Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Wojciech Czyzewski and Version 2 by Lindsay Dong.
Traumatic Brain Injury (TBI) represents a significant health concern, necessitating advanced therapeutic interventions. Following injury, astrocytes exhibit reactive transformations, differentiating into pro-inflammatory (A1) and neuroprotective (A2) phenotypes.
- astroglia
- astrocytes
- traumatic brain injury
1. Introduction
Astrocytes, characterized by their heterogeneity, play a multifaceted role essential to cerebral function. These cells are instrumental in maintaining ion homeostasis, modulating the clearance of neurotransmitters, and regulating cerebral blood flow and water dynamics. Additionally, they are pivotal in the upkeep of synaptic structures and significantly contribute to the integrity of the blood-brain barrier [1][2][1,2]. Traumatic brain injury (TBI) is defined as brain damage caused by external mechanical force and it is the leading cause of death and disability especially in children and young adults [3][4][5][3,4,5]. It concerns approximately 64–74 million people annually [6]. In the United States alone, approximately 1.7 million people experience TBI, and over 5.3 million suffer from a trauma-related disability [7] reaching a 3% mortality rate [8]. This results in costs of roughly 56–221 billion US $ which are spent on diagnosis, treatment, and rehabilitation of patients with TBI each year [9]. The global incidence of TBI from any cause and all severity is estimated at 939 per 100,000 people. Brain injuries are traditionally classified by the Glasgow coma scale (GCS) according to their severity into mild (13–15 GCS points ), moderate (12–9 GCS points), and severe (<9 GCS points) [10][11][10,11]. The first group includes the least serious injuries and accounts for 80–90% of the total. They are usually caused by blunt, non-penetrating head trauma. Symptoms are usually transient and not very specific. They may include headache, mild cognitive symptoms, memory problems, nausea, and vomiting [4][6][4,6]. Moderate and severe TBI are associated with greater force of the trauma and their effect are often visible in imaging tests. The most severe of the groups is also associated with a very high mortality rate, both in the acute phase and later, reaching 35–75% [5][12][13][5,12,13]. However, despite its much lower prevalence, it is moderate and severe TBI that pose a serious problem for medicine. It is estimated that they cause approximately 90% of total TBI medical costs [14]. Many efforts have been implemented to improve the prognosis of patients as much as possible; however, no specific neuroprotective measures have been identified that can affect the mortality of patients after TBI on a broad scale [15]. Current guidelines for the management of trauma patients are primarily aimed at reducing secondary brain damage caused by a cascade of physiological processes that are the body’s response to injury. For this reason, it is so important to properly understand these relationships. As studies have shown, astrocytes play a key role in this process through the mechanism of reactive astrogliosis. According to preceding studies, restoration and regeneration following brain trauma is as complex as it is challenging due to various aspects [16]. Numerous cellular groups and biochemical compounds participate in this process, among which astrocytes are a key component. During the acute phase, injury results not only from the direct impact of external force upon the body but also from the reactive responses of astrocytes and microglia cells. These cells undergo alterations in their transcriptional and morphological profiles, leading to the initiation of both pro-inflammatory and anti-inflammatory processes. These physiological adaptations are designed to facilitate neuronal regeneration and the clearance of damaged tissue [17][18][17,18]. Upon exposure to specific stimuli, frequently originating from central nervous system (CNS) injuries or pathologies, astrocytes undergo a significant transformation into a reactive state. This process, termed “reactive astrogliosis”, is characterized by substantial changes in gene expression, cellular morphology, and functional dynamics [19]. Multiple theoretical frameworks have been proposed to elucidate the mechanisms underlying astrocyte responses to cerebral injury. Confronted with brain trauma, astroglial cells exhibit notable alterations in both functionality and phenotypic characteristics [20][21][22][20,21,22]. Although this reactive state of astrocytes can be conducive to healing and recovery, exemplified by functions such as stabilizing the blood-brain barrier (BBB), facilitating neurogenesis, and secreting neurotrophic compounds, it also has the potential to produce adverse effects. A significant consequence is the formation of a glial scar, traditionally regarded as an impediment to CNS repair. This scar, emerging from the collective response of reactive astrocytes, is a primary factor exacerbating neuronal regeneration. The fibrotic scar tissue not only presents a physical and chemical barrier but also disrupts the process of synaptogenesis [23][24][23,24].
