Chloroquine (CQ) and its derivate hydroxychloroquine (HCQ), the compounds with recognized ability to suppress autophagy, have been tested in experimental works and in clinical trials as adjuvant therapy for the treatment of tumors of different origin to increase the efficacy of cytotoxic agents. Such a strategy can be effective in overcoming the resistance of cancer cells to standard chemotherapy or anti-angiogenic therapy. The majority of experimental studies has shown that CQ/HCQ can effectively sensitize cancer cells to cytotoxic agents and increase the potential of chemotherapy, however, the results of clinical trials are often inconsistent. Although pharmacological suppression of autophagy remains a promising tool for increasing the efficacy of standard chemotherapy, the development of more specific inhibitors is required.
- chloroquine
- hydroxychloroquine
- autophagy
- chemotherapy
- cultured cancer cells
- animal xenografts
- clinical trials
1. Cellular Chloroquine Effects
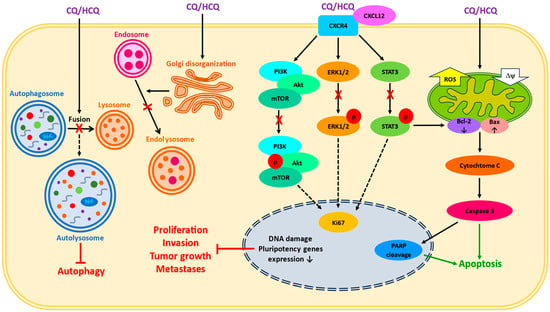
2. Chloroquine as a Single Treatment
3. Chloroquine and Chemotherapy Drugs
3.1. Chloroquine and Doxorubicin (DOX)
3.2. Chloroquine and Paclitaxel (PTX)
3.3. Chloroquine- and Platinum-Based Anticancer Drugs
3.4. Chloroquine and Gemcitabine (GEM)
3.5. Chloroquine and Tyrosine Kinase Inhibitors
3.6. Chloroquine and PI3K/Akt/mTOR Inhibitors
3.7. Chloroquine and Other Agents
3.8. Chloroquine in Multi-Drug Combinations
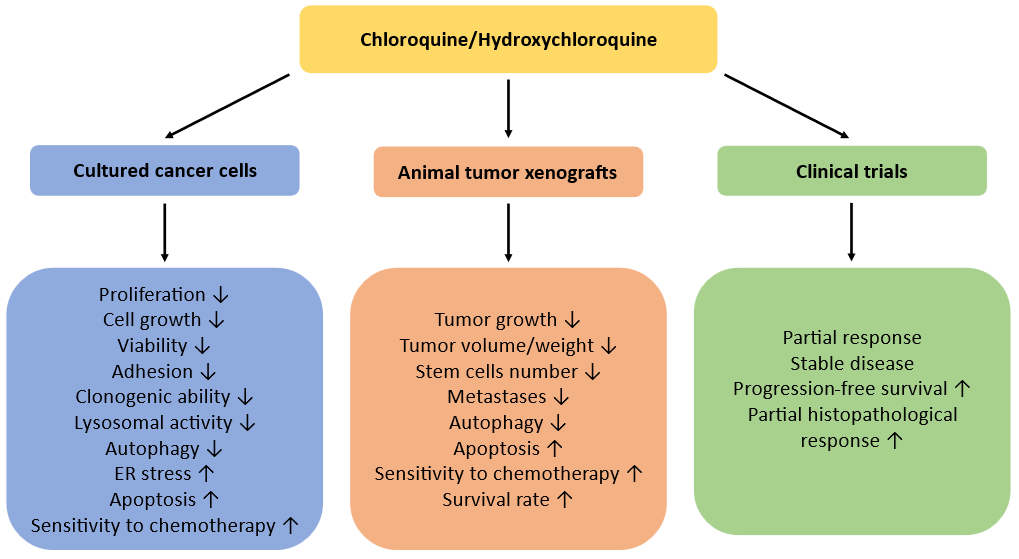
Fig.ure 2
. Anticancer effects of CQ/HCQ in experimental studies and in clinical trials.
References
- De Sanctis, J.B.; Charris, J.; Blanco, Z.; Ramírez, H.; Martínez, G.P.; Mijares, M.R. Molecular mechanisms of chloroquine and hydroxychloroquine used in cancer therapy. Anticancer Agents Med. Chem. 2023, 23, 1122–1144.
- Fong, W.; To, K.K.W. Repurposing chloroquine analogs as an adjuvant cancer therapy. Recent Pat. Anticancer Drug Discov. 2021, 16, 204–221.
- Low, L.E.; Kong, C.K.; Yap, W.H.; Siva, S.P.; Gan, S.H.; Siew, W.S.; Ming, L.C.; Lai-Foenander, A.S.; Chang, S.K.; Lee, W.L.; et al. Hydroxychloroquine: Key therapeutic advances and emerging nanotechnological landscape for cancer mitigation. Chem. Biol. Interact. 2023, 13, 110750.
