You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Homeyra Piri.
The environmental sustainability of agricultural and industrial vehicles, as well as of the transportation sector, represents one of the most critical challenges to the sustainable development of a nation. In recent decades, compression-ignition engines have been widely used in on-road and off-road vehicles due to their better fuel economy, autonomy, compactness, and mechanical performance (spec. the high torque values). Due to the consistent environmental impact of fossil fuels, scientists are searching for alternative energy sources while preserving the beneficial features of diesel engines. The utilization of blends of diesel fuel, biodiesel, and bioethanol fuel (referred to as “ternary blends”) is among the most promising solutions for replacing fossil fuels in the near term, allowing, at the same time, us to continue using existing vehicles until new technologies are developed, consolidated and adapted to the agricultural and industrial sector. These ternary blends can lower exhaust emissions without creating major problems for existing fuel-feeding systems, typically designed for low-viscosity fossil fuels. One of the concerns in using liquid biofuels, specifically biodiesel, is the high chemical affinity with conventional and bio-based lubricants, so the main parameters of lubricants can vary significantly after a long operation of the engine. The comprehensive literature review presented in this article delves into the techchnical challenges and the main research pathways are presented herein.
- compression-ignition engines
- biodiesel
- biofuels
- alternative energy
- lubricants
- nanoparticles
1. Why Biofuels Are Used in Agricultural and Industrial Heavy-Duty Engines
A viable alternative to liquid fossil fuels must manage the so-called “Energy trilemma” competitive features, which are defined based on three main dimensions: (a) energy security, (b) energy equity, and (c) environmental sustainability. The uninterrupted availability of energy resources at an affordable price is important from an economic point of view, which actually defines “energy security”, so energy security means its availability, its compatibility with the environment, and its reasonable price, which leads to economic development [1][2][3]. Expanding the use of renewable resources, namely liquid biofuels for the application in Internal Combustion Engines (ICEs), also improves the energy autarchy and reduces the dependence on other countries [4]. The significance and consequences of using biofuel alternatives may be related to the following factors:
-
By employing more renewable fuels produced locally, concerns about the dependency on fossil fuels may be allayed [5].
-
It is possible to enhance the energy and environmental performance of the ICE, due to some superior physical and chemical characteristics of alternative fuels as compared to fossil fuels [6][7][8]. Notwithstanding some fossil fuels (e.g., diesel fuel) have a higher lower heating value than biodiesel and vegetable oil, this metric alone does not capture the full advantages brought by biofuels to engine performance and environmental impact. Biofuels, such as biodiesel, indeed, offer several advantages, including renewability, biodegradability, and lower emissions of greenhouse gases during combustion. These attributes contribute to a reduced environmental footprint and increased energy security, which are critical considerations in the transition towards sustainable energy sources.
-
Biodiesel and alcohols have higher oxygen content compared to diesel fuel. This characteristic ensures the promotion of complete combustion [9]. Unfortunately, this characteristic can also result in the faster degradation of some properties of lubricant and materials coming into contact with biofuels, due to an increased solvency characteristic of higher blending rates. The search for a trade-off highlights the need for ongoing research to optimize biofuel formulations and engine designs to mitigate these effects.
-
The maximum heat release rate (HRR) is kind of lower for biodiesel–diesel–ethanol blends and rises with the ethanol proportion in diesel–ethanol blends [6]. In diesel–ethanol blends, the ignition delay rises as the ethanol proportion rises, while it falls marginally in biodiesel–diesel–ethanol blends or when a cetane number (CN) improver is added [10][11][12]. In blends of diesel fuel and ethanol, cylinder pressure rises with increasing ethanol content, whereas it either slightly falls or remains similar in blends of biodiesel and diesel with ethanol [13][14].
-
In terms of engine performance, when compared to the use of fossil (diesel) fuel, the brake-specific fuel consumption is greater in all the investigated circumstances; the brake thermal efficiency increases or is comparable; and the expressed power is very similar to or slightly lower. Some changes in exhaust gas temperatures were noted; the indicated mean effective pressure also shows minor variations or decreases when fuel blends contain more than 35% ethanol [6][12][13][14][15].
-
Due to the increased demand for diesel fuel, renewable biodiesel from affordable sources, which can supply the need, is required. Realistically, the use of sustainable biodiesel in large quantities may immediately enhance engine performance and emission characteristics [16].
-
The use of biodiesel in diesel engines reduces GHG emissions, and more reductions are possible with the anticipated growth in biodiesel production and fuel consumption [17].
1.1. Classification of Liquid Biofuels Used in Agricultural and Industrial Heavy-Duty Engines
Biofuel is a sustainable fuel that, in general, may be either liquid or gaseous and is derived from biomasses [18]. Biofuels must be suitable for use with current engines and fuel specifications [19]. Biofuels used to replace non-renewable energy fuels mainly come from agricultural and vital crops, forests, and waste streams [20]. Liquid biofuels include ethanol, which may replace gasoline in many late-model vehicles, and biodiesel, which can replace diesel fuel in agricultural and industrial heavy-duty engines. For this reason, biofuels are particularly useful in supplying energy to the transportation sector [21]. Biofuels are classified into different generations based on the used feed stock and conversion method [22]. The classification of biofuels is shown in Table 1.
Table 1.
Classification of biofuels based on their generation technologies
.
