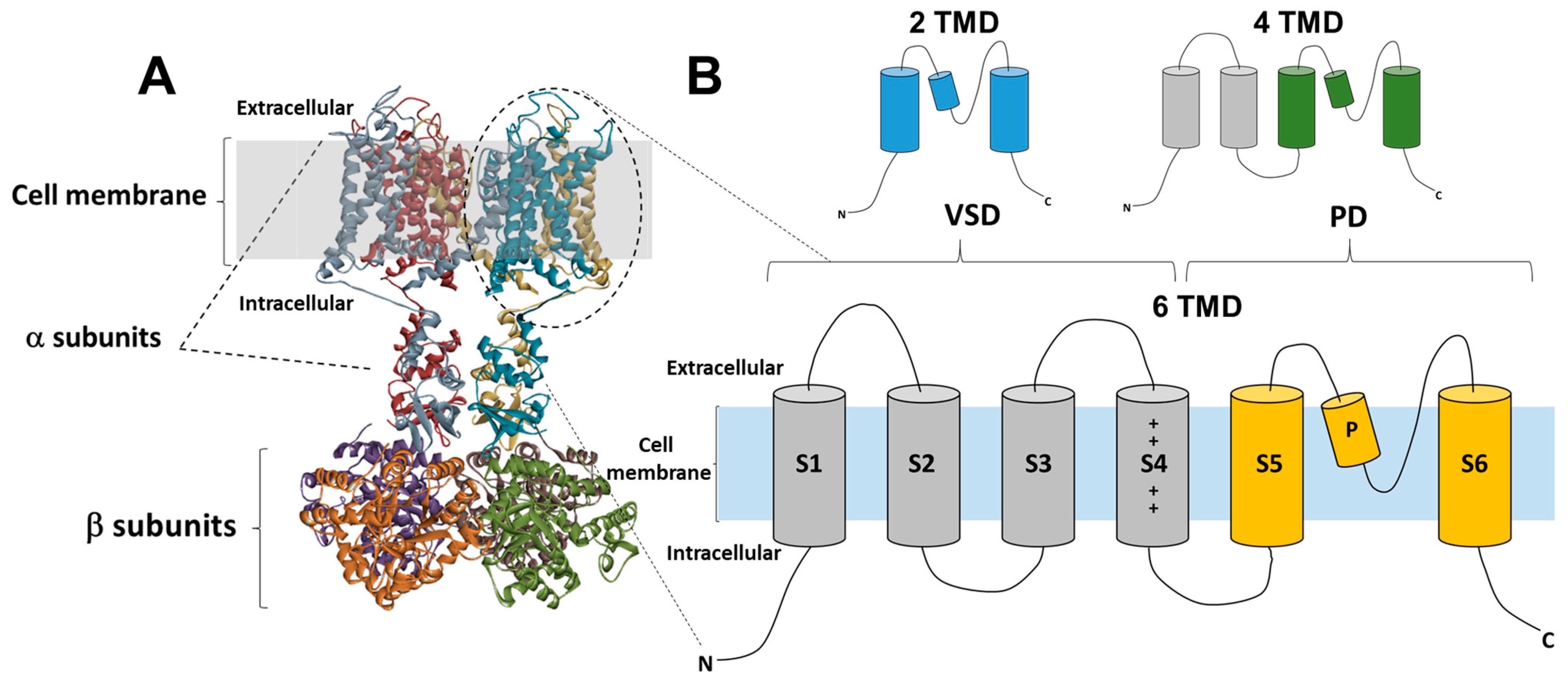
3. Snake Venom
The composition of snake venoms is characterized by a complex mixture of peptides, proteins, and non-protein components [2,44][2][44]. Despite the variations seen among different snake species and their geographical distribution, it is noteworthy that a significant proportion of the protein component in snakes exhibits enzymatic activity. The pro-inflammatory effects of the venom are commonly ascribed mostly to metalloproteases (SVMPs) and phospholipases A2 (PLA2s) [2,3][2][3]. Conversely, non-enzymatic proteins exacerbate the intensity of the envenomation. The venoms produced by snakes have the ability to selectively target particular receptors, ion channels, or plasma proteins, or extracellular components, thereby functioning as agonists, antagonists, or modulators. The pharmaceutical effects described would disrupt the individual’s physiological processes, leading to a variety of hazardous outcomes [45]. Included in this group of proteins, besides SVMPs and PLA2s, are disintegrins, C-type lectins, three-finger toxins, bradykinin-potentiating peptides (BPPs), Kunitz-type polypeptide, cysteine-rich secretory proteins (CRISPs), serpins, ICK peptides, and serine proteases (SVSPs) [9]. The components that specifically target Kv channels aim to disrupt the normal functioning of these channels, resulting in a decrease in the excitability of the affected tissues and ultimately leading to paralysis in the individual.4. Snake Venom Neurotoxins as Therapeutics
Snake venom is composed of a diverse combination of proteins and peptides, several of which have demonstrated promising medicinal possibilities [6,8,10,11,13,190,191][6][8][10][11][13][46][47]. The potential therapeutic applications of snake venom neurotoxins have been investigated owing to their capacity to selectively bind to certain receptors within the nervous system [192][48]. Neurotoxins possess the potential for both therapeutic and deleterious effects, contingent upon their distinct method of action and administered dosage. Nonetheless, the process of generating therapeutic treatments using neurotoxins derived from snake venom has numerous hurdles. One of the primary obstacles encountered in the development of snake venom neurotoxins as therapeutic agents lies in the attainment of adequate quantities suitable for clinical application [193][49]. The difficulties in acquiring adequate quantities of purified toxins from crude snake venom for scientific investigation and therapeutics can be mitigated by implementing venomics technologies, such as reverse-phase high-performance liquid chromatography (RP-HPLC) and followed by LC-MS/MS-based toxin identification [8]. Moreover, the future of advancements and discoveries lies in the use of efficient biotechnologies, such as cloning and large-scale toxin expression systems, and the optimization of drug delivery by toxin conjugation to monoclonal antibodies and nanoparticles [6,12][6][12]. Other approaches could involve the rational design of modified venom toxins with reduced toxicity and increased protection against proteolytic degradation [6,12][6][12]. A further obstacle lies in the imperative task of guaranteeing the safety of these toxins, given their high potency and the inherent risk of injury if mishandled [194][50]. In spite of the aforementioned hurdles, certain neurotoxins derived from snake venom have already been transformed into medicinal agents [9]. The utilization of snake venom neurotoxins exhibits considerable potential as a medicinal intervention for pain management. For instance, the antihypertensive drug Captopril (Capoten) was the first peptide derived from the venom of the Bothrops jararaca snake to receive FDA approval in 1981 [12,195][12][51]. This was then followed by the approval of Enalapril by the FDA in 1985 [9]. Both drugs are generated from bradykinin-potentiating peptides and function as angiotensin-converting enzyme inhibitors. They are administered to regulate hypertension and to avoid or ameliorate congestive heart failure [8,9][8][9]. This paved the way for more drug discoveries, such as Tirofiban and Eptifibatide, the selective competitive inhibitors for fibrinogen receptors [10]. Certain neurotoxins contained in snake venom have been discovered to specifically inhibit particular types of ion channels in sensory neurons, effectively impeding the transmission of pain signals to the brain [196][52]. These ion channels play a crucial role in a diverse range of pain syndromes, encompassing neuropathic pain, inflammatory pain, and cancer pain. In addition to the neurotoxins discussed in this review from various protein families (Table 1), DTXs exhibited notable efficacy and specificity against Kv1 channels. Such criteria of DTX make them useful tools to study the presynaptic Kv1 channel populations in healthy tissue and the integrity of brain’s connectomes in neurodegenerative diseases [7]. The synthesis of selective ligands against Kv1 channels, which are commonly seen in demyelinated neurons, has been achieved by rational design using a chemo-informatic approach. This strategy involves the design of chemical analogs to increase neural conduction in these neurons [197][53]. Nevertheless, there have been no reports of any pharmaceutical medication derived from a neurotoxic found in snake venom that specifically targets Kv channels for therapeutic purposes. Notably, Kv channel modulators derived from snake venom of various sizes (Table 1) show promise for biological uses. Due to their ability to precisely modulate various Kv channels, they have considerable potential as cardiovascular and neurological disease treatments and research tools. More research is needed to identify the molecular targets for several toxins, such as the members of PLA2, CRISPs, and three-finger toxins families.|
Snake Polypeptides |
Source |
Molecular Mass (kDa) |
Potassium Channel Targets |
References |
|---|---|---|---|---|
|
BPTI-Kunitz type |
||||
|
DTXs |
Dendroapis angusticeps Dendroapis polylepis Dendroapis viridis |
7 |
Kv1.1, Kv1.2, and Kv1.6 antagonists |
|
|
BF9 |
Bungarus fasciatus |
9 |
Kv1.3 antagonist |
|
|
PLA2 |
||||
|
Crotamine |
Crotalus durissus terrificus |
4.8 |
Kv1.1, Kv1.2, and Kv1.3 antagonists |
|
|
β-Bungarotoxin |
Bungarus multicinctus |
22 |
Inhibition of Kv currents (including Kv1.2 and Kv1.1). |
|
|
Natratoxin |
Naja atra |
13 |
A-type Kv channels antagonist |
|
|
MiDCA1 |
Micrurus dumerilii carinicauda |
15.5 |
Kv2.1 antagonist |
|
|
Taipoxin |
Oxyuranus s. scutellatus |
4.6 |
Inhibition of slowly activating Kv channels |
|
|
Notexin |