- Noguchi, M.; Hirata, N.; Tanaka, T.; Suizu, F.; Nakajima, H.; Chiorini, J.A. Autophagy as a modulator of cell death machinery. Cell Death Dis. 2020, 11, 517.
- Russell, R.C.; Guan, K.L. The multifaceted role of autophagy in cancer. EMBO J. 2022, 41, e110031.
- Chern, Y.J.; Tai, I.T. Adaptive response of resistant cancer cells to chemotherapy. Cancer Biol. Med. 2020, 17, 842–863.
- Zhang, T.; Yu, J.; Cheng, S.; Zhang, Y.; Zhou, C.H.; Qin, J.; Luo, H. Research progress on the anticancer molecular mechanism of targets regulating cell autophagy. Pharmacology 2023, 108, 224–237.
- Rahman, M.A.; Saikat, A.S.; Rahman, M.S.; Islam, M.; Parvez, M.A.; Kim, B. Recent update and drug target in molecular and pharmacological insights into autophagy modulation in cancer treatment and future progress. Cells 2023, 12, 458.
- Niemann, B.; Puleo, A.; Stout, C.; Markel, J.; Boone, B.A. Biologic functions of hydroxychloroquine in disease: From COVID-19 to cancer. Pharmaceutics 2022, 14, 2551.
- Kim, E.L.; Wüstenberg, R.; Rübsam, A.; Schmitz-Salue, C.; Warnecke, G.; Bücker, E.M.; Pettkus, N.; Speidel, D.; Rohde, V.; Schulz-Schaeffer, W.; et al. Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells. Neuro-Oncology 2010, 12, 389–400.
- Lakhter, A.J.; Sahu, R.P.; Sun, Y.; Kaufmann, W.K.; Androphy, E.J.; Travers, J.B.; Naidu, S.R. Chloroquine promotes apoptosis in melanoma cells by inhibiting BH3 domain-mediated PUMA degradation. J. Investig. Dermatol. 2013, 133, 2247–2254.
- Balic, A.; Sørensen, M.D.; Trabulo, S.M.; Sainz, B., Jr.; Cioffi, M.; Vieira, C.R.; Miranda-Lorenzo, I.; Hidalgo, M.; Kleeff, J.; Erkan, M.; et al. Chloroquine targets pancreatic cancer stem cells via inhibition of CXCR4 and hedgehog signaling. Mol. Cancer Ther. 2014, 13, 1758–1771.
- Hu, T.; Li, P.; Luo, Z.; Chen, X.; Zhang, J.; Wang, C.; Chen, P.; Dong, Z. Chloroquine inhibits hepatocellular carcinoma cell growth in vitro and in vivo. Oncol. Rep. 2016, 35, 43–49.
- Nakano, K.; Masui, T.; Yogo, A.; Uchida, Y.; Sato, A.; Kasai, Y.; Nagai, K.; Anazawa, T.; Kawaguchi, Y.; Uemoto, S. Chloroquine induces apoptosis in pancreatic neuro- endocrine neoplasms via endoplasmic reticulum stress. Endocr. Relat. Cancer 2020, 27, 431–439.
- Müller, A.; Weyerhäuser, P.; Berte, N.; Jonin, F.; Lyubarskyy, B.; Sprang, B.; Kantelhardt, S.R.; Salinas, G.; Opitz, L.; Schulz-Schaeffer, W.; et al. Concurrent Activation of Both Survival-Promoting and Death-Inducing Signaling by Chloroquine in Glioblastoma Stem Cells: Implications for Potential Risks and Benefits of Using Chloroquine as Radiosensitizer. Cells 2023, 12, 1290.
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 48, 1435–1455.
- Choi, D.S.; Blanco, E.; Kim, Y.S.; Rodriguez, A.A.; Zhao, H.; Huang, T.H.M.; Chen, C.L.; Jin, G.; Landis, M.D.; Burey, L.A.; et al. Chloroquine eliminates cancer stem cells through deregulation of Jak2 and DNMT1. Stem Cells 2014, 32, 2309–2323.
- Liang, D.H.; Choi, D.S.; Ensor, J.E.; Kaipparettu, B.A.; Bass, B.L.; Chang, J.C. The autophagy inhibitor chloroquine targets cancer stem cells in triple negative breast cancer by inducing mitochondrial damage and impairing DNA break repair. Cancer Lett. 2016, 376, 249–258.
- Fauzi, Y.R.; Nakahata, S.; Chilmi, S.; Ichikawa, T.; Nueangphuet, P.; Yamaguchi, R.; Nakamura, T.; Shimoda, K.; Morishita, K. Antitumor effects of chloroquine/hydroxychloroquine mediated by inhibition of the NF-κB signaling pathway through abrogation of autophagic p47 degradation in adult T-cell leukemia/lymphoma cells. PLoS ONE 2021, 16, e0256320.
