Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Mona Zou and Version 1 by Xiaowei Luo.
Acremonium fungi is one of the greatest and most complex genera in Hyphomycetes, comprising 130 species of marine and terrestrial sources. The past decades have witnessed substantial chemical and biological investigations on the diverse secondary metabolites from the Acremonium species. To date, over 600 compounds with abundant chemical types as well as a wide range of bioactivities have been obtained from this genus, attracting considerable attention from chemists and pharmacologists.
- Acremonium
- secondary metabolites
- chemical structures
- bioactivities
1. Terpenoids
A total of 124 terpenoids have been reported in Acremonium fungi within the period 2016–2023, consisting of 31 sesquiterpenoids, 15 diterpenoids, 5 triterpenoids, and 78 meroterpenoids and miscellaneous types, while 99 compounds were found to have bioactivities. Remarkably, there are 20 new sesquiterpenoids, 11 new diterpenoids, 18 new meroterpenoids, and miscellaneous types.
1.1. Sesquiterpenoids
Several mophilane-type sesquiterpenoids, acremeremophilanes A–O (1–15), along with seven known analogues, were isolated from the deep-sea sediments derived from Acremonium sp. TVG-S004-0211 (Figure 1). Compounds 2–5 and 14 exhibited inhibition of lipopolysaccharide (LPS)-induced nitric oxide (NO) production in RAW 264.7 macrophages with IC50 values ranging from 8 to 45 μM [15][1].
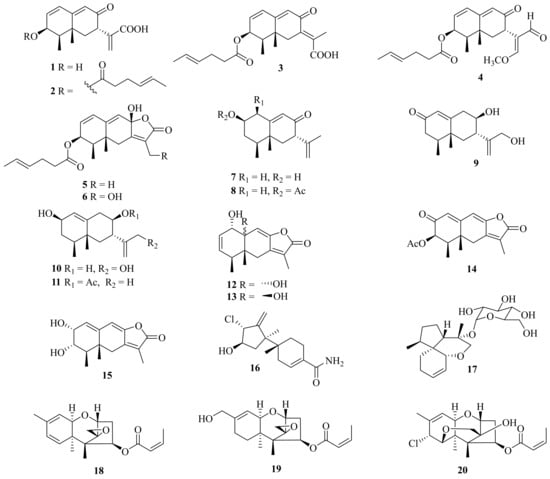
Figure 1. Chemical structures of sesquiterpenoids (1–20).
One new sesquiterpenoid, marinobazzanan (16), was isolated from marine sediment-derived Acremonium sp. CNQ-049, which showed an inhibition of cancer cell migration and invasion at non-toxic concentrations of 1, 2.5, and 5 μM by down-regulating transcription factors of Snail, Slug, and Twist. In addition, marinobazzananan reduced cell motility by down-regulating the expression level of KITENIN and by up-regulating the expression level of KAI 1, and it further reduced the number of metastatic nodules in the intraperitoneal xenograft mouse model [16][2]. Moreover, one new acorane-type sesquiterpene glycoside, isocordycepoloside A (17), was isolated from the fungus Acremonium sp. SF-7394 [17][3].
Meanwhile, three new trichothecenes, including two trichothecenes, 7-dehydro-8-dehydroxytrichothecinol B (18) and 8-deoxy-16-hydroxytrichothecinol B (19), along with one trichothecene analogue, 4-((Z)-but-2-enoyloxy)-8-chloro-12-hydroxy-7,13-epoxytrichothec-9-ene (20), and four known analogues, were isolated from the fungus A. crotocinigenum BCC 20012. Among them, the known compound trichothecin exerted the strongest antimalarial activity against Plasmodium falciparum K1 with an IC50 value of 0.05 mg/mL, and possessed cytotoxic activity against Vero cells with an IC50 value of 0.13 mg/mL [18][4].
1.2. Diterpenes
A chemical investigation of the marine-derived fungus A. striatisporum KMM 4401 resulted in the isolation of ten new diterpene glycosides, virescenosides Z9–Z18 (21–30), together with four known analogues [12][5] (Figure 2). One new diterpene, acrepseudoterin (31), was isolated from the fungus Acremonium sp. SF-7394. Acrepseudoterin inhibited the enzyme activity in a dose-dependent manner with an IC50 value of 22.8 ± 1.1 μM, which was identified as a competitive inhibitor of PTP1B [17][3].