2. Role of Astroglia in Brain Injury
2.1. Blood Brain Barrier
One of the influences of astrocytes on the homeostasis of the CNS is their effect on the blood-brain barrier. Astrogliosis acts protectively on surrounding neural tissue through the maintenance of the blood-brain barrier (BBB), repair of damaged tissues, production of neuroprotective compounds, and mediation of inflammation [22][25][26][22,27,28]. This process is possible because of the communication with endothelial cells [27][28][29][29,30,31]. Astrocytes also oversee the functioning of the BBB through specialized astrocytic extensions known as “endfeet” that are equipped with the potassium channel Kir 4.1 and Aquaporin-4, which play a crucial role in regulating ion and water balance, contributing to BBB integrity [30][32]. In the event of insult to the brain, astrocyte-derived factors possess a key role in recovery and disruption of the blood-brain barrier. The astrocyte-derived factors that influence vascular permeability encompass vascular endothelial growth factors, glutamate, matrix metalloproteinases, nitric oxide, and endothelin-1. These elements heighten the permeability of the blood-brain barrier, ultimately leading to its disruption.2.2. Immunological Response
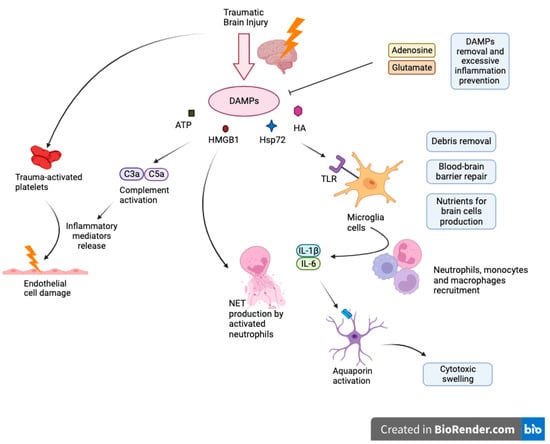
Activated astrocytes are important regulators of immune response. In response to damage, astroglial cells release neurotrophic factors that act to protect the brain [31][33]. For example, brain-derived neurotrophic factor (BDNF) secreted by astroglial cells has been shown to combat drug-related neurotoxic effects and neuronal degeneration associated with normal aging. Suboptimal levels of BDNF have been associated with neuronal death [32][33][34,35]. Another neuroprotective contribution is through the release of 17β-estradiol (E2) compounds from activated astrocytes [34][35][36,37]. E2 is important in mediating inflammatory responses and in reducing microglial activation in the brain [36][37][38,39]. Overactivation of microglia can lead to a feedback loop which induces more reactive astrogliosis and further inflammatory damage as a result [38][39][40][40,41,42]. Also, astrocytes function as an inhibitor of neuroinflammation through various physiological pathways such as TGF-β signaling or estrogen receptor signaling pathway [41][42][43,44]. Even though astroglia can downregulate inflammatory responses, its activation can also increase inflammation. Studies have found that activated astrocytes are associated with higher amounts of inflammatory markers [43][44][45,46]. Cytokines released from astrocytes also promote inflammation through microglial cells after traumatic brain injury [45][46][47,48]. Glial cell necrosis mitigates cell’s functionality what triggers the release and subsequent accumulation of molecules known as Damage-Associated Molecular Patterns (DAMPs), such as intracellular ions, nucleic acids, high mobility group box 1 protein (HMGB1), heat shock protein 72 (Hsp72), HA and ATP. These DAMPs activate immune receptors, notably Toll-Like Receptors (TLR), Receptors for Advanced Glycation End Products (RAGE) or purinergic receptors on myeloid cells encompassing macrophages, glial cells, dendritic cells, and astrocytes which cause their activation as well as inflammasome assembly (NALP1) that advocates generation of cytokines as IL-18 or IL-1Β [47][53]. Upon activation, resident microglia undertake cleanup of cellular debris, restore the integrity of the damaged blood-brain barrier, and aim to deliver essential nutrients requisite for neuronal cells. However, microglia exhibit a high degree of flexibility, and their role in a non-infectious immune response is determined by their activation status, the extent of the damage, interplay with adjacent cells, and the makeup of the immune cells that have infiltrated the area. Further release of proinflammatory cytokines such as IL-1β and IL-6 facilitates the recruitment of peripheral immune cells into the site of injury. When neutrophils traverse the BBB, they exacerbate leukocyte activation, proinflammatory cytokines levels, and incite brain tissue swelling Figure 1.