- Fukuda, T.; Oda, K.; Wada-Hiraike, O.; Sone, K.; Inaba, K.; Ikeda, Y.; Miyasaka, A.; Kashiyama, T.; Tanikawa, M.; Arimoto, T.; et al. The anti-malarial chloroquine suppresses proliferation and overcomes cisplatin resistance of endometrial cancer cells via autophagy inhibition. Gynecol. Oncol. 2015, 137, 538–545.
- Lin, Y.C.; Lin, J.F.; Wen, S.I.; Yang, S.C.; Tsai, T.F.; Chen, H.E.; Chou, K.Y.; Hwang, T.I. Chloroquine and hydroxychloroquine inhibit bladder cancer cell growth by targeting basal autophagy and enhancing apoptosis. Kaohsiung J. Med. Sci. 2017, 33, 215–223.
- Mulcahy Levy, J.M.; Zahedi, S.; Griesinger, A.M.; Morin, A.; Davies, K.D.; Aisner, D.L.; Kleinschmidt-DeMasters, B.K.; Fitzwalter, B.E.; Goodall, M.L.; Thorburn, J.; et al. Autophagy inhibition overcomes multiple mechanisms of resistance to BRAF inhibition in brain tumors. Elife 2017, 6, e19671.
- Pagotto, A.; Pilotto, G.; Mazzoldi, E.L.; Nicoletto, M.O.; Frezzini, S.; Pastò, A.; Amadori, A. Autophagy inhibition reduces chemoresistance and tumorigenic potential of human ovarian cancer stem cells. Cell Death Dis. 2017, 8, e2943.
- Duarte, D.; Vale, N. New trends for antimalarial drugs: Synergism between antineoplastics and antimalarials on breast cancer cells. Biomolecules 2020, 10, 1623.
- El-Gowily, A.H.; Loutfy, S.A.; Ali, E.M.; Mohamed, T.M.; Mansour, M.A. Tioconazole and chloroquine act synergistically to combat doxorubicin-induced toxicity via inactivation of PI3K/AKT/mTOR signaling mediated ROS-dependent apoptosis and autophagic flux inhibition in MCF-7 breast cancer cells. Pharmaceuticals 2021, 14, 254.
- Kazakova, D.; Shimamura, M.; Kurashige, T.; Hamada, K.; Nagayama, Y. Re-evaluation of the role of autophagy in thyroid cancer treatment. Endocr. J. 2022, 69, 847–862.
- Mattioli, R.; Ilari, A.; Colotti, B.; Mosca, L.; Fazi, F.; Colotti, G. Doxorubicin and other anthracyclines in cancers: Activity, chemoresistance and its overcoming. Mol. Asp. Med. 2023, 93, 101205.
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin-an agent with multiple mechanisms of anticancer activity. Cells 2023, 12, 659.
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339.
- Kumar, A.; Singh, U.K.; Chaudhary, A. Targeting autophagy to overcome drug resistance in cancer therapy. Future Med. Chem. 2015, 7, 1535–1542.
- Usman, R.M.; Razzaq, F.; Akbar, A.; Farooqui, A.A.; Iftikhar, A.; Latif, A.; Hassan, H.; Zhao, J.; Carew, J.S.; Nawrocki, S.T.; et al. Role and mechanism of autophagy-regulating factors in tumorigenesis and drug resistance. Asia Pac. J. Clin. Oncol. 2021, 17, 193–208.
- Zhou, Y.; Chen, E.; Tang, Y.; Mao, J.; Shen, J.; Zheng, X.; Xie, S.; Zhang, S.; Wu, Y.; Liu, H.; et al. MiR-223 overexpression inhibits doxorubicin-induced autophagy by targeting FOXO3a and reverses chemoresistance in hepatocellular carcinoma cells. Cell Death Dis. 2019, 10, 843.
- Yu, P.; Wang, H.Y.; Tian, M.; Li, A.X.; Chen, X.S.; Wang, X.L.; Zhang, Y.; Cheng, Y. Eukaryotic elongation factor-2 kinase regulates the cross-talk between autophagy and pyroptosis in doxorubicin-treated human melanoma cells in vitro. Acta Pharmacol. Sin. 2019, 40, 1237–1244.
- Duarte, D.; Nunes, M.; Ricardo, S.; Vale, N. Combination of Antimalarial and CNS Drugs with Antineoplastic Agents in MCF-7 Breast and HT-29 Colon Cancer Cells: Biosafety Evaluation and Mechanism of Action. Biomolecules 2022, 12, 1490.
- Abdel-Mohsen, M.A.; Abdel Malak, C.A.; El-Shafey, E.S. Influence of copper (I) nicotinate complex and autophagy modulation on doxorubicin-induced cytotoxicity in HCC1806 breast cancer cells. Adv. Med. Sci. 2019, 64, 202–209.
- Bano, N.; Ansari, M.I.; Kainat, K.M.; Singh, V.K.; Sharma, P.K. Chloroquine synergizes doxorubicin efficacy in cervical cancer cells through flux impairment and down regulation of proteins involved in the fusion of autophagosomes to lysosomes. Biochem. Biophys. Res. Commun. 2023, 656, 131–138.