Oxyuranus s. scutellatus |
Inhibition of slowly activating Kv channels |
||
|
Crotoxin |
Crotalus durissus terrificus |
24 |
Inhibition of slowly activating Kv channels |
|
|
CRISPs |
||||
|
BaltCRP |
Bothrops alternatus |
24 |
Inhibition of Kv1.1, Kv1.3, Kv2.1, and Shaker channels |
|
|
Natrin |
Naja atra |
25 |
Inhibition of the Kv1.3 and BKCa channels |
|
|
Stecrisp |
Trimeresurus stejnegeri |
25 |
Possible Kv channels |
|
|
SVSPs |
||||
|
Collinein-1 |
Crotalus durissus collilineatus |
29.5 |
Inhibitor for hEAG1 (Kv10.1) channel (anticancer effect)Mild inhibitor for hERG1 (Kv11.1) channel |
|
|
Gyroxin_B1.3 |
Crotalus durissus terrificus |
28 |
Inhibitor for hEAG1 (Kv10.1) channel |
|
|
BjSP |
Bothrops jararaca |
28 |
Inhibitor for hEAG1 (Kv10.1) channel |
|
|
Three-finger toxins |
||||
|
Cardiotoxin-I |
Naja kaouthia |
6.7 |
Possible antagonist for KATP channel involved in stimulating insulin release |
|
|
Crotalphine |
Crotalus durissus terrificus |
1.5 |
Possible agonist for K+ channels mediating antinociceptive effect |
References
- Auerbach, P.S. Venomous and Poisonous Animals: A Handbook for Biologists, Toxicologists and Toxinologists, Physicians and Pharmacists. Wilderness Environ. Med. 2003, 14, 281.
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex Cocktails: The Evolutionary Novelty of Venoms. Trends Ecol. Evol. 2013, 28, 219–229.
- Chan, Y.S.; Cheung, R.C.F.; Xia, L.; Wong, J.H.; Ng, T.B.; Chan, W.Y. Snake Venom Toxins: Toxicity and Medicinal Applications. Appl. Microbiol. Biotechnol. 2016, 100, 6165–6181.
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite Envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063.
- Gutiérrez, J.M.; Williams, D.; Fan, H.W.; Warrell, D.A. Snakebite Envenoming from a Global Perspective: Towards an Integrated Approach. Toxicon 2010, 56, 1223–1235.
- Teodoro, A.; Gonçalves, F.J.M.; Oliveira, H.; Marques, S. Venom of Viperidae: A Perspective of Its Antibacterial and AntitumorPotential. Curr. Drug Targets 2022, 23, 126–144.
- Diniz-Sousa, R.; da S. Caldeira, C.A.; Pereira, S.S.; Da Silva, S.L.; Fernandes, P.A.; Teixeira, L.M.C.; Zuliani, J.P.; Soares, A.M. Therapeutic applications of snake venoms: An invaluable potential of new drug candidates. Int. J. Biol. Macromol. 2023, 238, 124357.
- Offor, B.C.; Piater, L.A. Snake Venom Toxins: Potential Anticancer Therapeutics. J. Appl. Toxicol. 2023. early view.
- Oliveira, A.L.; Viegas, M.F.; da Silva, S.L.; Soares, A.M.; Ramos, M.J.; Fernandes, P.A. The Chemistry of Snake Venom and Its Medicinal Potential. Nat. Rev. Chem. 2022, 6, 451–469.
- Teixeira, S.C.; Da Silva, M.S.; Gomes, A.A.S.; Moretti, N.S.; Lopes, D.S.; Ferro, E.A.V.; Rodrigues, V.D.M. Panacea within a Pandora’s Box: The Antiparasitic Effects of Phospholipases A2 (PLA2s) from Snake Venoms. Trends Parasitol. 2022, 38, 80–94.
- Almeida, J.R.; Palacios, A.L.V.; Patiño, R.S.P.; Mendes, B.; Teixeira, C.A.S.; Gomes, P.; Da Silva, S.L. Harnessing Snake Venom Phospholipases A2 to Novel Approaches for Overcoming Antibiotic Resistance. Drug Dev. Res. 2019, 80, 68–85.
- Li, L.; Huang, J.; Lin, Y. Snake Venoms in Cancer Therapy: Past, Present and Future. Toxins 2018, 10, 346.
- Zhou, K.; Luo, W.; Liu, T.; Ni, Y.; Qin, Z. Neurotoxins Acting at Synaptic Sites: A Brief Review on Mechanisms and Clinical Applications. Toxins 2022, 15, 18.