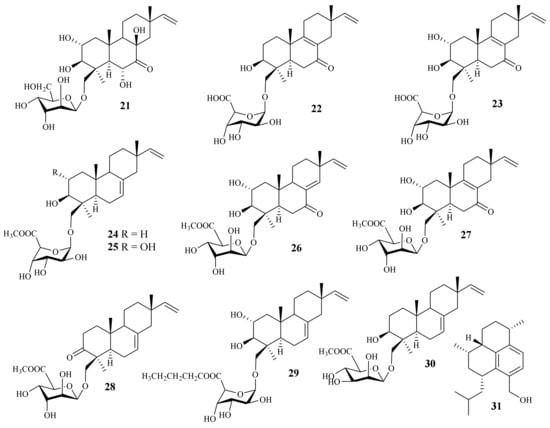
Figure 2. Chemical structures of sesquiterpenoids (21–31).
1.3. Meroterpenoids
Twenty-five ascochlorin derivatives, biosynthesized through the farnesylation of orsellinic acid [19][6], were obtained from the coral-derived A. sclerotigenum GXIMD 02501, including 13 new compounds, acremochlorins A–M (32–44) (Figure 3). Compounds 32 and 44, two novel potent human dihydroorotate dehydrogenase (hDHODH) inhibitors, induced the apoptosis of triple-negative breast cancer (TNBC) cells by up-regulating the levels of cleaved-PARP1 and cleaved-caspase7, and further effectively inhibited tumor growth in a patient-derived TNBC xenograft model without significant weight loss or obvious toxicity in mice, showing higher safety than that of brequinar [10][7].
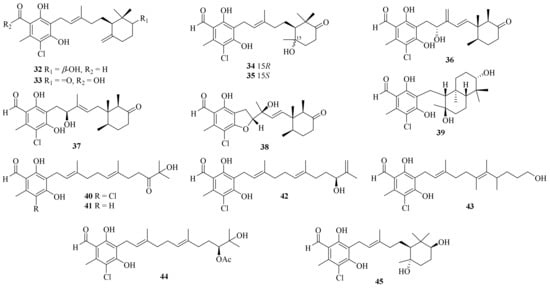
Figure 3. Chemical structures of meroterpenoids (32–45).
Meanwhile, ascofuranone and ascochlorin, two representative ascochlorin derivatives, were also reported as potential lead candidates for drug development targeting the hDHODH of cancer cells living under a tumor microenvironment [20][8]. Moreover, two known potential anti-tumor ascochlorins, 3-bromoascochlorin (BAS) and ilicicolin A (Ili-A), were also obtained from the coral-derived fungus A. sclerotigenum GXIMD 02501. BAS could induce the apoptosis, invasion, and migration of H446 and H69AR cells, and it further suppressed the tumor growth of a small cell lung cancer xenograft mouse model by inhibiting the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway [21][9]. Moreover, Ili-A showed efficacious activity against prostate cancer cells by abrogating EZH2/AR-mediated processes and demonstrated a synergistic anti-prostate cancer effect combined with enzalutamide in vivo, revealing a novel EZH2 inhibitor for the treatment of castration-resistant prostate cancer [22][10].
Ascofuranone and its derivatives, obtained from A. egyptiacum, were found as the first dual inhibitors of fumarate and oxygen respiration in Echinococcus multilocularis by targeting mitochondrial complexes II and III, suggesting potential lead compounds in the development of anthelminthic drugs [23][11]. One new ascochlorin, acremochlorin N (45), and a pair of new natural enantiomers, 3-phenylcyclopentane-1,2-diol (±-46) (Figure 4), together with nine known analogues were isolated from marine sediment-derived A. furcatum CS-280. All the isolates showed significant anti-Vibrio activities, especially against Vibrio harveyi and V. alginolyticus. Moreover, the presence of chlorine atoms in the ascochlorins could significantly enhance their antibacterial activity [24][12].
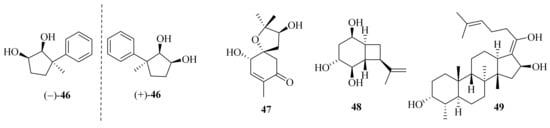
Figure 4. Chemical structures of miscellaneous terpenoids (46–49).
Meanwhile, four known ascochlorins, including ascochlorin, 10′-deoxy10′α-hydroxyascochlorin, 4′,5′-dihydro-4′-hydroxyascochlorin, and ascofuranone, were obtained from the sponge-derived Acremonium sp. IMB18-086. Ascochlorin and ascofuranone showed significant antibacterial activity against Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Bacillus subtilis, and Candida albicans. Moreover, they showed significant cytotoxicity against A549 and/or HepG2 cell lines with IC50 values of 0.9–5.8 μM [25][13].