- Sato, K.; Ota, N.; Endo, S.; Nakata, A.; Yamashita, H.; Tatsunami, R. Effect of chloroquine on doxorubicin-induced apoptosis in A549 cells. Anticancer Res. 2022, 42, 4025–4035.
- Utkusavas, A.; Gurel Gurevin, E.; Yilmazer, N.; Uvez, A.; Oztay, F.; Bulut, H.; Ustunova, S.; Esener, O.B.B.; Sonmez, K.; Erol Kutucu, D.; et al. Effects of combined administration of doxorubicin and chloroquine on lung pathology in mice with solid Ehrlich ascites carcinoma. Biotech. Histochem. 2022, 97, 555–566.
- Gurel-Gurevin, E.; Kiyan, H.T.; Esener, O.B.B.; Aydinlik, S.; Uvez, A.; Ulukaya, E.; Dimas, K.; Armutak, E.I. Chloroquine used in combination with chemotherapy synergistically suppresses growth and angiogenesis in vitro and in vivo. Anticancer Res. 2018, 38, 4011–4020.
- Zhao, H.; Yu, J.; Zhang, R.; Chen, P.; Jiang, H.; Yu, W. Doxorubicin prodrug-based nanomedicines for the treatment of cancer. Eur. J. Med. Chem. 2023, 258, 115612.
- Lee, J.; Choi, M.K.; Song, I.S. Recent advances in doxorubicin formulation to enhance pharmacokinetics and tumor targeting. Pharmaceuticals 2023, 16, 802.
- Gabizon, A.A. Pegylated liposomal doxorubicin: Metamorphosis of an old drug into a new form of chemotherapy. Cancer Investig. 2001, 19, 424–436.
- Chiang, C.F.; Hsu, Y.H.; Liu, C.C.; Liang, P.C.; Miaw, S.C.; Lin, W.L. Pulsed-wave ultrasound hyperthermia enhanced nanodrug delivery combined with chloroquine exerts effective antitumor response and postpones recurrence. Sci. Rep. 2019, 9, 12448.
- Chiang, C.F.; Wang, Z.Z.; Hsu, Y.H.; Miaw, S.C.; Lin, W.L. Exercise improves the outcome of anticancer treatment with ultrasound-hyperthermia-enhanced nanochemotherapy and autophagy inhibitor. PLoS ONE 2023, 18, e0288380.
- Dos Reis, S.B.; de Oliveira Silva, J.; Garcia-Fossa, F.; Leite, E.A.; Malachias, A.; Pound-Lana, G.; Mosqueira, V.C.F.; Oliveira, M.C.; de Barros, A.L.B.; de Jesus, M.B. Mechanistic insights into the intracellular release of doxorubicin from pH-sensitive liposomes. Biomed. Pharmacother. 2021, 134, 110952.
- Xu, S.; Zhong, Y.; Nie, C.; Pan, Y.; Adeli, M.; Haag, R. Activation co-delivery of doxorubicin and chloroquine by polyglycerol functionalized MoS2 nanosheets for efficient multidrug-resistant cancer therapy. Macromol. Biosci. 2021, 21, e2100233.
- Wang, J.; Qiu, L. Drug-induced self-assembled nanovesicles for doxorubicin resistance reversal via autophagy inhibition and delivery synchronism. Theranostics 2022, 12, 3977–3994.
- Sousa-Pimenta, M.; Estevinho, L.M.; Szopa, A.; Basit, M.; Khan, K.; Armaghan, M.; Ibrayeva, M.; Sönmez Gürer, E.; Calina, D.; Hano, C.; et al. Chemotherapeutic properties and side-effects associated with the clinical practice of tepene alkaloids: Paclitaxel, docetaxel, and cabazitaxel. Front. Pharmacol. 2023, 14, 1157306.
- Liu, Y.; Zhao, F.; Wang, Q.; Zhao, Q.; Hou, G.; Meng, Q. Current perspectives on paclitaxel: Focus on its production, delivery and combination therapy. Mini Rev. Med. Chem. 2023, 23, 1780–1796.
- Škubník, J.; Svobodová Pavlíčková, V.; Ruml, T.; Rimpelová, S. Autophagy in cancer resistance to paclitaxel: Development of combination strategies. Biomed. Pharmacother. 2023, 161, 114458.
- Yuan, Z.; Cai, J.; Du, Q.; Ma, Q.; Xu, L.; Cai, Y.; Zhong, X.; Guo, X. chloroquine sensitizes esophageal carcinoma EC109 cells to paclitaxel by inhibiting autophagy. Crit. Rev. Eukaryot. Gene Expr. 2023, 33, 43–53.
- Anand, K.; Niravath, P.; Patel, T.; Ensor, J.; Rodriguez, A.; Boone, T.; Wong, S.T.; Chang, J.C. A Phase II Study of the Efficacy and Safety of Chloroquine in Combination with Taxanes in the Treatment of Patients with Advanced or Metastatic Anthracycline-refractory Breast Cancer. Clin. Breast Cancer 2021, 21, 199–204.