- Gutman, G.A.; Chandy, K.G.; Grissmer, S.; Lazdunski, M.; Mckinnon, D.; Pardo, L.A.; Robertson, G.A.; Rudy, B.; Sanguinetti, M.C.; Stühmer, W.; et al. International Union of Pharmacology. LIII. Nomenclature and Molecular Relationships of Voltage-Gated Potassium Channels. Pharmacol. Rev. 2005, 57, 473–508.
- Alexander, S.P.; Mathie, A.; Peters, J.A.; Veale, E.L.; Striessnig, J.; Kelly, E.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; et al. The Concise Guide to Pharmacology 2021/22: Ion Channels. Br. J. Pharmacol. 2021, 178, S157–S245.
- Yellen, G. The Moving Parts of Voltage-Gated Ion Channels. Quart. Rev. Biophys. 1998, 31, 239–295.
- Armstrong, C.M. Voltage-Gated K Channels. Sci. Signal. 2003, 2003, re10.
- Lewis, R.J.; Garcia, M.L. Therapeutic Potential of Venom Peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802.
- Zelanis, A.; Keiji Tashima, A. Unraveling Snake Venom Complexity with ‘Omics’ Approaches: Challenges and Perspectives. Toxicon 2014, 87, 131–134.
- Finol-Urdaneta, R.K.; Belovanovic, A.; Micic-Vicovac, M.; Kinsella, G.K.; McArthur, J.R.; Al-Sabi, A. Marine Toxins Targeting Kv1 Channels: Pharmacological Tools and Therapeutic Scaffolds. Mar. Drugs 2020, 18, 173.
- Gilquin, B.; Bourgoin, M.; Ménez, R.; Le Du, M.-H.; Servent, D.; Zinn-Justin, S.; Ménez, A. Motions and Structural Variability within Toxins: Implication for Their Use as Scaffolds for Protein Engineering. Protein Sci. 2003, 12, 266–277.
- Mouhat, S.; Jouirou, B.; Mosbah, A.; De Waard, M.; Sabatier, J.-M. Diversity of Folds in Animal Toxins Acting on Ion Channels. Biochem. J. 2004, 378, 717–726.
- Keynes, R.D. The Ionic Channels in Excitable Membranes. In Novartis Foundation Symposia; Wolstenholme, G.E.W., Fitzsimons, D.W., Eds.; Wiley: Hoboken, NJ, USA, 1975; Volume 31, pp. 191–203. ISBN 978-0-470-66323-3.
- Rudy, B. Diversity and Ubiquity of K Channels. Neuroscience 1988, 25, 729–749.
- Vacher, H.; Mohapatra, D.P.; Trimmer, J.S. Localization and Targeting of Voltage-Dependent Ion Channels in Mammalian Central Neurons. Physiol. Rev. 2008, 88, 1407–1447.
- Barry, D.M.; Trimmer, J.S.; Merlie, J.P.; Nerbonne, J.M. Differential Expression of Voltage-Gated K+ Channel Subunits in Adult Rat Heart: Relation to Functional K+ Channels? Circ. Res. 1995, 77, 361–369.
- Kalman, K.; Nguyen, A.; Tseng-Crank, J.; Dukes, I.D.; Chandy, G.; Hustad, C.M.; Copeland, N.G.; Jenkins, N.A.; Mohrenweiser, H.; Brandriff, B.; et al. Genomic Organization, Chromosomal Localization, Tissue Distribution, and Biophysical Characterization of a Novel Mammalian Shaker-Related Voltage-Gated Potassium Channel, Kv1.7. J. Biol. Chem. 1998, 273, 5851–5857.
- Matsubara, H.; Liman, E.R.; Hess, P.; Koren, G. Pretranslational Mechanisms Determine the Type of Potassium Channels Expressed in the Rat Skeletal and Cardiac Muscles. J. Biol. Chem. 1991, 266, 13324–13328.
- Tan, Y.P.; Llano, I. Modulation by K+ Channels of Action Potential-evoked Intracellular Ca2+ Concentration Rises in Rat Cerebellar Basket Cell Axons. J. Physiol. 1999, 520, 65–78.
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-Gated Potassium Channels as Therapeutic Targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001.
- Long, S.B.; Tao, X.; Campbell, E.B.; MacKinnon, R. Atomic Structure of a Voltage-Dependent K+ Channel in a Lipid Membrane-like Environment. Nature 2007, 450, 376–382.
- Long, S.B.; Campbell, E.B.; MacKinnon, R. Crystal Structure of a Mammalian Voltage-Dependent Shaker Family K+ Channel. Science 2005, 309, 897–903.
- Kurata, H.T.; Fedida, D. A Structural Interpretation of Voltage-Gated Potassium Channel Inactivation. Prog. Biophys. Mol. Biol. 2006, 92, 185–208.