| Generation | Description | Used Feedstocks | Production Technologies | Examples |
|---|---|---|---|---|
| 1st | Biofuels are produced using ingredients including grain, sugar, animal fats, and vegetable oils. | Sugarcane, corn, soybeans, wheat, barley | Fermentation and distillation for ethanol, transesterification for biodiesel | Ethanol, biodiesel, biobutanol, bioethanol |
| 2nd | Often referred to as advanced biofuels, these are fuels that may be produced from several forms of (waste) biomass, including plant and animal resources. | Switchgrass, wood chips, agricultural waste, municipal waste, forest residues | enzymatic hydrolysis and fermentation for ethanol, transesterification for biodiesel | Cellulosic ethanol, biomethane |
| 3rd | Biofuels are generated from aquatic autotrophic organisms. Microalgal organisms in particular have a superb capacity to produce important chemical and food products, which is primarily responsible for the manufacture of biodiesel. | Algae, cyanobacteria | Synthetic biology, metabolic engineering, and fermentation for hydrocarbons and biohydrogen | Algae-based biofuels |
| 4th | It is created from modified algae even though it is still in the experimental laboratory stage. Algae are undergoing metabolic modification in this way to raise their oil content, boost their capacity to trap carbon, and improve the cultivation, harvesting, and fermentation processes. Additionally, certain species of algae biomass use metabolic engineering to increase the lipid content and accelerate growth. | Synthetic biology and genetic engineering, bioengineered cyanobacteria, yeasts, fungus, or algae | Biomethanation, and power-to-liquid technologies | Synthetic biofuels |
1.1.1. Biodiesel, First-Generation Biofuels
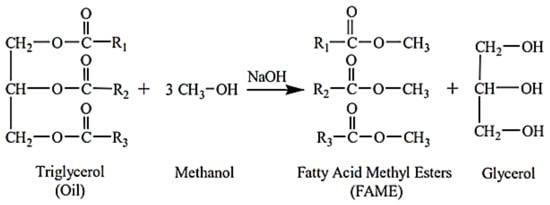
Biodiesel is a biofuel derived from renewable sources, such as vegetable oils and animal fats. It is the result of a chemical process (transesterification of vegetable oils with ethyl or methyl alcohol) or other biological components [25]. The main issue with using bio-oils directly in conventional diesel engines is their excessive viscosity. Pyrolysis, microemulsion, dilution, and transesterification are techniques and processes that use different non-edible raw materials to reduce the viscosity of biodiesel. Transesterification is a viable process adopted so far for viscosity reduction [17]. Alkyl esters are created by the transesterification process, which involves mixing vegetable oils with alcohol to make alkyl esters in the presence of a catalyst. Methanol and ethanol are the alcohols in this process that are most readily accessible and affordable [24]. The process of transesterification to produce biodiesel is shown in Figure 1, and the physical–chemical properties of biodiesel from different feedstocks are briefly compared in Table 2.
Table 2.
Physical–chemical properties of biodiesel from different feedstocks
.
| Property | Unit | Diesel (EN 590) |
Soybean Oil | Canola Oil | Palm Oil | Jatropha Oil | Rapeseed Oil | Animal Fat (Tallow) | Used Cooking Oil | Sunflower Oil | SAF-Flower Oil | Yellow Grease | Coconut Oil | Corn | Cottonseed Oil | Rice Bran Oil |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density at 15 °C | [kg·L−1] | 0.82/0.84 | 0.88 | 0.87 | 0.87 | 0.86 | 0.87 | 0.87 | 0.87 | 0.86 | 0.88 | 0.89 | 0.86 | 0.86 | 0.88 | 0.88 |
| Viscosity at 40 °C | [mm2 |
1.1.2. Alcohols Methanol, Ethanol, Butanol
Alcohols are typically produced chemically from coal (methanol, CH3OH) or synthetically from plant waste (ethanol, C2H5OH), both of which may be made from non-petroleum sources. Due to the issue of combining alcohol with gasoline at high rates (phase separation, fuel watering, and corrosion on engine components), up to 10% to 15%, alcohol may be combined with gasoline without causing any damage to the engine. Additionally, alcohol blends boost volumetric efficiency by decreasing intake temperature as a consequence of eliminating heat from the aspired air since they have a greater latent heat of vaporization than gasoline. The characteristics of methanol, ethanol, butanol, and gasoline are summarized in Table 3 [34].
Table 3.
Properties of methanol, ethanol, butanol, and gasoline
.
| Property | Measurement Unit | Fuel | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methanol (CH | 3 | OH) | Ethanol (C | 2 | H | 5 | OH) | n-Butanol (C | 4 | H | 9 | OH) | Gasoline (C | 8 | H15) | |||||||
| Density at 15 °C | [kg·m−3] | 791.3 | 789.4 | 809.1 | 750.0 | |||||||||||||||||
| ·s | −1] | 2.0/4.5 | 4.1 | 4.4 | 4.5 | 4.5 | 4.4 | 4.2 | 3.6 | 4.2 | 4.1 | 4.8 | 5.5 | 4.1 | 4.1 | |||||||
| Molecular weight | 5.1 | |||||||||||||||||||||
| [kg·kmol | −1] | 32.04 | 46.07 | 74.12 | 114.23 | Flash point FP | [°C] | >+55.0 | +140.1 | +172.3 | +176.7 | +175.5 | +169.5 | +124.0 | +160.0 | +180.3 | +174.0 | +161.0 | +113.8 | +171.0 | +210.0 | +161.0 |
| Vapor pressure | [mmHg] | 127.0 | 55.0 | 7.0 | 562.5 | |||||||||||||||||
| −1 | ||||||||||||||||||||||
| ] | ||||||||||||||||||||||
| 1100 | ||||||||||||||||||||||
| 838 | ||||||||||||||||||||||
| 585 | ||||||||||||||||||||||
| 180–350 | ||||||||||||||||||||||
| Surface tension at 20 °C | [mN·m−1] | |||||||||||||||||||||
]. ICEs and direct methanol fuel cells (DMFCs) may both utilize methanol, which is regarded as the most desired alternative fuel as a replacement for gasoline. M85 (85% methanol, 15% gasoline) and M10 (10% methanol, 90% gasoline) are common methanol blends [34][45]. The following is a summary of the issues raised by the usage of methanol in internal engines:
-
Strong corrosive effect on metal parts: Figure 2 summarizes the scanning electron microscopy (SEM) images taken of the engine components’ surfaces before and after they were exposed to fuel samples for 180 days. The photographs demonstrate that the polished surface of the pistons has less corrosion damage than the surface exposed to fuel samples [47].