- Forgie, B.N.; Prakash, R.; Telleria, C.M. Revisiting the anti-cancer toxicity of clinically approved platinating derivatives. Int. J. Mol. Sci. 2022, 23, 15410.
- Mason, S.R.; Willson, M.L.; Egger, S.J.; Beith, J.; Dear, R.F.; Goodwin, A. Platinum-based chemotherapy for early triple-negative breast cancer. Cochrane Database Syst. Rev. 2023, 9, CD014805.
- Lu, E.; Gareev, I.; Yuan, C.; Liang, Y.; Sun, J.; Chen, X.; Beylerli, O.; Sufianov, A.; Zhao, S.; Yang, G. The mechanisms of current platinum anticancer drug resistance in the glioma. Curr. Pharm. Des. 2022, 28, 1863–1869.
- Chen, T.; Zeng, C.; Li, Z.; Wang, J.; Sun, F.; Huang, J.; Lu, S.; Zhu, J.; Zhang, Y.; Sun, X.; et al. Investigation of chemoresistance to first-line chemotherapy and its possible association with autophagy in high-risk neuroblastoma. Front. Oncol. 2022, 12, 1019106.
- Zhu, J.; Zheng, Y.; Zhang, H.; Zhu, J.; Sun, H. Low concentration of chloroquine enhanced efficacy of cisplatin in the treatment of human ovarian cancer dependent on autophagy. Am. J. Transl. Res. 2017, 9, 4046–4058.
- Zhang, H.Q.; Fang, N.; Liu, X.M.; Xiong, S.P.; Liao, Y.Q.; Jin, W.J.; Song, R.F.; Wan, Y.Y. Antitumor activity of chloroquine in combination with cisplatin in human gastric cancer xenografts. Asian Pac. J. Cancer Prev. 2015, 16, 3907–3912.
- Guo, X.L.; Li, D.; Hu, F.; Song, J.R.; Zhang, S.S.; Deng, W.J.; Sun, K.; Zhao, Q.D.; Xie, X.Q.; Song, Y.J.; et al. Targeting autophagy potentiates chemotherapy-induced apoptosis and proliferation inhibition in hepatocarcinoma cells. Cancer Lett. 2012, 320, 171–179.
- Wear, D.; Bhagirath, E.; Balachandar, A.; Vegh, C.; Pandey, S. Autophagy inhibition via hydroxychloroquine or 3-methyladenine enhances chemotherapy-induced apoptosis in neuro-blastoma and glioblastoma. Int. J. Mol. Sci. 2023, 24, 12052.
- Karim, N.A.; Ullah, A.; Ahmad, I.; Bahassi, E.; Olowokure, O.; Khaled, A.; Davis, H.; Morris, J.C. A phase I trial to determine the safety and tolerability of autophagy inhibition using chloroquine or hydroxychloroquine in combination with carboplatin and gemcitabine in patients with advanced solid tumors. Front. Oncol. 2022, 12, 811411.
- Abdel-Karim, N.; Gaber, O.; Aljohani, H.M.; Eldessouki, I.; Bahassi, E.M.; Morris, J. Exosomes as a surrogate marker for autophagy in peripheral blood, correlative data from phase I study of chloroquine in combination with carboplatin/gemcitabine in advanced solid tumors. Asian Pac. J. Cancer Prev. 2019, 20, 3789–3796.
- Du, H.; Yang, W.; Chen, L.; Shi, M.; Seewoo, V.; Wang, J.; Lin, A.; Liu, Z.; Qiu, W. Role of autophagy in resistance to oxaliplatin in hepatocellular carcinoma cells. Oncol. Rep. 2012, 27, 143–150.
- Selvakumaran, M.; Amaravadi, R.K.; Vasilevskaya, I.A.; O'Dwyer, P.J. Autophagy inhibition sensitizes colon cancer cells to antiangiogenic and cytotoxic therapy. Clin. Cancer Res. 2013, 19, 2995–3007.
- Shi, Y.; Lin, G.; Zheng, H.; Mu, D.; Chen, H.; Lu, Z.; He, P.; Zhang, Y.; Liu, C.; Lin, Z.; et al. Biomimetic nanoparticles blocking autophagy for enhanced chemotherapy and metastasis inhibition via reversing focal adhesion disassembly. J. Nanobiotechnol. 2021, 19, 447.
- Beutel, A.K.; Halbrook, C.J. Barriers and opportunities for gemcitabine in pancreatic cancer therapy. Am. J. Physiol. Cell Physiol. 2023, 324, C540–C552.
- Wang, F.-T.; Wang, H.; Wang, Q.-W.; Pan, M.-S.; Li, X.-P.; Sun, W.; Fan, Y.-Z. Inhibition of autophagy by chloroquine enhances the antitumor activity of gemcitabine for gallbladder cancer. Cancer Chemother. Pharmacol. 2020, 86, 221–232.