Acremine S (47) was isolated from the sponge Mycale sp. derived fungus A. persicinum KUFA 1007 and showed inhibitory activity against butyrylcholine esterase, which was three folds higher than that of galantamine [26][14]. Hexahydroacremonintriol (48), along with an analogue, acremonin A glucoside, were obtained from a tropical sinkhole derived from A. masseei CICY026. Both displayed insecticidal activity against Myzus persicae and/or Rhopalosiphum padi with settling inhibition ranging from 48% to 67% [27][15]. One new fusidic acid derivative, acremonidiol A (49), and three known analogs were obtained from the endophytic fungus A. pilosum F47. Among these, fusidic acid displayed a strong inhibitory effect on Gram-positive bacterium S. aureus, and the acetylation of the hydroxyl group at C-16 was crucial for antibacterial activity [28][16].
2. Peptides
A total of 45 peptides have been reported from Acremonium fungi during the period 2016–2023, including 33 new compounds, while 19 bioactive compounds were found.
2.1. Linear Peptides
One new linear peptide, acremopeptin (50), and a known one, adenopeptin, were obtained from the soil-derived fungus Acremonium sp. PF1450 [29][17]. Moreover, four new peptaibiotics, acremotins A–D (51–54) (Figure 5), along with a known peptaibiotic XR586 were isolated from the soil-derived fungus A. persicinum SC0105. Acremotins A–D showed strong inhibitory activity against Gram-positive bacteria, while the MIC values of acremotin D against S. aureus and MRSA were 12.5 and 6.25 μg/mL, respectively. Moreover, acremotins A–D and XR586 also showed cytotoxicity against three human cancer cell lines (A549, HeLa, and HepG2), with IC50 values ranging from 1.2 to 21.6 μM [30][18].
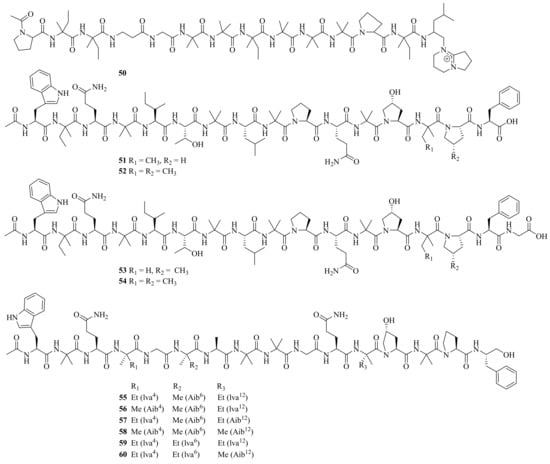
Figure 5. Chemical structures of linear peptides (50–60).
Six new 16-residue peptaibols, acremopeptaibols A–F (55–60), along with PF1171A, were isolated from the cultures of the sponge-associated fungus Acremonium sp. IMB18-086. Compounds 55 and 59 showed significant antibacterial activity against S. aureus, MRSA, B. subtilis, and C. albicans, with MIC values ranging from 16 to 64 μM [25][13]
Six new linear pentadecapeptides, emerimicins V–X (61–66) (Figure 6), were obtained from the soil-derived fungus A. tubakii MT053262. Emerimicins V (61) and VI (62) displayed strong toxicity toward Zebrafish embryos. In addition, emerimicin V showed certain activity against Enterococcus faecalis, MRSA, and vancomycin-resistant Enterococcus faecium with MIC values of 64, 32, and 64 μg/mL, respectively [31][19].
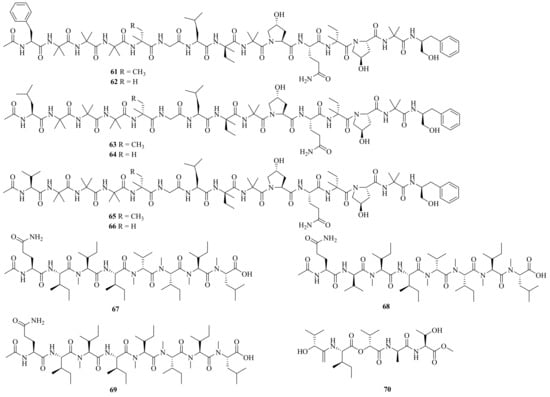
Figure 6. Chemical structures of linear peptides (61–70).