Figure 1.
Cascade of neuroinflammation in Traumatic Brain Injury: DAMPs, glial activation, and systemic consequences.
2.3. Role in Synthesis and Function of Synapses
In addition to providing support to neuronal cells, astrocytes also exist as basic components of the neuronal circuit known as the tripartite synapse and are essential to the structural integrity of synaptic transmission in the brain [48][49][50][51][57,58,59,60]. Tripartite synapses refer to the integrative communication between the presynaptic neurons, postsynaptic neurons, and the surrounding glial cells [52][53][61,62]. In the pathogenesis of altered consciousness or neurodegenerative diseases, astrocytic interactions within tripartite synapses seem to play a role in mood and behavior alterations and the predisposition to neurological diseases [54][55][56][63,64,65].
Astroglia serves a morphological and functional role in these synapses [48][57][57,66]. As part of the structural components of the tripartite synapse, astrocytes act as a controller for the active metabolic milieu surrounding neurons [51][58][59][60][60,67,68,69]. The metabolic milieu in the CNS is important in the formation, maintenance, and function of synapses. Perisynaptic astroglia regulate the CNS milieu through various mechanisms and pathways such as altering extracellular ions, regulating pH, managing waste, and assisting in the exchange of signaling molecules [61][62][63][70,71,72].
Studies have found that under circumstances where astroglia function is compromised, the chemical environment can become neurotoxic and damaging to the surrounding neuronal tissue, thereby negatively affecting synaptic transmission [38][64][40,73].
In conjunction with synaptogenesis, astroglial cells can also control synaptic plasticity [65][66][94,95]. Astrocytes release neuromediators that induce an increase in the formation of excitatory synapses which are associated with the expression of synaptic plasticity [67][96]. The induction of synaptic plasticity plays a role in memory formation [68][69][97,98]. Contrarily, astrocytes also have the potential to inhibit neural plasticity [70][99].
Astrocytes are also essential for neurovascular coupling (NVC), which coordinates communication between neurons and blood vessels in the brain. These versatile cells contribute significantly to proper blood vessel density and branching during development, as well as in conditions like ischemic stroke, traumatic spine injury, and by analogy TBI. In response to neurotransmitter release, like glutamate and ATP, astrocytes elevate Ca2+ levels, releasing vasoactive molecules onto blood vessels to drive NVC [71][102].
Astrocytes also dynamically regulate cerebral vessel diameter through Ca2+-dependent mechanisms, releasing substances such as EETs (epoxyeicosatrienoic acids) and PGE2 to dilate vessels or 20-HETE (20-hydroxytetraenoic acid) to constrict them. Additionally, they help maintain ion balance by absorbing excess extracellular K+ during neuronal activity, which triggers Ca2+ increases, activates BK channels, and releases K+ onto vascular mural cells.
Astroglia also plays a role in synapse maturation. Several astrocytic-derived genes are associated with proteins that participate in the maturation of synapses and neuronal circuits [72][104].