- Hara, K.; Horikoshi, Y.; Morimoto, M.; Nakaso, K.; Sunaguchi, T.; Kurashiki, T.; Nakayama, Y.; Hanaki, T.; Yamamoto, M.; Sakamoto, T.; et al. TYRO3 promotes chemoresistance via increased LC3 expression in pancreatic cancer. Transl. Oncol. 2023, 28, 101608.
- Matsumoto, S.; Nakata, K.; Sagara, A.; Guan, W.; Ikenaga, N.; Ohuchida, K.; Nakamura, M. Efficient pre-treatment for pancreatic cancer using chloroquine-loaded nanoparticles targeting pancreatic stellate cells. Oncol. Lett. 2021, 22, 633.
- Chen, X.; Tao, Y.; He, M.; Deng, M.; Guo, R.; Sheng, Q.; Wang, X.; Ren, K.; Li, T.; He, X.; et al. Co-delivery of autophagy inhibitor and gemcitabine using a pH-activatable core-shell nanobomb inhibits pancreatic cancer progression and metastasis. Theranostics 2021, 11, 8692–8705.
- AlMasri, S.S.; Zenati, M.S.; Desilva, A.; Nassour, I.; Boone, B.A.; Singhi, A.D.; Bartlett, D.L.; Liotta, L.A.; Espina, V.; Loughran, P.; et al. Encouraging long-term survival following autophagy inhibition using neoadjuvant hydroxychloroquine and gemcitabine for high-risk patients with resectable pancreatic carcinoma. Cancer Med. 2021, 10, 7233–7241.
- Kronick, O.; Chen, X.; Mehra, N.; Varmeziar, A.; Fisher, R.; Kartchner, D.; Kota, V.; Mitchell, C.S. Hematological adverse events with tyrosine kinase inhibitors for chronic myeloid leukemia: A systematic review with meta-analysis. Cancers 2023, 15, 4354.
- Golčić, M.; Jones, R.L.; Huang, P.; Napolitano, A. Evaluation of systemic treatment options for gastrointestinal stromal tumours. Cancers 2023, 15, 4081.
- Mishima, Y.; Terui, Y.; Mishima, Y.; Taniyama, A.; Kuniyoshi, R.; Takizawa, T.; Kimura, S.; Ozawa, K.; Hatake, K. Autophagy and autophagic cell death are next targets for elimination of the resistance to tyrosine kinase inhibitors. Cancer Sci. 2008, 99, 2200–2208.
- Crowley, L.C.; O'Donovan, T.R.; Nyhan, M.J.; McKenna, S.L. Pharmacological agents with inherent anti-autophagic activity improve the cytotoxicity of imatinib. Oncol. Rep. 2013, 29, 2261–2268.
- Gupta, A.; Roy, S.; Lazar, A.J.; Wang, W.L.; McAuliffe, J.C.; Reynoso, D.; McMahon, J.; Taguchi, T.; Floris, G.; Debiec-Rychter, M.; et al. Autophagy inhibition and antimalarials promote cell death in gastrointestinal stromal tumor (GIST). Proc. Natl. Acad. Sci. USA 2010, 107, 14333–14338.
- Zheng, S.; Shu, Y.; Lu, Y.; Sun, Y. Chloroquine combined with imatinib overcomes imatinib resistance in gastrointestinal stromal tumors by inhibiting autophagy via the MAPK/ERK pathway. Onco Targets Ther. 2020, 13, 6433–6441.
- Horne, G.A.; Stobo, J.; Kelly, C.; Mukhopadhyay, A.; Latif, A.L.; Dixon-Hughes, J.; McMahon, L.; Cony-Makhoul, P.; Byrne, J.; Smith, G.; et al. A randomised phase II trial of hydroxychloroquine and imatinib versus imatinib alone for patients with chronic myeloid leukaemia in major cytogenetic response with residual disease. Leukemia 2020, 34, 1775–1786.
- Hua, X.; Yin, Z.; Liang, J.; Chen, W.; Gong, H. Efficacy and safety comparison between Lenvatinib and Sorafenib in hepatocellular carcinoma treatment: A systematic review and meta-analysis of real-world study. Eur. J. Gastroenterol. Hepatol. 2024, 36(1), 120–128.
- Buttell, A.; Qiu, W. The action and resistance mechanisms of Lenvatinib in liver cancer. Mol. Carcinog. 2023, 62, 1918–1934.
- Xue, L.; Gong, Z.; Vlantis, A.C.; Chan, J.Y.; Meehan, K.; van Hasselt, C.A.; Li, D.; Zeng, X.; Wei, M.; Tong, M.C.; et al. Autophagy regulates anti-angiogenic property of lenvatinib in thyroid cancer. Am. J. Cancer Res. 2023, 13, 1457–1470.