| Cloud point | ||||||||||||||||||||||
| [°C] | ||||||||||||||||||||||
| −5/+3 | 0 | −3.25 | +14.25 | +5.66 | −3.50 | +13.00 | − | +1.33 | −4.00 | +8.00 | −1.60 | −4.00 | +1.70 | +0.55 | ||||||||
| Cold filter plugging point (CFPP) | [°C] | −15/−5 | −4 | − | +9 | − | −12 | +13 | − | |||||||||||||
| Boiling point | [°C] | +65.0 | +78.0 | +117.5 | +30.0/+190.0 | −2 | −6 | +1 | −5 | −8 | − | − | ||||||||||
| Research octane number (RON) | [-] | 110 | 119 | – | 97 | Cetane number, CN | [−] | >51.0 | 51.3 | 54.0 | 61.9 | 55.7 | 53.7 | 58.9 | 50.4 | 51.1 | 51.1 | 56.8 | 59.3 | 55.7 | 52.0 | 56.2 |
| Motor octane number (MON) | [-] | 92 | 92 | – | 86 | Iodine value | [gI2·(100g)−1] | − | 125.5 | 113.6 | 54.0 | 109.5 | 116.1 | 65.9 | − | 128.7 | 141.0 | 89.9 | 18.5 | 101.0 | 120.0 | 102.0 |
| Cetane number | [-] | 5 | 11 | 17 | 8 | Acid value | [mgKOH·g−1] | − | 0.18 | 0.49 | 0.20 | 0.24 | 0.26 | 0.38 | − | 0.35 | − | − | 0.18 | − | 0.5 | − |
| Stoichiometric AFR | [kg·kg−1] | 6.50 | 9.00 | 11.10 | 14.70 | Sulphur content | [ppm] | <10 | 2 | 2 | 2 | 5 | 4 | 7 | − | 2 | − | 5 | 3 | 4 | − | − |
| Lower heating value at 15 °C | [MJ·kg−1] | 19.80 | 26.40 | 33.09 | 41.30 | Pour point | [°C] | − | −3.2 | −8.0 | +14.3 | +6.0 | −11.0 | +10.0 | − | −2.0 | −7.0 |
].
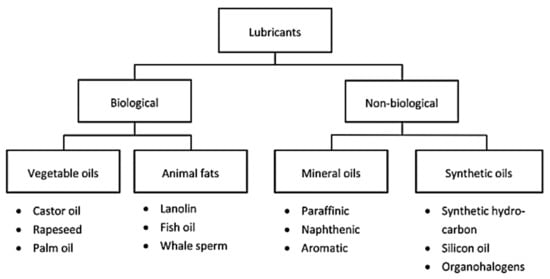
The classification of lubricants depends on the type of source and its properties, and they are generally synthesized from two different sources of base oil. As shown in Figure 3, biological and non-biological sources are two sources of base oil that have different properties and uses [66].
2.1. Non-Biological Lubricants
Non-biological or conventional lubricants are made from mineral or synthetic oils. In particular, mineral oil is a petroleum product used as a lubricant in the automotive, railway, and aviation industries, as well as cosmetic and health products [68]. Synthetic lubricant is a type of lubricant that is composed of chemical compounds (chemically modified petroleum components) that are synthesized artificially. Crude oil is the most common raw material, which is converted into a lubricant by distillation and then physically and chemically modified. Synthetic lubricants have an exceptional performance compared to conventional lubricants based on mineral materials [69]. In the comparison between synthetic and mineral lubricants, many advantages can be mentioned for synthetic lubricants, for example, better fluidity at low temperatures, oxidation stability, and thermal stability. The disadvantages of synthetic lubricants include higher prices (two to four times of mineral lubricants), as well as an increased risk of additive precipitation due to cold storage [70][71].
2.2. Biological Lubricants
Biolubricants represent a category of lubricants generated from renewable sources such as vegetable oils and animal fats, which may contain triglyceride esters but can also contain other bio-based chemicals. The most common oils for biolubricants are vegetable oils such as high oleic canopy oil, castor oil, palm oil, sunflower oil, canola oil, and so on [72]. Different applications of vegetable oils are shown in Table 4. As the studies reported in the cited table show, one of the most common applications of vegetable oils is lubrication. Biolubricants that have been synthesized from vegetable oils have higher levels of lubrication. Also, the flash point, volatility, and viscosity index show higher levels than conventional lubricants. Table 5 provides a general comparison between mineral oils and vegetable oils.
Table 4.
Various vegetable oils and their applications
.
| Vegetable Oil | Major Applications in Industry | |||
|---|---|---|---|---|
| Soybean Oil | Lubricant, hydraulic fluid, plasticizers, printing inks, pesticides, disinfectants, and in the manufacture of soap, plastics, and synthetic rubber. | |||
| Canola Oil | The production of biodiesel and as a lubricant in the food industry. Hydraulic oils, tractor transmission fluids, metalworking fluids. | |||
| Palm Oil | The production of soaps, candles, and as a lubricant in the textile and machinery industries. | |||
| Sunflower Oil | The production of paints, varnishes, and as a lubricant in the machinery industry. Grease, diesel fuel substitutes. | |||
| Peanut Oil | Lubricant in the machinery and textile industries, and in the production of soaps and cosmetics. | |||
| Olive Oil | Automotive lubricants. The production of soaps and as a lubricant in the food industry. | |||
| Coconut Oil | The production of soaps, cosmetics, as a lubricant in the food industry, and engine oils. | |||
| Flaxseed Oil | The production of paints, varnishes, and as a lubricant in the machinery industry. | |||
| Corn Oil | The production of biodiesel and as a lubricant in the food industry. | |||
| +3.0 | −8.3 | −2.0 | −12.5 | −6.8 |
| Higher heating value | [MJ·kg−1] | 22.88 | ||
| Cottonseed Oil | 29.85 | The production of soaps and as a lubricant in the machinery and textile industries. | 36.07 | |
| Rapeseed Oil | The production of biodiesel and as a lubricant in the machinery industry. Air compressor-farm equipment. | |||
| Sesame Oil | The production of soaps, cosmetics and as a lubricant in the food industry | |||
| Castor Oil | The production of soaps, cosmetics, and as a lubricant in the machinery industry. Gear lubricants, greases. | |||
| Grape seed Oil | The production of paints, varnishes, and as a lubricant in the machinery industry. | |||
| Rice Bran Oil | The production of biodiesel and as a lubricant in the food industry. Cosmetics, soap making. | |||
| Tallow oil | The production of candles, soaps, lubricants, plastics. | |||
| Cuphea oil | Used in cosmetics and personal care products, motor oil. | |||
| Crambe oil | Lubricant and industrial lubricant. Intermediate chemicals, surfactants | |||
| Jojoba oil | Used in cosmetics, personal care products, and as a lubricant. | |||
| Linseed oil | The production of paints, varnishes, stains, and lacquers. | |||
| Safflower oil | Used in cosmetics, personal care products, and as a lubricant. |
Table 5.