- Pau, V.; Zhou, Y.; Ramu, Y.; Xu, Y.; Lu, Z. Crystal Structure of an Inactivated Mutant Mammalian Voltage-Gated K+ Channel. Nat. Struct. Mol. Biol. 2017, 24, 857–865.
- Valiyaveetil, F.I. A Glimpse into the C-Type-Inactivated State for a Potassium Channel. Nat. Struct. Mol. Biol. 2017, 24, 787–788.
- Aldrich, R.W. Fifty Years of Inactivation. Nature 2001, 411, 643–644.
- Fan, C.; Sukomon, N.; Flood, E.; Rheinberger, J.; Allen, T.W.; Nimigean, C.M. Ball-and-Chain Inactivation in a Calcium-Gated Potassium Channel. Nature 2020, 580, 288–293.
- Matthies, D.; Bae, C.; Toombes, G.E.; Fox, T.; Bartesaghi, A.; Subramaniam, S.; Swartz, K.J. Single-Particle Cryo-EM Structure of a Voltage-Activated Potassium Channel in Lipid Nanodiscs. eLife 2018, 7, e37558.
- Rettig, J.; Heinemann, S.H.; Wunder, F.; Lorra, C.; Parcej, D.N.; Oliver Dolly, J.; Pongs, O. Inactivation Properties of Voltage-Gated K+ Channels Altered by Presence of β-Subunit. Nature 1994, 369, 289–294.
- Rhodes, K.J.; Keilbaugh, S.A.; Barrezueta, N.X.; Lopez, K.L.; Trimmer, J.S. Association and Colocalization of K+ Channel Alpha- and Beta-Subunit Polypeptides in Rat Brain. J. Neurosci. 1995, 15, 5360–5371.
- Isacoff, E.Y.; Jan, Y.N.; Jan, L.Y. Evidence for the Formation of Heteromultimeric Potassium Channels in Xenopus Oocytes. Nature 1990, 345, 530–534.
- Koch, R.O.; Wanner, S.G.; Koschak, A.; Hanner, M.; Schwarzer, C.; Kaczorowski, G.J.; Slaughter, R.S.; Garcia, M.L.; Knaus, H.-G. Complex Subunit Assembly of Neuronal Voltage-Gated K+ Channels. J. Biol. Chem. 1997, 272, 27577–27581.
- Shamotienko, O.G.; Parcej, D.N.; Dolly, J.O. Subunit Combinations Defined for K+ Channel Kv1 Subtypes in Synaptic Membranes from Bovine Brain. Biochemistry 1997, 36, 8195–8201.
- Bieber, A.L. Metal and Nonprotein Constituents in Snake Venoms. In Snake Venoms; Lee, C.-Y., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 1979; Volume 52, pp. 295–306. ISBN 978-3-642-66915-6.
- McCauley, T.G.; Hamaguchi, N.; Stanton, M. Aptamer-Based Biosensor Arrays for Detection and Quantification of Biological Macromolecules. Anal. Biochem. 2003, 319, 244–250.
- King, G.F. Venoms as a Platform for Human Drugs: Translating Toxins into Therapeutics. Expert. Opin. Biol. Ther. 2011, 11, 1469–1484.
- Ang, W.F.; Koh, C.Y.; Kini, R.M. From Snake Venoms to Therapeutics: A Focus on Natriuretic Peptides. Pharmaceuticals 2022, 15, 1153.
- Osipov, A.; Utkin, Y. What Are the Neurotoxins in Hemotoxic Snake Venoms? Int. J. Mol. Sci. 2023, 24, 2919.
- Bordon, K.C.F.; Perino, M.G.; Giglio, J.R.; Arantes, E.C. Isolation, Enzymatic Characterization and Antiedematogenic Activity of the First Reported Rattlesnake Hyaluronidase from Crotalus durissus terrificus Venom. Biochimie 2012, 94, 2740–2748.
- Chuang, P.-C.; Chang, K.-W.; Cheng, F.-J.; Wu, M.-H.; Tsai, M.-T.; Li, C.-J. Risk Factors Associated with Snake Antivenom Reaction and the Role of Skin Test. Acta Trop. 2020, 203, 105293.
- Cushman, D.W.; Ondetti, M.A. History of the Design of Captopril and Related Inhibitors of Angiotensin Converting Enzyme. Hypertension 1991, 17, 589–592.
- Alama, A.; Bruzzo, C.; Cavalieri, Z.; Forlani, A.; Utkin, Y.; Casciano, I.; Romani, M. Inhibition of the Nicotinic Acetylcholine Receptors by Cobra Venom α-Neurotoxins: Is There a Perspective in Lung Cancer Treatment? PLoS ONE 2011, 6, e20695.