- Zheng, Y.; Huang, C.; Lu, L.; Yu, K.; Zhao, J.; Chen, M.; Liu, L.; Sun, Q.; Lin, Z.; Zheng, J.; et al. STOML2 potentiates metastasis of hepatocellular carcinoma by promoting PINK1-mediated mitophagy and regulates sensitivity to lenvatinib. J. Hematol. Oncol. 2021, 14, 16.
- Peng, D.; Cai, Y.; Chen, G.; Hou, M.; Luo, X.; Dongzhi, Z.; Xie, H.; Liu, Y. Efficacy and safety of apatinib versus sorafenib/placebo in first-line treatment for intermediate and advanced primary liver cancer: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1101063.
- Feng, H.; Cheng, X.; Kuang, J.; Chen, L.; Yuen, S.; Shi, M.; Liang, J.; Shen, B.; Jin, Z.; Yan, J.; et al. Apatinib-induced protective autophagy and apoptosis through the AKT-mTOR pathway in anaplastic thyroid cancer. Cell Death Dis. 2018, 9, 1030.
- Wang, Y.M.; Xu, X.; Tang, J.; Sun, Z.Y.; Fu, Y.J.; Zhao, X.J.; Ma, X.M.; Ye, Q. Apatinib induces endoplasmic reticulum stress-mediated apoptosis and autophagy and potentiates cell sensitivity to paclitaxel via the IRE-1α-AKT-mTOR pathway in esophageal squamous cell carcinoma. Cell Biosci. 2021, 11, 124.
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138.
- Wylaź, M.; Kaczmarska, A.; Pajor, D.; Hryniewicki, M.; Gil, D.; Dulińska-Litewka, J. Exploring the role of PI3K/AKT/mTOR inhibitors in hormone-related cancers: A focus on breast and prostate cancer. Biomed. Pharmacother. 2023, 168, 115676.
- Singh, S.; Barik, D.; Lawrie, K.; Mohapatra, I.; Prasad, S.; Naqvi, A.R.; Singh, A.; Singh, G. Unveiling novel avenues in mtor-targeted therapeutics: Advancements in glioblastoma treatment. Int. J. Mol. Sci. 2023, 24, 14960.
- Ishibashi, Y.; Nakamura, O.; Yamagami, Y.; Nishimura, H.; Fukuoka, N.; Yamamoto, T. Chloroquine Enhances Rapamycin-induced Apoptosis in MG63 Cells. Anticancer Res. 2019, 39, 649–654.
- Masaki, N.; Aoki, Y.; Obara, K.; Kubota, Y.; Bouvet, M.; Miyazaki, J.; Hoffman, R.M. Targeting autophagy with the synergistic combination of chloroquine and rapamycin as a novel effective treatment for well-differentiated liposarcoma. Cancer Genom. Proteom. 2023, 20, 317–322.
- Masaki, N.; Aoki, Y.; Kubota, Y.; Obara, K.; Miyazaki, J.; Hoffman, R.M. Chloroquine combined with rapamycin arrests tumor growth in a patient-derived orthotopic xenograft (PDOX) mouse model of dedifferentiated liposarcoma. In Vivo 2022, 36, 2630–2637.
- Jiang, B.; Cui, Y.; Ma, X.; Zhang, Y.; Feng, X.; Yang, T.; Feng, L.; Guo, W.; Li, Y.; Wang, T.; et al. Crosstalk between autophagy inhibitor and salidroside-induced apoptosis: A novel strategy for autophagy-based treatment of hepatocellular cancer. Int. Immunopharmacol. 2023, 124 Pt B, 111040.
- Jiang, B.; Feng, L.; Yang, T.; Guo, W.; Li, Y.; Wang, T.; Liu, C.; Su, H. Combination of chloroquine diphosphate and salidroside induces human liver cell apoptosis via regulation of mitochondrial dysfunction and autophagy. Mol. Med. Rep. 2023, 27, 37.
- Abdelwahab, M.; Saeed, H.; Elnikhely, N.; Nematalla, H. Synergistic effect of Dactolisib/Lys05 combination on autophagy in A549 cells. Acta Biochim. Pol. 2023, 70, 615–622.
- Grimaldi, A.; Santini, D.; Zappavigna, S.; Lombardi, A.; Misso, G.; Boccellino, M.; Desiderio, V.; Vitiello, P.P.; Di Lorenzo, G.; Zoccoli, A.; et al. Antagonistic effects of chloroquine on autophagy occurrence potentiate the anticancer effects of everolimus on renal cancer cells. Cancer Biol. Ther. 2015, 16, 567–579.
- Haas, N.; Appleman, L.J.; Stein, M.; Redlinger, M.; Wilks, M.; Xu, X.; Onorati, A.; Kalavacharla, A.; Kim, T.; Zhen, C.J.; et al. Autophagy Inhibition to Augment mTOR Inhibition: A Phase I/II Trial of Everolimus and Hydroxychloroquine in Patients with Previously Treated Renal Cell Carcinoma. Clin. Cancer Res. 2019, 25, 2080–2087.