Comparison between mineral oils and vegetable oils
.
| Characteristics | Vegetable Oils | Mineral Oils | |||
|---|---|---|---|---|---|
| Source | Extracted from seeds, nuts, or fruits of plants | Derived from petroleum | |||
| Chemical structure | Complex mixtures of fatty acids, triglycerides, and other compounds | Complex mixtures of hydrocarbons | |||
| Density @ 20 °C (kg·m−3) | 910–940 | 820–900 | |||
| 48.00 | |||||
| Relative density | |||||
| 22.1 | |||||
| 22.3 | |||||
| 24.6 | |||||
| 21.6 | |||||
| Dynamic viscosity at 20 °C | [mPa·s] | 0.57 | 1.20 | 2.80 | 0.60 |
| Volumetric energy content | [MJ·m−3] | 15,871 | 21,291 | 26,795 | 31,746 |
| Specific CO2 emissions | [g·MJ−1] | 68.44 | 70.99 | 71.90 | 73.95 |
| Auto ignition temperature | [K] | 738 | 698 | 616 | 465/743 |
| Adiabatic flame temperature | [K] | 2143 | 2193 | 2262 | ~2275 |
One of the most popular chemical compounds employed as a hydrogen transporter is methanol (CH3OH), which is a simple oxygenated hydrocarbon (also known as methyl alcohol and wood alcohol). Its symbol is MeOH and it is the simplest aliphatic alcohol [44][45][46
- .
-
Reduction in HC and CO exhaust, while NOx emission and formaldehyde formation increase 5 times (when using M85 fuel) [34].
-
Because of its propensity to react with water and separate from petrol, it results in a heterogeneous combination.
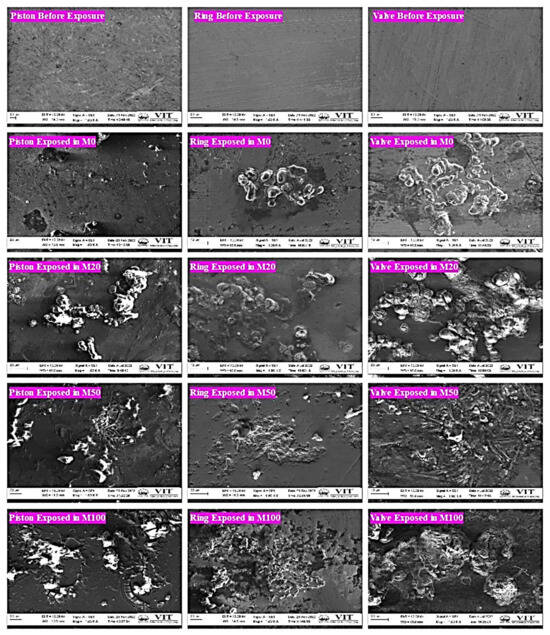
Figure 2.
SEM images of metal samples before and after being exposed to fuels.
Ethanol with the chemical formula C2H6O (abbreviation of EtOH: ethyl alcohol, also called grain alcohol, drinking alcohol, or simple alcohol) is an organic compound. Ethanol is produced naturally through the fermentation process of sugars by yeasts or through petrochemical processes such as ethylene hydration [49]. The fuel blend of ethanol, e.g., E85 (85% ethanol, 15% gasoline) and E10 (10% ethanol, 90% gasoline), are broadly used. E10 fuel blend is also known as “gasohol” [34]. Butanol (C4H9OH), commonly known as butyl alcohol, is a four-carbon alcohol that is largely employed as a solvent and as an intermediary in chemical synthesis. It may also be used as a fuel. Biobutanol, which may be either n-butanol or isobutanol, is the term for butanol produced biologically. By fermenting biomass, particularly wood waste that is enriched in cellulose, butanol may be obtained [50]. The advantages of butanol compared to other alcohols (methanol and ethanol) are as follows [34]:
-
Higher cetane number;
-
Lower corrosion;
-
Lower ignition temperature;
-
Lower evaporation enthalpy [51].
1.2. The Main Fluid Dynamic and Thermodynamic Properties of Fuels for ICEs
Several fluid dynamic and thermodynamic properties of a fuel can significantly impact the performance of ICEs. One such property is viscosity, which affects the behavior of fuel injection. As the ambient temperature decreases, the viscosity increases, so measures must be taken to prevent a reduction in the engine performance. Higher viscosity of fuel leads to poor atomization and weak evaporation of fuel, larger droplets, and more penetration of fuel spray into the cylinder, in particular during cold starts at low temperatures. As the study [6] has shown, on the other hand, a high viscosity also reduces the fuel flow rate and causes insufficient fuel delivery. Very high viscosity has effects such as pump disturbance, bad combustion, more diffusion, and increased engine lubricant dilution, which were reported in this study.
Density is also one of the properties that increase with decreasing temperature. Higher density means that the same volume of injected fuel corresponds to a greater mass, which affects the air-to-fuel ratio and the total energy content inside the cylinder; all these phenomena happen at low ambient temperatures and during cold starts [6]. Cetane number is a measure of fuel ignition delay time in diesel engines and, hence, is related to the quality characteristics of fuel combustion and auto-ignition in the engine. It also affects the cold start of the engine [6]. Using fuels with a higher cetane number accelerates the ignition process, resulting in a shorter engine start-up and reduced cranking time. The volatile properties are described by the vapor and distillation pressure curves. Evaporation characteristics have an effect on the structure of the spray, which has consequences on the air–fuel mixture [52]. Distillation properties have a significant effect on fuel spray penetration and mixture formation [53]. The flash point (FP) temperature of a petroleum product is the lowest temperature (at a pressure of 101.3 kPa) in which the substance vapors (fuel) form a combustible mixture with air so that, by bringing a small flame close to it, it ignites and extinguishes almost instantaneously. The more volatile the fuel, the lower the FP [52]. Low FP is needed for safety and better fuel transfer. The three characteristics of fuel that affect the performance of CI engines in cold ambient temperatures are as follows: the cloud point (CP) is the lowest temperature at which the first crystals start to form, solid fuel particles start to emerge, and clear liquid begins to cloud. The term “Cold Filter Plugging Point” (CFPP) refers to the lowest temperature (°C) at which, under certain circumstances, a given amount of diesel fuel will still flow through a standardized filtration system at a given time [54]. The lowest temperature at which a liquid, particularly a lubricant, flows under certain circumstances is known as the pour point (PP) of that liquid (i.e., the temperature beyond which the oil can be easily pumped) [52]. Low-Temperature Flow Test (LTFT) was conducted in the United States and Canada to predict fuel performance at low temperatures. The type of fuel for an engine should be selected according to the seasonal characteristics and weather of that region. Lower and higher heating values of fuel (LHV and HHV) are the amounts of heat that are released from burning fuel at a certain temperature and pressure by considering or not considering the heat used to vaporize water [55][56]. It is very important to develop a model and technique to measure LHV and HHV of compounds. The water content of a fuel is an important factor that can lead to clogging the flow of fuel to the engine at low temperatures. It is highly suggested that the fuel must be free of water. Other disadvantages of water in fuel include increased corrosion, acceleration of oxidation, and strengthening of microbial growth [57].