- Al-Sabi, A.; Daly, D.; Hoefer, P.; Kinsella, G.K.; Metais, C.; Pickering, M.; Herron, C.; Kaza, S.K.; Nolan, K.; Dolly, J.O. A Rational Design of a Selective Inhibitor for Kv1.1 Channels Prevalent in Demyelinated Nerves That Improves Their Impaired Axonal Conduction. J. Med. Chem. 2017, 60, 2245–2256.
- Hopkins, W.F.; Miller, J.L.; Miljanich, G.P. Voltage-Gated Potassium Channel Inhibitors. Curr. Pharm. Des. 1996, 2, 389–396.
- Grissmer, S.; Nguyen, A.N.; Aiyar, J.; Hanson, D.C.; Mather, R.J.; Gutman, G.A.; Karmilowicz, M.J.; Auperin, D.D.; Chandy, K.G. Pharmacological Characterization of Five Cloned Voltage-Gated K+ Channels, Types Kv1.1, 1.2, 1.3, 1.5, and 3.1, Stably Expressed in Mammalian Cell Lines. Mol. Pharmacol. 1994, 45, 1227–1234.
- Grupe, A.; Schröter, K.H.; Ruppersberg, J.P.; Stocker, M.; Drewes, T.; Beckh, S.; Pongs, O. Cloning and Expression of a Human Voltage-Gated Potassium Channel. A Novel Member of the RCK Potassium Channel Family. EMBO J. 1990, 9, 1749–1756.
- Imredy, J.P.; Chen, C.; MacKinnon, R. A Snake Toxin Inhibitor of Inward Rectifier Potassium Channel ROMK1. Biochemistry 1998, 37, 14867–14874.
- Yang, W.; Feng, J.; Wang, B.; Cao, Z.; Li, W.; Wu, Y.; Chen, Z. BF9, the First Functionally Characterized Snake Toxin Peptide with Kunitz-Type Protease and Potassium Channel Inhibiting Properties: Kunitz-Type Protease and Potassium Channel Inhibitor from Snake. J. Biochem. Mol. Toxicol. 2014, 28, 76–83.
- Coronado, M.A.; Gabdulkhakov, A.; Georgieva, D.; Sankaran, B.; Murakami, M.T.; Arni, R.K.; Betzel, C. Structure of the Polypeptide Crotamine from the Brazilian Rattlesnake Crotalus durissus terrificus. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1958–1964.
- Peigneur, S.; Orts, D.J.B.; Prieto da Silva, A.R.; Oguiura, N.; Boni-Mitake, M.; de Oliveira, E.B.; Zaharenko, A.J.; de Freitas, J.C.; Tytgat, J. Crotamine Pharmacology Revisited: Novel Insights Based on the Inhibition of KV Channels. Mol. Pharmacol. 2012, 82, 90–96.
- Kondo, K.; Narita, K.; Lee, C.-Y. Chemical Properties and Amino Acid Composition of β1-Bungarotoxin from the Venom of Bungarus multicinctus (Formosan Banded Krait). J. Biochem. 1978, 83, 91–99.
- Kondo, K.; Toda, H.; Narita, K. Characterization of Phospholipase A Activity of β 1-Bungarotoxin from Bungarus multicinctus Venom. J. Biochem. 1978, 84, 1291–1300.
- Petersen, M.; Penner, R.; Pierau, F.K.; Dreyer, F. Beta-Bungarotoxin Inhibits a Non-Inactivating Potassium Current in Guinea Pig Dorsal Root Ganglion Neurones. Neurosci. Lett. 1986, 68, 141–145.
- Bräu, M.E.; Dreyer, F.; Jonas, P.; Repp, H.; Vogel, W. A K+ Channel in Xenopus Nerve Fibres Selectively Blocked by Bee and Snake Toxins: Binding and Voltage-Clamp Experiments. J. Physiol. 1990, 420, 365–385.
- Guillemare, E.; Honore, E.; Pradier, L.; Lesage, F.; Schweitz, H.; Attali, B.; Barhanin, J.; Lazdunski, M. Effects of the Level of mRNA Expression on Biophysical Properties, Sensitivity to Neurotoxins, and Regulation of the Brain Delayed-Rectifier K+ Channel Kv1.2. Biochemistry 1992, 31, 12463–12468.
- Hu, P.; Sun, L.; Zhu, Z.-Q.; Hou, X.-W.; Wang, S.; Yu, S.-S.; Wang, H.-L.; Zhang, P.; Wang, M.; Niu, L.-W.; et al. Crystal Structure of Natratoxin, a Novel Snake Secreted phospholipaseA2 Neurotoxin from Naja Atra Venom Inhibiting A-Type K+ Currents. Proteins 2008, 72, 673–683.