- Rangwala, R.; Chang, Y.C.; Hu, J.; Algazy, K.M.; Evans, T.L.; Fecher, L.A.; Schuchter, L.M.; Torigian, D.A.; Panosian, J.T.; Troxel, A.B.; et al. Combined MTOR and autophagy inhibition: Phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy 2014, 10, 1391–1402.
- Erkisa, M.; Aydinlik, S.; Cevatemre, B.; Aztopal, N.; Akar, R.O.; Celikler, S.; Yilmaz, V.T.; Ari, F.; Ulukaya, E. A promising therapeutic combination for metastatic prostate cancer: Chloroquine as autophagy inhibitor and palladium(II) barbiturate complex. Biochimie 2020, 175, 159–172.
- Cook, K.; Wärri, A.; Soto-Pantoja, D.R.; Clarke, P.A.; Cruz, M.I.; Zwart, A.; Clarke, R. Chloroquine inhibits autophagy to potentiate antiestrogen responsiveness in ER+ breast cancer. Clin. Cancer Res. 2014, 20, 3222–3232.
- Briceño, E.; Reyes, S.; Sotelo, J. Therapy of glioblastoma multiforme improved by the antimutagenic chloroquine. Neurosurg. Focus 2003, 14, e3.
- Sotelo, J.; Briceño, E.; López-González, M.A. Adding chloroquine to conventional treatment for glioblastoma multiforme: A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2006, 144, 337–343.
- Deng, Q.; Tao, S.; Huang, H.; Lv, Q.; Wang, W. Chloroquine Supplementation for the Treatment of Glioblastoma: A Meta-analysis of Randomized Controlled Studies. Clin. Neuropharmacol. 2023, 46, 1–5.
- Vogl, D.T.; Stadtmauer, E.A.; Tan, K.-S.; Heitjan, D.F.; Davis, L.E.; Pontiggia, L.; Rangwala, R.; Piao, S.; Chang, Y.C.; Scott, E.C.; et al. Combined Autophagy and Proteasome Inhibition: A Phase 1 Trial of Hydroxychloroquine and Bortezomib in Patients With Relapsed/Refractory Myeloma. Autophagy 2014, 10, 1380–1390.
- Cocco, S.; Leone, A.; Roca, M.S.; Lombardi, R.; Piezzo, M.; Caputo, R.; Ciardiello, C.; Costantini, S.; Bruzzese, F.; Sisalli, M.J.; et al. Inhibition of autophagy by chloroquine prevents resistance to PI3K/AKT inhibitors and potentiates their antitumor effect in combination with paclitaxel in triple negative breast cancer models. J. Transl. Med. 2022, 20, 290.
- Loh, J.S.; Rahim, N.A.; Tor, Y.S.; Foo, J.B. Simultaneous proteasome and autophagy inhibition synergistically enhances cytotoxicity of doxorubicin in breast cancer cells. Cell Biochem. Funct. 2022, 40, 403–416.
- Delaney, J.R.; Patel, C.B.; Willis, K.M.; Haghighiabyaneh, M.; Axelrod, J.; Tancioni, I.; Lu, D.; Bapat, J.; Young, S.; Cadassou, O.; et al. Haploinsufficiency Networks Identify Targetable Patterns of Allelic Deficiency in Low Mutation Ovarian Cancer. Nat. Commun. 2017, 8, 14423.
- Malhotra, J.; Jabbour, S.; Orlick, M.; Riedlinger, G.; Guo, Y.; White, E.; Aisner, J. Phase Ib/II study of hydroxychloroquine in combination with chemotherapy in patients with metastatic non-small cell lung cancer (NSCLC). Cancer Treat. Res. Commun. 2019, 21, 100158.
- Zeh, H.J.; Bahary, N.; Boone, B.A.; Singhi, A.D.; Miller-Ocuin, J.L.; Normolle, D.P.; Zureikat, A.H.; Hogg, M.E.; Bartlett, D.L.; Lee, K.K.; et al. A randomized phase II preoperative study of autophagy inhibition with high-dose hydroxychloroquine and gemcitabine/nab-paclitaxel in pancreatic cancer patients. Clin. Cancer Res. 2020, 26, 3126–3134.
- Fei, N.; Wen, S.; Ramanathan, R.; Hogg, M.E.; Zureikat, A.H.; Lotze, M.T.; Bahary, N.; Singhi, A.D.; Zeh, H.J.; Boone, B.A. SMAD4 loss is associated with response to neoadjuvant chemotherapy plus hydroxychloroquine in patients with pancreatic adenocarcinoma. Clin. Transl. Sci. 2021, 14, 1822–1829.
- Karasic, T.; O'Hara, M.H.; Loaiza-Bonilla, A.; Reiss, K.A.; Teitelbaum, U.R.; Borazanci, E.; De Jesus-Acosta, A.; Redlinger, C.; Burrell, J.A.; Laheru, D.A.; et al. Effect of Gemcitabine and nab-Paclitaxel with or Without Hydroxychloroquine on Patients with Advanced Pancreatic Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 993–998.