2. Lubrication and Complications of Interaction of Biofuels with Lubricants
Lubrication is a method in which the use of chemicals (lubricants) reduces the wear of surfaces in relative movement with each other and improves pressure transfer between opposite surfaces. Lubricants, (anti-friction agents) whose quality and type play a key role in reducing friction, support better performance of the device and reduce frequent breakdowns [58]. An excellent lubricant should have features such as a high boiling point, a low freezing point, a high viscosity index, strong thermal stability, hydraulic stability, corrosion resistance, and high oxidation resistance. Furthermore, the lubricant reduces the temperature in the metal contact areas [59] and, therefore, also contributes to cooling the hot parts. One of the important uses of lubricants is as motor oil for gasoline and diesel engines. They mainly reduce the friction and wear of materials between moving parts, thus improving the efficiency of equipment and machinery and playing a significant role in saving fuel and energy. One of the factors that have a negative effect on lubricants is temperature. Increasing the temperature reduces properties such as viscosity and can degrade the lubricant [60][61][62][63]. Contamination agents and wear particles of metal parts are other factors that deteriorate the lubricant’s performance. Lubricants lose their properties with increasing time and decreasing desirable properties, so they should be replaced periodically with new lubricants. The two main reasons why lubricants are unsuitable for further use include the accumulation of pollutants and their chemical changes, while their base oil does not deteriorate and is only contaminated [64].
Their base oil is mostly vegetable oils or synthetic liquids (hydrogenated polyolefins, esters, silicones, fluorocarbons, etc.). Lubricants generally include 90% base oil and 10% additives, i.e., components that are able to reduce friction and wear, increase viscosity, and improve their overall resistance to corrosion, oxidation, ageing, and pollution [65
| Viscosity index (-) | ||
| 80–220 | 95–105 | |
| Pour point (°C) | −15 to −30 | −15 to −60 |
| Flash point (°C) | >150 | >150 |
| Oxidation stability | Neutral | Stable |
| Solubility in water | ||
| Insoluble | Insoluble | |
| Sludge forming tendency | Can form sludge when exposed to air and moisture | Minimal sludge formation |
| Seal swelling tendency | Slender | Slender |
| Shear stability | Stable | Stable |
| Environmental impact | Renewable, biodegradable, and less harmful to the environment | Non-renewable, non-biodegradable, and can have a negative impact on the environment |
Biolubricants are biodegradable, have good thermal stability (higher heat content than mineral oil) and low pour and cloud points, and contain a large amount of unsaturated fatty acids and minimum sulphur content [79]. According to the literature [58][73][79][80], biolubricants can effectively replace mineral lubricants in many applications. Their applications include gearbox oil, hydraulic oils, engine oils, two-stroke engine lubricants, tractors, insulation oils, aircraft oil, grease, metal grinding oils, or multipurpose oils [78].
Environmental worries and constraints towards conventional lubricants have led to a wider use [81] of biological lubricants that are environmentally friendly and produced from non-edible plant resources. Furthermore, biolubricants are also potential candidates for automotive applications notwithstanding the severe service conditions they undergo in an internal combustion engine [59].
The experimental results in a research study showed that after comparing rapeseed-based biolubricant and synthetic lubricant, both of which were contaminated with biodiesel, in the same operating conditions, the performance of biolubricant was reported to be better in terms of wear and friction [82]. Vegetable oils can be a source of alternative lubricants. Among the advantages of biolubricants, we can mention renewability, compatibility with the environment, biodegradability, and less toxicity [83]. Transesterification may address the shortcomings of vegetable oils as biolubricants, particularly the higher viscosity if not processed as indicated. The most notable drawbacks of vegetable oils used as lubricants include poor performance at low temperatures, low oxidation, thermal stability, and gummy effect [84][85]. One of the technical issues and the most important features of biolubricants is their viscosity range, which should be improved, and for that improvement, environmentally friendly viscosity modifiers are suggested. Styrene–butadiene–styrene (SBS) and ethylene-vinyl acetate (EVA) copolymers were utilized to expand the range of viscosity in biolubricants, which improved and increased kinematic viscosity between 150–250 cSt and 26–36 cSt at 40 °C and 100 °C temperatures [86]. The tribological properties of a biolubricant formulated by jatropha oil (10–50% by volume) with base lubricant SAE 40 were investigated. The blend of jatropha oil with base lubricant performed very well as a biolubricant additive. The results showed that the addition of 10% jatropha oil in the base lubricant had the best performance in terms of reduction in wear, friction coefficient, viscosity, increase in temperature, wear scar diameter, and flash temperature parameter [87].
2.3. Important Properties for Lubricating Oil Performance
The performance of lubricating oil is affected by numerous crucial characteristics. These characteristics are necessary to guarantee efficient lubrication and protection of equipment and engines. Among the essential characteristics are the following:
-
Viscosity. The most important characteristic of lubricants, which is measured as the fluid resistance to flow, directly related to the minimization of friction losses, is viscosity. The viscosity of the lubricating oil in an engine can decrease or increase due to the dilution operated by the fuel and according to the type of fuel used (diesel fuel causes it to decrease and heavy diesel fuel causes it to increase) [88]. Another factor that increases viscosity is oil aging due to progressive oxidation and thermal degradation [89]. One of the effects of too-high viscosity is an excessive resistance to flow, and one of the effects of too-low viscosity is the excessive wear of moving organs due to the lubricant film not being preserved between the moving surfaces
-
In comparison to conventional fuels, biofuels often include more water [6] and oxygen [110][88][90]. In general, increasing the temperature of the lubricant leads to a decrease in viscosity [91], but, if the viscosity index is high (above 200), that lubricant has an outstanding advantage because, in this case, the viscosity will not be affected by temperature [92].