Four new peptides, acrepeptins A–D (67–70), and three known analogs, destruxin B, guangomide A, and guangomide B, were obtained from a marine algicolous fungus Acremonium sp. NTU492. Acrepeptins A (67) and B (68) exhibited significant inhibitory activity on NO production in LPS-activated microglia BV-2 cells, with IC50 values of 12.0 ± 2.3 and 10.6 ± 4.0 mM, respectively [32][20].
2.2. Cyclic Peptides
Four new hydroxamate-containing cyclopeptides, acremonpeptides A–D (71–74), together with a known one, Al (III)-acremonpeptide D, were obtained from the marine fungus A. persicinum SCSIO 115. Compounds 71, 72, and Al (III)-acremonpeptide D exhibited moderate antiviral activity against HSV-1 with EC50 values of 16, 8.7, and 14 μM, respectively [33][21] (Figure 7). Meanwhile, a new cyclic depsipeptide, acremonamide (75), was isolated from a marine-derived fungus Acremonium sp. strain CNQ-049 [34][22].
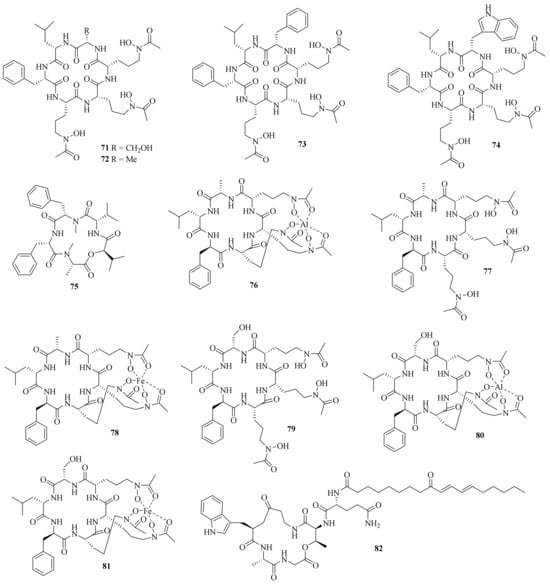
Figure 7. Chemical structures of cyclic peptides (71–82).
Six new hydroxamate siderophore cyclohexapeptides, Al (III)-acremonpeptide E (76), acremonpeptide E (77), Fe (III)-acremonpeptide E (78), acremonpeptide F (79), Al (III)-acremonpeptide F (80), and Fe (III)-acremonpeptide F (81), and one new cyclic pentapeptolide, aselacin D (82), together with a known compound, aselacin C, were isolated from the sponge-derived fungus A. persicinum F10. Compounds 76 and 80 showed pronounced antifungal activity against Aspergillus fumigatus and A. niger with a shared MIC value of 1 μg/mL, and both showed no cytotoxicity against human embryonic lung fibroblasts (MRC-5) at a concentration of 30 μM [35][23].
3. Polyketides
A total of 60 polyketides have been reported from the genus Acremonium within the period, including 23 new compounds and 18 bioactive compounds.
One new dibenzoquinone, 2,7-dihydroxy-3,6,9-trimethyl-9H-xanthene-1,4,5,8-tetraone (83) (Figure 8), and a known analog, 3,3′,6,6′-tetrahydroxy-4,4′-dimethyl-1,1′-bi-p-benzoquinon, were obtained from the fungus A. cavaraeanum CA022 [36][24]. Meanwhile, a chemical examination of marine sponge Mycale sp. derived fungus A. persicinum KUFA 1007 led to the isolation of one new compound, acremine T (84) [26][14].
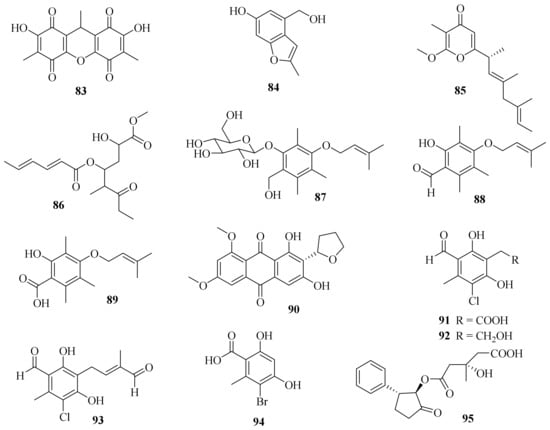
Figure 8. Chemical structures of polyketides (83–95).