- Belo, C.A.D.; Toyama, M.H.; Toyama, D.D.O.; Marangoni, S.; Moreno, F.B.; Cavada, B.S.; Fontana, M.D.; Hyslop, S.; Carneiro, E.M.; Boschero, A.C. Determination of the Amino Acid Sequence of a New Phospholipase A(2) (MIDCA1) Isolated from Micrurus dumerilii carinicauda Venom. Protein J. 2005, 24, 147–153.
- Schütter, N.; Barreto, Y.C.; Vardanyan, V.; Hornig, S.; Hyslop, S.; Marangoni, S.; Rodrigues-Simioni, L.; Pongs, O.; Dal Belo, C.A. Inhibition of Kv2.1 Potassium Channels by MiDCA1, A Pre-Synaptically Active PLA2-Type Toxin from Micrurus dumerilii carinicauda Coral Snake Venom. Toxins 2019, 11, 335.
- Dreyer, F.; Penner, R. The Actions of Presynaptic Snake Toxins on Membrane Currents of Mouse Motor Nerve Terminals. J. Physiol. 1987, 386, 455–463.
- Faure, G.; Xu, H.; Saul, F.A. Crystal Structure of Crotoxin Reveals Key Residues Involved in the Stability and Toxicity of This Potent Heterodimeric β-Neurotoxin. J. Mol. Biol. 2011, 412, 176–191.
- Cendron, L.; Mičetić, I.; Polverino de Laureto, P.; Paoli, M. Structural Analysis of Trimeric Phospholipase A2 Neurotoxin from the Australian Taipan Snake Venom. FEBS J. 2012, 279, 3121–3135.
- Westerlund, B.; Nordlund, P.; Uhlin, U.; Eaker, D.; Eklund, H. The Three-Dimensional Structure of Notexin, a Presynaptic Neurotoxic Phospholipase A2 at 2.0 Å Resolution. FEBS Lett. 1992, 301, 159–164.
- Bernardes, C.P.; Menaldo, D.L.; Zoccal, K.F.; Boldrini-França, J.; Peigneur, S.; Arantes, E.C.; Rosa, J.C.; Faccioli, L.H.; Tytgat, J.; Sampaio, S.V. First Report on BaltCRP, a Cysteine-Rich Secretory Protein (CRISP) from Bothrops alternatus Venom: Effects on Potassium Channels and Inflammatory Processes. Int. J. Biol. Macromol. 2019, 140, 556–567.
- Wang, J.; Shen, B.; Guo, M.; Lou, X.; Duan, Y.; Cheng, X.P.; Teng, M.; Niu, L.; Liu, Q.; Huang, Q.; et al. Blocking Effect and Crystal Structure of Natrin Toxin, a Cysteine-Rich Secretory Protein from Naja atra Venom That Targets the BKCa Channel. Biochemistry 2005, 44, 10145–10152.
- Wang, F.; Li, H.; Liu, M.; Song, H.; Han, H.; Wang, Q.; Yin, C.; Zhou, Y.; Qi, Z.; Shu, Y.; et al. Structural and Functional Analysis of Natrin, a Venom Protein That Targets Various Ion Channels. Biochem. Biophys. Res. Commun. 2006, 351, 443–448.
- Guo, M.; Teng, M.; Niu, L.; Liu, Q.; Huang, Q.; Hao, Q. Crystal Structure of the Cysteine-Rich Secretory Protein Stecrisp Reveals That the Cysteine-Rich Domain Has a K+ Channel Inhibitor-like Fold. J. Biol. Chem. 2005, 280, 12405–12412.
- Boldrini-França, J.; Pinheiro-Junior, E.L.; Arantes, E.C. Functional and Biological Insights of rCollinein-1, a Recombinant Serine Protease from Crotalus Durissus Collilineatus. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e147118.
- Boldrini-Franca, J.; Pinheiro-Junior, E.L.; Peigneur, S.; Pucca, M.B.; Cerni, F.A.; Borges, R.J.; Costa, T.R.; Carone, S.E.I.; Fontes, M.R.D.M.; Sampaio, S.V.; et al. Beyond Hemostasis: A Snake Venom Serine Protease with Potassium Channel Blocking and Potential Antitumor Activities. Sci. Rep. 2020, 10, 4476.
- Jahnke, W.; Mierke, D.F.; Béress, L.; Kessler, H. Structure of Cobra Cardiotoxin CTXI as Derived from Nuclear Magnetic Resonance Spectroscopy and Distance Geometry Calculations. J. Mol. Biol. 1994, 240, 445–458.
- Koster, J.C.; Permutt, M.A.; Nichols, C.G. Diabetes and Insulin Secretion. Diabetes 2005, 54, 3065–3072.
- Konno, K.; Picolo, G.; Gutierrez, V.P.; Brigatte, P.; Zambelli, V.O.; Camargo, A.C.M.; Cury, Y. Crotalphine, a Novel Potent Analgesic Peptide from the Venom of the South American Rattlesnake Crotalus durissus terrificus. Peptides 2008, 29, 1293–1304.