- , which can cause, for example, fuel system components to corrode [6]. Older engines that might not have been built to manage the increasing levels of water and oxygen of actual fuels may find this to be especially problematic. Fuel leaks and other issues brought on by corrosion can cause serious damage to an engine [111]. The high temperature of combustion leads to an increase in the acidity of biodiesel and the chemical reaction between biodiesel and the surface of the injection nozzle, and these processes of oxidation and corrosion increase the level of wear [112].
- Thermal stability. Thermal stability is another essential lubricant property, especially when vegetable oil is used as a lubricant under high-temperature conditions. The onset temperature, which can be defined as the temperature at which lubricant begins to decompose, determines thermal stability. Thermal stability is primarily determined by the chemical composition and fatty acid composition (FAC) of a hydrocarbon [93][94].
- Compared to traditional fuels, biofuels have a larger potential for pollutant build-up, including dust, debris, and other impurities [113]. Due to chemical differences, biodiesel has a higher boiling point and a more limited range compared to diesel, which leads to accumulation in crankshaft oil [114]. These impurities may block diesel fuel filters [115][116] and injectors, thus reducing engine output and energy efficiency [117]. Biofuels may also be more susceptible to clogs and other issues with the fuel system [118][119][120][121].
| <100 | ||||||||||||||||||||||
| 100–200 | ||||||||||||||||||||||
| >200 | ||||||||||||||||||||||
| Sodium (Na) | <50 | 50–200 | >200 | |||||||||||||||||||
| Lead (Pb) | <30 | 30–75 | >75 | |||||||||||||||||||
| Hydrolytic stability | Not stable; can break down in the presence of water | Stable | ||||||||||||||||||||
| Tin (Sn) | <20 | 20–30 | >30 | Cold flow behavior | May solidify or become thicker at low temperatures | Less affected by low temperatures | ||||||||||||||||
| Silicon (Si) | <20 | 20–50 | >50 | |||||||||||||||||||
| [−] | − | 0.882 | 0.883 | 0.873 | 0.876 | 0.879 | 0.878 | − | 0.878 | 0.879 | 0.879 | 0.874 | 0.883 | 0.885 | − | |||||||
| Flash point at closed cup | [°C] | +12 | +13 | +29 | −45 | Lower heating value | [MJ·kg−1] | 42.7 | 37.0 | 38.9 | 37.3 | 37.7 | 37.6 | 37.2 | − | 35.3 | − | 37.6 | 35.2 | 39.9 | 37.5 | 38.7 |
| Higher heating value | [MJ·kg−1 | |||||||||||||||||||||
| Oxygen content by mass | [%] | 49.93 | 34.73 | 21.58 | 0.00 | ] | − | 39.7 | 41.3 | 40.6 | 40.7 | 41.1 | 37.0 | − | 40.6 | 42.2 | 39.4 | 38.1 | 43.1 | − | − | |
| Hydrogen content by mass | [%] | 12.58 | 13.13 | 13.60 | ~14.00 | Average chain length | [−] | − | 17.9 | 18.2 | 17.2 | 18.3 | 17.9 | 17.3 | − | 18.1 | 17.8 | 18.5 | 13.4 | 17.6 | − | − |
| Carbon content by mass | [%] | 37.48 | 52.14 | 64.82 | ~86.00 | Average unsaturation | [−] | − | 1.50 | 1.34 | 0.62 | 1.15 | 1.31 | 0.59 | − | 1.59 | 1.63 | 1.06 | 0.12 | 1.46 | − | − |
- Oxidation resistance
- . Oxidation is a chemical reaction that occurs when oil is exposed to oxygen and heat, resulting in thickening of the oil, sediment formation, and acidity. To preserve its efficacy and prevent engine or machinery damage, lubricating oil should have a high oxidation resistance [95].
- Wear protection
- If exposed to cold temperatures, biofuels could be more likely to gel or form wax, which might lead to fuel system clogging and engine stalling. In colder areas where winter temperatures can drop dramatically this can be very hazardous
- [
- Table 6
- , engine operators can determine if the engine is experiencing abnormal degradation or other issues that may necessitate maintenance
- [
- ]
- [
- ]
- .
Table 6.
Limits of metal content in the industry independently of the brand name and kilometers or working hours for diesel engines
[90]
.
| Metal Component | Normal (ppm) | Abnormal (ppm) | Critical (ppm) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aluminum (Al) | <20 | 20–30 | >30 | ||||||||||||||
| Chrome (Cr) | <10 | 10–25 | >25 | ||||||||||||||
| Copper (Cu) | <30 | 30–75 | >75 | ||||||||||||||
| Nickel (Ni) | <10 | 10–20 | >20 | ||||||||||||||
| Iron (Fe) | |||||||||||||||||
| Vapor density (STP) | |||||||||||||||||
| [kg·m | |||||||||||||||||
| −3 | |||||||||||||||||
| ] | |||||||||||||||||
| 1.42 | |||||||||||||||||
| 2.06 | 2.60 | 3.88 | |||||||||||||||
| Boiling point | [°C] | − | − | − | − | − | − | − | − | − | − | − | − | − | +280/+400 | − | |
| Heat of vaporization | [kJ·kgStoichiometric air-fuel ratio (AFR) | [−] | − | − | − | − | − | − | − | − | − | − | − | − | − | 12.5 | − |
- . Strong film-forming lubricants can efficiently separate contact surfaces, resulting in minimal wear of interacting surfaces and low friction in mixed and boundary lubrication regimes. Therefore, the indicators of wear and friction are necessary to comprehend a lubricant’s efficacy
- [
- ].
- ]
- .
- Corrosion and rust protection
- . Metal surfaces should be protected by lubricating oil from rust and corrosion brought on by moisture or acidic combustion by-products. It ought to have additives that provide a barrier of defense against the development of corrosive chemicals
- [
- 97]
- In comparison to traditional fuels, biofuels have the potential to minimize greenhouse gas emissions while maintaining equal engine power output; however, the actual emissions profile can vary greatly depending on the kind of biofuel, the method of production, and the engine technology. For instance, some types of biofuels may increase emissions of specific pollutants including nitrogen oxides (NO
- x
- )
- [
- ]
- [
- Foam resistance
- . One of the most fundamental physical properties of lubricants is their resistance to foam formation. In the formulation of a lubricant, detergent and anti-oxidation additives may contribute to the formation of foam. Foaming results in discontinuous lubrication, and the addition of anti-foaming agents prevents the formation of stable foam in oil. Antifoaming agents reduce the surface tension of air bubbles by breaking them into smaller bubbles or eliminating them on the surface, thereby promoting the rapid decomposition of foam
- [
- ].