A chemical investigation on the endophytic fungus A. citrinum SS-g13 yielded a new γ-pyrone derivative, acrepyrone A (85), and three known sorbicillinoids, trichodimerol, dihydrotrichodimerol, and tetrahydrotrichodimerol [8][25]. A chemical investigation of the endophytic fungus A. citrinum MMF4 derived from the root of the mangrove plant Kandelia obovate resulted in the isolation of one new compound, triacremoniate (86), along with a known compound, acrepyrone A. Compound 86 had significant inhibitory effects on the proliferation of HeLa cells, with an IC50 value of 30.5 ± 1.99 μM [37][26].
Three new zinniol analogues, pleoniols A–C (87–89), along with a known compound were isolated from a mixed fermentation of two endophytic fungi, Pleosporales sp. F46 and A. pilosum F47, both of which originated from the pedicel of the medicinal plant Mahonia fortune [38][27]. Four dimethylated anthraquinone derivatives, including one new compound, 6,8-di-O-methylbipolarin (90), and three known compounds, aversin, 6,8-di-O-methylaverufin, and 6,8-di-O-methylnidurufin, were obtained from the marine-derived fungus A. vitellinum MH726097. Compound 90 showed the strongest insecticidal activity against the third instar larvae of Helicoverpa armigera, with a LC50 value of 0.72 mg/mL [39][28].
Three new chlorinated orsellinic aldehyde derivatives, orsaldechlorins A–C (91–93), and one new natural brominated orsellinic acid, 5-bromo-2,4-dihydroxy-6-methylbenzoic acid (94), along with ten known biosynthetically related derivatives were further characterized from the Beibu Gulf coral-associated fungus A. sclerotigenum GXIMD 02501. Most of them inhibited LPS-induced NF-κB activation in RAW 264.7 cells at a concentration of 20 μM. Notably, compounds 91 and 92 showed inhibitions of RANKL-induced osteoclast differentiation in bone marrow macrophages without cytotoxicity [40][29].
One new compound, fusidione (95), along with a known one, microperfuranone, were isolated from the sea-water-derived fungus A. fusidioides RZ01. Fusidione displayed inhibitory activity against HL-60 cells with an IC50 value of 44.9 μM [41][30].
A new benzoyl compound, 1-(2′-benzoyl-3,4-dihydroxy-1′-methoxycyclobut-2′-enyl)-3,4,5-trihydroxy-2-methylnona-2,6-dien-1-one (96) (Figure 9), was obtained from the endophytic fungus Acremonium sp. of Garcinia griffithii [42][31]. One new polyketide, acrefurcatone A (97), was isolated from the deep-sea cold-seep sediment-derived fungus A. furcatum CS-280, which showed strong activity against Pseudomonas aeruginosa with an MIC value of 8 μg/mL [24][12].
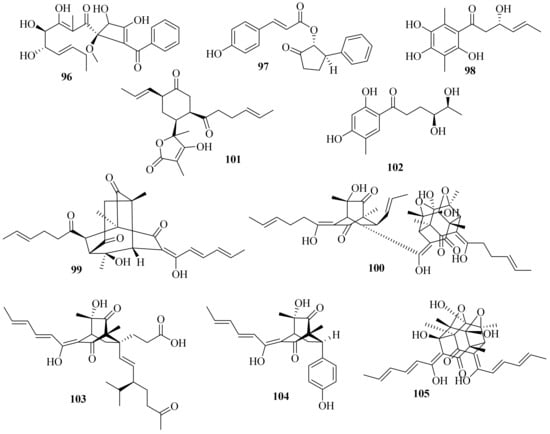
Figure 9. Chemical structures of polyketides (96–105).
A chemical investigation of marine sediment-derived fungus Acremonium sp. resulted in the isolation of two new compounds, 3(S)-hydroxy-1-(2,4,5-trihydroxy-3,6dimethylphenyl)-hex-4E-en-1-one (98) and acremonilactone (99), along with eight known compounds. Among them, (2E,4E)-1-(2,6dihydroxy-3,5-dimethyl-phenyl) hexa-2,4-dien-1-one, sorbicillin, and tetrahydrotrichodimerol showed inhibitory activity against S. aureus, with a shared MIC value of 128 μg/mL. In addition, compounds 98 and trichodimerol showed 2,2-diphenyl-1-trinitrophenylhydrazine (DPPH) free radical scavenging activity with inhibition rates of 96.50% and 84.95% at a concentration of 0.5 mg/mL, respectively [43][32].