- Brigatte, P.; Konno, K.; Gutierrez, V.P.; Sampaio, S.C.; Zambelli, V.O.; Picolo, G.; Curi, R.; Cury, Y. Peripheral Kappa and Delta Opioid Receptors Are Involved in the Antinociceptive Effect of Crotalphine in a Rat Model of Cancer Pain. Pharmacol. Biochem. Behav. 2013, 109, 1–7.
- Shi, G.; Liu, Y.; Lin, H.; Yang, S.; Feng, Y.; Reid, P.F.; Qin, Z. Involvement of Cholinergic System in Suppression of Formalin-Induced Inflammatory Pain by Cobratoxin. Acta Pharmacol. Sin. 2011, 32, 1233–1238.
- Chen, Z.; Zhang, H.; Gu, Z.; Chen, B.; Han, R.; Reid, P.F.; Raymond, L.N.; Qin, Z. A Long-Form Alpha-Neurotoxin from Cobra Venom Produces Potent Opioid-Independent Analgesia1. Acta Pharmacol. Sin. 2006, 27, 402–408.
- Smith, H.H.; Deer, T.R. Safety and Efficacy of Intrathecal Ziconotide in the Management of Severe Chronic Pain. Ther. Clin. Risk Manag. 2009, 5, 521–534.
- Vink, S.; Alewood, P. Targeting Voltage-Gated Calcium Channels: Developments in Peptide and Small-Molecule Inhibitors for the Treatment of Neuropathic Pain: VGCC Ligands and Pain. Br. J. Pharmacol. 2012, 167, 970–989.
- Almeida, J.R.; Resende, L.M.; Watanabe, R.K.; Carregari, V.C.; Huancahuire-Vega, S.; Caldeira, C.D.S.; Coutinho-Neto, A.; Soares, A.M.; Vale, N.; Gomes, P.D.C.; et al. Snake Venom Peptides and Low Mass Proteins: Molecular Tools and Therapeutic Agents. Curr. Med. Chem. 2017, 24, 3254–3282.
- Kini, R.M.; Koh, C.Y. Snake Venom Three-Finger Toxins and Their Potential in Drug Development Targeting Cardiovascular Diseases. Biochem. Pharmacol. 2020, 181, 114105.
- Meyer, C.; Hahn, U.; Rentmeister, A. Cell-Specific Aptamers as Emerging Therapeutics. J. Nucleic Acids 2011, 2011, 904750.
- Nirthanan, S.; Awal, W.; Niranjan, N.R. Snake α-Neurotoxins and the Nicotinic Acetylcholine Receptor. In Snake Venoms; Inagaki, H., Vogel, C.-W., Mukherjee, A.K., Rahmy, T.R., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 215–252. ISBN 978-94-007-6409-5.
- Sridharan, S.; Kini, R.M.; Richards, A.M. Venom Natriuretic Peptides Guide the Design of Heart Failure Therapeutics. Pharmacol. Res. 2020, 155, 104687.
- Chakrabarty, D.; Chanda, C. Snake Venom Disintegrins. In Snake Venoms; Inagaki, H., Vogel, C.-W., Mukherjee, A.K., Rahmy, T.R., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 437–449. ISBN 978-94-007-6409-5.
- Golubkov, V.; Hawes, D.; Markland, F.S. Anti-Angiogenic Activity of Contortrostatin, a Disintegrin from Agkistrodon contortrix contortrix Snake Venom. Angiogenesis 2003, 6, 213–224.
- Jang, S.H.; Ryu, P.D.; Lee, S.Y. Dendrotoxin-κ Suppresses Tumor Growth Induced by Human Lung Adenocarcinoma A549 Cells in Nude Mice. J. Vet. Sci. 2011, 12, 35–40.
- Dubovskii, P.; Utkin, Y. Antiproliferative Activity of Cobra Venom Cytotoxins. Curr. Top. Med. Chem. 2015, 15, 638–648.
- Vonk, F.J.; Jackson, K.; Doley, R.; Madaras, F.; Mirtschin, P.J.; Vidal, N. Snake Venom: From Fieldwork to the Clinic: Recent Insights into Snake Biology, Together with New Technology Allowing High-Throughput Screening of Venom, Bring New Hope for Drug Discovery. Bioessays 2011, 33, 269–279.
- Zhou, Q.; Sherwin, R.P.; Parrish, C.; Richters, V.; Groshen, S.G.; Tsao-Wei, D.; Markland, F.S. Contortrostatin, a Dimeric Disintegrin from Contortrix contortrix, Inhibits Breast Cancer Progression. Breast Cancer Res. Treat. 2000, 61, 249–259.