- The ignition and combustion characteristics of biofuels may differ from those of conventional fuels, which can impact engine performance and emissions. Some biofuels, for instance, may have lower volatility
- [
- ]
- Compatibility with seals and materials
- . It is essential that lubricating oil is compatible with the seals and materials of the machinery. It should not cause seals, gaskets, or other components to expand, contract, or deteriorate
- [
- ]
- .
- or higher boiling points
- [
- ]
- than conventional fuels. This can result in issues such as misfires
- Water content
- . The presence of water
- [
- ]
- in the lubricant will cause many complications. It worsens the rheological properties, reduces the ability of lubrication and insulation, reduces the possibility of bearing load transfer, speeds up the oxidation process of the oil, rinses out the improvers, increases the amount of sediment, and causes corrosion
-
Flash point. The flash point is the lowest temperature at which a liquid may generate sufficient vapor to combine with air to ignite. Commercial products must comply with specific flash points to guarantee safe handling, transit, and use because this can pose a major fire risk. The precise flash point specifications, which might change depending on the product and how it will be used, are frequently set by supervisory authorities or organizations that develop industry standards [88][103][104].
-
Content of metal particles. Metals, non-metals, and chemicals can belong to one of the main categories of wear metals, oil elements, pollutants, and additives. The limits of chemical components accepted in the industry for diesel engines are summarized according to Table 6. Due to the wear and tear of engine components, metal particles are discharged into the lubricant during engine operation. In addition to dust, dirt, and combustion by-products, lubricants can contain other contaminants. As a guide for monitoring the metal particle content of the lubricating oil, the limits presented in Table 6 are used. They represent the permissible maximum levels of each metal component in the lubricant, which can indicate abnormal engine wear or other problems. By frequently monitoring the metal particle content in the lubricant and comparing it to the limits presented in
-
Total Base Number (TBN). TBN refers to a lubricant’s capacity to neutralize acids, measured by the quantity of potassium hydroxide (KOH) needed to neutralize one gram of the lubricant sample, expressed in milligrams. TBN is a crucial parameter in petroleum products, and its value fluctuates based on the specific use case [106][107].
-
Total Acid Number (TAN). TAN is a crucial parameter for evaluating the overall acidity of a substance, determined by the quantity of potassium hydroxide required to neutralize the acids present in one gram of lubricant. TAN testing plays a vital role in assessing additive depletion, acidic contamination, and oxidation in lubricants, contributing to the proactive preservation of equipment. This measurement encompasses both low-pH organic acids and high-pH inorganic acids within the lubricant. An increase in TAN indicates lubricant oxidation, which may result from factors such as time or operational temperature [108][109].
3. Possible Problems in Using Biofuels in Internal Combustion Engines
Impact of Biofuels on Engine Lubricant: Performance, Dilution, and Degradation
During long-term engine operation, fuel is considered to be a significant influencing factor for engine oil conditions [138]. Biofuels may contain higher levels of oxygen (10% to 45%) [139], which can increase lubricant oxidation and degradation, thereby diminishing their efficacy and lifespan [138][140][141]. In addition, certain types of biofuels may contain contaminants or impurities that interact with lubricants, resulting in additive depletion and viscosity changes [141][142]. For example, the physical properties of biodiesel, which are different from diesel, such as higher surface tension, lower volatility, and higher relative density, cause the formation of larger droplets downstream of the injector [143] and more collisions with the fuel wall during injection into the combustion chamber. They also lead to higher dilution of biodiesel than diesel [144]. Based on the studies and experiments [145][146], it is possible to conclude that biodiesel has other destructive effects in addition to changes in lubricating oil viscosity. Diluting lubricating oil with aged biodiesel and its degradation products (oxidized biodiesel) can cause interaction with zinc dialkyldithiophosphate (ZDDP) anti-wear additives in lubricating oil, which lead to increased engine wear [144]. Also, the presence of biodiesel increases TAN and decreases the TBN of the lubricant [141]. Researchers showed that the use of biofuels in long-term engine durability tests also leads to engine damage due to more carbon deposits and lubricating oil pollution [147].
References
- Khan, A.A.; Kamal, T.A. Biofuel Second Generation and Energy Security: An Overview. Int. J. Sci. Eng. Res. 2016, 7, 1306–1320. Available online: http://www.ijser.org (accessed on 20 November 2022).
- World Energy Council. World Energy Trilemma Index. 2019. Available online: https://www.worldenergy.org/transition-toolkit/world-energy-trilemma-index (accessed on 20 November 2022).
- Bietresato, M.; Caligiuri, C.; Bolla, A.; Renzi, M.; Mazzetto, F. Proposal of a Predictive Mixed Experimental- Numerical Approach for Assessing the Performance of Farm Tractor Engines Fuelled with Diesel- Biodiesel-Bioethanol Blends. Energies 2019, 12, 2287.
- Guo, M.; Song, W.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sustain. Energy Rev. 2015, 42, 712–725.
- Kabeyi, M.J.B.; Olanrewaju, O.A. Sustainable Energy Transition for Renewable and Low Carbon Grid Electricity Generation and Supply. Front. Energy Res. 2022, 9, 1032.
- Niculescu, R.; Clenci, A.; Iorga-Siman, V. Review on the Use of Diesel–Biodiesel–Alcohol Blends in Compression Ignition Engines. Energies 2019, 12, 1194.
- Yasin, M.H.M.; Ali, M.A.; Mamat, R.; Yusop, A.F.; Ali, M.H. Physical properties and chemical composition of biofuels. In Second and Third Generation of Feedstocks: The Evolution of Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 291–320.
- MHafiz; Hafizil, M.; Yasin, M.; Salleh, M.R.; Ali, M.H.; Mamat, R. Characterization of Physical Properties for Diesel-alcohol and Biodiesel-alcohol Blends Fuel, Mixture Formation and Combustion Process Characterization of Physical Properties for Diesel-alcohol and Biodiesel-alcohol Blends. Fuel Mix. Form. Combust. Process 2022, 4, 1–5. Available online: www.fazpublishing.com/fmc (accessed on 20 November 2022).
- Khan, M.; Sharma, R.; Kadian, A.K.; Hasnain, S.M.M. An assessment of alcohol inclusion in various combinations of biodiesel-diesel on the performance and exhaust emission of modern-day compression ignition engines—A review. Mater. Sci. Energy Technol. 2022, 5, 81–98.