A chemical investigation of the terrestrial plant Fructus mori derived A. citrinum SS-g13 produced three new sorbicillinoids, trisorbicillinone E (100), acremosorbicillinoids A and B (101 and 102), and one new natural product, 2S,3S-acetyl-β-methyltryptophan, along with eight known sorbicillinoids. Among them, dihydrobisvertinolone showed significant cholesterol efflux-enhancing activity [44][33].
Moreover, three new sorbicillinoid derivatives, acresorbicillinols A–C (103–105), along with five known compounds were obtained from the marine-derived fungus A. chrysogenum C10. Compounds 104 and 105 displayed moderate activity against S. aureus and Cryptococcus neoformans with IC50 values of 86.93 ± 1.72 and 69.06 ± 10.50 μM, respectively. Moreover, compound 105 demonstrated strong DPPH free radical scavenging activity, with the IC50 value ranging from 11.53 ± 1.53 to 60.29 ± 6.28 μM in 24 h [45][34]. A chemical investigation of the deep-sea-derived A. alternatum provided two known bisorbicillinoids, tetrahydrotrichodimerol and dihydrotrichodimerol [46][35].
References
- Cheng, Z.; Zhao, J.; Liu, D.; Proksch, P.; Zhao, Z.; Lin, W. Eremophilane–type sesquiterpenoids from an Acremonium sp. fungus isolated from deep–sea sediments. J. Nat. Prod. 2016, 79, 1035–1047.
- Pulat, S.; Hillman, P.F.; Kim, S.; Asolkar, R.N.; Kim, H.; Zhou, R.; Tas, I.; Gamage, C.D.B.; Varli, M.; Park, S.Y.; et al. Marinobazzanan, a bazzanane-type sesquiterpenoid, suppresses the cell motility and tumorigenesis in cancer cells. Mar. Drugs 2023, 21, 153.
- Kim, H.J.; Li, X.J.; Kim, D.C.; Kim, T.K.; Sohn, J.H.; Kwon, H.; Lee, D.; Kim, Y.C.; Yim, J.H.; Oh, H. PTP1B inhibitory secondary metabolites from an antarctic fungal strain Acremonium sp. SF-7394. Molecules 2021, 26, 5505.
- Bunyapaiboonsri, T.; Yoiprommarat, S.; Lapanun, S.; Balram, U.; Chanthaket, R.; Klaysuban, A.; Suetrong, S. Trichothecenes from the fungus Acremonium crotocinigenum BCC 20012. Phytochem. Lett. 2016, 18, 39–43.
- Zhuravleva, O.I.; Antonov, A.S.; Oleinikova, G.K.; Khudyakova, Y.V.; Popov, R.S.; Denisenko, V.A.; Pislyagin, E.A.; Chingizova, E.A.; Afiyatullov, S.S. Virescenosides from the holothurian–associated fungus Acremonium striatisporum Kmm 4401. Mar. Drugs 2019, 17, 616.
- Araki, Y.; Awakawa, T.; Matsuzaki, M.; Cho, R.; Matsuda, Y.; Hoshino, S.; Shinohara, Y.; Yamamoto, M.; Kido, Y.; Inaoka, D.K.; et al. Complete biosynthetic pathways of ascofuranone and ascochlorin in Acremonium egyptiacum. Proc. Natl. Acad. Sci. USA 2019, 116, 8269–8274.
- Luo, X.W.; Cai, G.D.; Guo, Y.F.; Gao, C.H.; Huang, W.F.; Zhang, Z.H.; Lu, H.M.; Liu, K.; Chen, J.H.; Xiong, X.F.; et al. Exploring marine–derived ascochlorins as novel human dihydroorotate dehydrogenase inhibitors for treatment of triple–negative breast cancer. J. Med. Chem. 2021, 64, 13918–13932.
- Miyazaki, Y.; Inaoka, D.K.; Shiba, T.; Saimoto, H.; Sakura, T.; Amalia, E.; Kido, Y.; Sakai, C.; Nakamura, M.; Moore, A.L.; et al. Selective cytotoxicity of dihydroorotate dehydrogenase inhibitors to human cancer cells under hypoxia and nutrient-deprived conditions. Front. Pharmacol. 2018, 9, 997.
- Zhang, Z.H.; Zhang, Y.D.; Yang, C.J.; Wang, Q.Y.; Wang, H.; Zhang, Y.T.; Deng, W.B.; Nie, Y.C.; Liu, Y.H.; Luo, X.W.; et al. Antitumor effects of 3-bromoascochlorin on small cell lung cancer via inhibiting MAPK pathway. Cell Biol. Int. 2021, 45, 2380–2390.
- Guo, L.; Luo, X.W.; Yang, P.; Zhang, Y.T.; Huang, J.L.; Wang, H.; Guo, Y.F.; Huang, W.F.; Chen, Z.Q.; Wang, S.S.; et al. Ilicicolin a exerts antitumor effect in castration-resistant prostate cancer via suppressing EZH2 signaling pathway. Front. Pharmacol. 2021, 12, 723729.
- Enkai, S.; Kouguchi, H.; Inaoka, D.K.; Shiba, T.; Hidaka, M.; Matsuyama, H.; Sakura, T.; Yagi, K.; Kita, K. Killing two birds with one stone: Discovery of dual inhibitors of oxygen and fumarate respiration in zoonotic parasite, echinococcus multilocularis. Antimicrob. Agents Chemother. 2023, 67, e01428-22.
- Chen, X.D.; Yang, S.Q.; Li, X.M.; Wang, B.G.; Li, X. Antibacterial polyketides and ascochlorins from deep-sea cold-seep-derived fungus Furcasterigmium furcatum (syn. Acremonium furcatum). Deep. Sea Res. Part I 2023, 199, 104114.
- Hao, X.M.; Li, S.S.; Ni, J.; Wang, G.Y.; Li, F.; Li, Q.; Chen, S.Z.; Shu, J.C.; Gan, M.L. Acremopeptaibols A–F, 16-residue peptaibols from the sponge-derived Acremonium sp. IMB18–086 cultivated with heat–killed Pseudomonas aeruginosa. J. Nat. Prod. 2021, 84, 2990–3000.
- Alves, A.J.S.; Pereira, J.A.; Dethoup, T.; Cravo, S.; Mistry, S.; Silva, A.M.S.; Pinto, M.M.M.; Kijjoa, A. A new meroterpene, a new benzofuran derivative and other constituents from cultures of the marine sponge–associated fungus Acremonium persicinum KUFA 1007 and their anticholinesterase activities. Mar. Drugs 2019, 17, 379.
- Ruiz-Jimenez, A.L.; Ruiz-Sánchez, E.; Heredia, G.; Tapia-Tussell, R.; González-Coloma, A.; Peraza-Jiménez, K.; Moo-Koh, F.A.; Medina-Baizabal, I.L.; Hernandez-Romero, Y.; Mena-Rejón, G.J.; et al. Identification of insect-deterrent metabolites from Acremonium masseei strain CICY026, a saprophytic fungus from a sinkhole in yucatán. Microorganisms 2019, 7, 712.
- Tian, C.; Gao, H.; Peng, X.P.; Li, G.; Lou, H.X. Fusidic acid derivatives from the endophytic fungus Acremonium pilosum F47. J. Asian Nat. Prod. Res. 2021, 23, 1148–1155.
- Iijima, M.; Amemiya, M.; Sawa, R.; Kubota, Y.; Kunisada, T.; Momose, I.; Kawadal, M.; Shibasaki, M. Acremopeptin, a new peptaibol from Acremonium sp. PF1450. J. Antibiot. 2017, 70, 791–794.
- Wang, C.; Wu, P.; Yao, L.; Xue, J.H.; Xu, L.X.; Li, H.X.; Deng, W.Q.; Wei, X.Y. Acremotins A–D, peptaibiotics produced by the soil-derived fungus Acremonium persicinum SC0105. J. Antibiot. 2018, 71, 927–938.
- Wu, G.; Dentinger, B.T.M.; Nielson, J.R.; Peterson, R.T.; Winter, J.M. Emerimicins V–X, 15-residue peptaibols discovered from an Acremonium sp. through integrated genomic and chemical approaches. J. Nat. Prod. 2021, 84, 1113–1126.
- Hsiao, G.; Wang, S.W.; Chiang, Y.R.; Chi, W.C.; Kuo, Y.H.; Do Anh, P.; Chen, C.Y.; Lee, T.H. Anti-inflammatory effects of peptides from a marine algicolous fungus Acremonium sp. NTU492 in BV-2 microglial cells. J. Food Drug Anal. 2020, 28, 89–97.
- Luo, M.H.; Zang, R.C.; Wang, X.; Chen, Z.M.; Song, X.X.; Ju, J.H.; Huang, H.B. Natural hydroxamate-containing siderophore acremonpeptides A–D and an aluminum complex of acremonpeptide D from the marine–derived Acremonium persicinum SCSIO 115. J. Nat. Prod. 2019, 82, 2594–2600.
- Kim, S.; Lee, C.W.; Park, S.Y.; Asolkar, R.N.; Kim, H.; Kim, G.J.; Oh, S.J.; Kim, Y.; Lee, E.Y.; Oh, D.C.; et al. Acremonamide, a cyclic pentadepsipeptide with wound-healing properties isolated from a marine-derived fungus of the genus Acremonium. J. Nat. Prod. 2021, 84, 2249–2255.
- Li, Y.X.; Li, Z.Y. Cyclopeptide derivatives from the sponge-derived fungus Acremonium persicinum F10. Mar. Drugs 2021, 19, 537.
- Xu, Q.; Zhang, M.M.; Yan, S.Z.; Cao, L.F.; Li, Q.; Lin, J.; Chen, S.L. Two dibenzoquinones from the fungus Acremonium cavaraeanum. Nat. Prod. Commun. 2017, 12, 1765–1768.
- Peng, X.P.; Li, G.; Ji, L.X.; Li, Y.X.; Lou, H.X. Acrepyrone A, a new γ–pyrone derivative from an endophytic fungus, Acremonium citrinum SS–g13. Nat. Prod. Res. 2020, 34, 1091–1096.
- Wang, X.Y.; Ye, X.S.; Gao, S.; Liu, J.X.; Tian, W.J.; Wang, G.H.; Chen, H.F.; Lin, T. Cytotoxic compound triacremoniate from marine fungus Acremonium citrinum. MMF4. Fitoterapia 2020, 147, 104766.
- Wang, Z.R.; Yang, H.X.; Peng, X.P.; Li, G.; Lou, H.X. Induced production of zinniol analogues by co-cultivation of two endophytic fungi in the same ecological niche. Phytochem. Lett. 2020, 35, 206–210.
- Yuan, X.L.; Wang, X.F.; Xu, K.; Li, W.; Chen, D.; Zhang, P. Characterization of a new insecticidal anthraquinone derivative from an endophyte of Acremonium vitellinum against Helicoverpa armigera. J. Agric. Food Chem. 2020, 68, 11480–11487.
- Lu, H.M.; Tan, Y.H.; Zhang, Y.T.; Li, Z.C.; Luo, X.W.; Chen, J.Y.; Gao, C.H.; Liu, Y.H. Osteoclastogenesis inhibitory phenolic derivatives produced by the Beibu Gulf coral-associated fungus Acremonium sclerotigenum GXIMD 02501. Fitoterapia 2022, 159, 105201.
- An, X.; Feng, B.M.; Chen, G.; Chen, S.F.; Wang, H.F.; Pei, Y.H. Isolation and identification of two new compounds from marine-derived fungus Acremonium fusidioides RZ01. Chin. J. Nat. Med. 2016, 14, 934–938.
- Elfita, E.; Munawar, M.; Muharni, M.; Pratiwi, G.; Rahmadania, R. A new benzoyl compound isolated from the endophytic fungi of kandis gajah (Garcinia griffithii) and asam kandis (Garcinia cowa). Makara J. Sci. 2016, 20, 167–172.
- An, C.L.; Ma, Q.Y.; Xie, Q.Y.; Yang, L.; Yuan, J.Z.; Dai, H.F.; Zhao, Y.X. Chemical constituents of the marine-derived fungus Acremonium sp. AN-13. J. Asian Nat. Prod. Res. 2023.
- Peng, X.P.; Li, G.; Wang, L.M.; Wang, Q.; Wang, C.; Ji, L.X.; Cao, C.X.; Lin, G.F.; Jiang, Z.Y.; He, Z.Q.; et al. Structurally various sorbicillinoids from an endophytic fungus Acremonium citrinum SS-g13. Front. Microbiol. 2022, 13, 800626.
- Duan, C.B.; Wang, S.Y.; Huo, R.Y.; Li, E.W.; Wang, M.; Ren, J.W.; Pan, Y.Y.; Liu, L.; Liu, G. Sorbicillinoid derivatives with the radical scavenging activities from the marine-derived fungus Acremonium chrysogenum C10. J. Fungi 2022, 8, 530.
- Li, Y.H.; Wu, J.; Xie, M.M.; Zhang, Y.; Yang, X.W. Chemical constituents of the deep-sea-derived Acremonium alternatum and their chemotaxonomic significance. Biochem. Syst. Ecol. 2022, 103, 104443.
More