- Li, W.; Ren, Y.; Wang, X.-B.; Miao, H.; Jiang, D.-M.; Huang, Z.-H. Combustion characteristics of a compression ignition engine fuelled with diesel—Ethanol blends. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2008, 222, 265–274.
- Wang, L.-J.; Song, R.-Z.; Zou, H.-B.; Liu, S.-H.; Zhou, L.-B. Study on combustion characteristics of a methanol—Diesel dual-fuel compression ignition engine. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2008, 222, 619–627.
- Tutak, W.; Jamrozik, A.; Pyrc, M.; Sobiepański, M. Investigation on combustion process and emissions characteristic in direct injection diesel engine powered by wet ethanol using blend mode. Fuel Process. Technol. 2016, 149, 86–95.
- Hansdah, D.; Murugan, S.; Das, L. Experimental studies on a DI diesel engine fueled with bioethanol-diesel emulsions. Alex. Eng. J. 2013, 52, 267–276.
- Yasin, M.H.M.; Mamat, R.; Yusop, A.F.; Aziz, A.; Najafi, G. Comparative Study on Biodiesel-methanol-diesel Low Proportion Blends Operating with a Diesel Engine. Energy Procedia 2015, 75, 10–16.
- Rakopoulos, D.C.; Rakopoulos, C.D.; Giakoumis, E.G.; Papagiannakis, R.G.; Kyritsis, D.C. Influence of properties of various common bio-fuels on the combustion and emission characteristics of high-speed DI (direct injection) diesel engine: Vegetable oil, bio-diesel, ethanol, n-butanol, diethyl ether. Energy 2014, 73, 354–366.
- Yalini, V.; Kannan, T.; Wilson, D.H. Optimization of Engine Performance through different piston shapes by Taguchi Method. Int. J. Innov. Technol. Explor. Eng. 2020, 9, 333–337.
- Ogunkunle, O.; Ahmed, N.A. A review of global current scenario of biodiesel adoption and combustion in vehicular diesel engines. Energy Rep. 2019, 5, 1560–1579.
- Magda, R.; Szlovák, S.; Tóth, J. The role of using bioalcohol fuels in sustainable development. In Bio-Economy and Agri-Production; Academic Press: Cambridge, MA, USA, 2021.
- Roberts, L.G.; Patterson, T.J. Biofuels. In Encyclopedia of Toxicology: Third Edition; Elsevier: Amsterdam, The Netherlands, 2014; pp. 469–475.
- Malode, S.J.; Prabhu, K.K.; Mascarenhas, R.J.; Shetti, N.P.; Aminabhavi, T.M. Recent advances and viability in biofuel production. Energy Convers. Manag. X 2020, 10, 100070.
- Hannah, L. Climate Change Biology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2022.
- Sharma, S.; Kundu, A.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Sustainable environmental management and related biofuel technologies. J. Environ. Manag. 2020, 273, 111096.
- Awogbemi, O.; Von Kallon, D.V.; Onuh, E.I.; Aigbodion, V.S. An Overview of the Classification, Production and Utilization of Biofuels for Internal Combustion Engine Applications. Energies 2021, 14, 5687.
- Bhuiya, M.M.K.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Azad, A.K. Prospects of 2nd generation biodiesel as a sustainable fuel—Part: 1 selection of feedstocks, oil extraction techniques and conversion technologies. Renew. Sustain. Energy Rev. 2016, 55, 1109–1128.
- Hemp as a Renewable Energy Source: Biomass, Ethanol, Biodiesel. Available online: www.bottegadellacanapa.it (accessed on 20 November 2022).
- Northrop, W.F. Particulate and Gas Phase Hydrocarbon Emissions from Partially Premixed Low Temperature Compression Ignition Combustion of Biodiesel. Automotive Emissions View Project Cloud Connected Delivery Vehicles: Boosting Fuel Economy Using Physics-Aware Spatiotemporal Data Analytics and Realtime Powertrain Control View Project. Available online: https://www.researchgate.net/publication/265047147 (accessed on 20 November 2022).
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169.
- Deviren, H.; Aydın, H. Production and physicochemical properties of safflower seed oil extracted using different methods and its conversion to biodiesel. Fuel 2023, 343, 128001.
- Veljković, V.B.; Biberdžić, M.O.; Banković-Ilić, I.B.; Djalović, I.G.; Tasić, M.B.; Nježić, Z.B.; Stamenković, O.S. Biodiesel production from corn oil: A review. Renew. Sustain. Energy Rev. 2018, 91, 531–548.
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Kumari, D. Chemical compositions, properties, and standards for different generation biodiesels: A review. Fuel 2019, 253, 60–71.
- Bacha, J.; Freel, J.; Gibbs, A.; Gibbs, L.; Hemighaus, G.; Hoekman, K.; Horn, J.; Ingham, M.; Jossens, L.; Kohler, D.; et al. Diesel Fuels Technical Review. 2007.
- Read the Specifications for EN 590 Diesel and EN 590 Gas Oil. Available online: https://www.crownoil.co.uk/fuel-specifications/en-590/ (accessed on 20 November 2022).
- Demirbas, A.; Baluabaid, M.A.; Kabli, M.; Ahmad, W. Diesel Fuel from Waste Lubricating Oil by Pyrolitic Distillation. Pet. Sci. Technol. 2014, 33, 129–138.
- Kumar, A.; Hardikk, A.; Editors, V. Energy, Environment, and Sustainability Series Editor: Avinash Kumar Agarwal Potential and Challenges of Low Carbon Fuels for Sustainable Transport. 2022. Available online: https://link.springer.com/bookseries/15901 (accessed on 20 November 2022).
- Vancoillie, J.; Sileghem, L.; Verhelst, S. Development and validation of a quasi-dimensional model for methanol and ethanol fueled SI engines. Appl. Energy 2014, 132, 412–425.
- Yates, A.; Bell, A.; Swarts, A. Insights relating to the autoignition characteristics of alcohol fuels. Fuel 2010, 89, 83–93.
- Jangi, M.; Li, C.; Shamun, S.; Tuner, M.; Bai, X. Modelling of Methanol Combustion in a Direct Injection Compression Ignition Engine using an Accelerated Stochastic Fields Method. Energy Procedia 2017, 105, 1326–1331.
- Obergruber, M.; Hönig, V.; Procházka, P.; Kučerová, V.; Kotek, M.; Bouček, J.; Mařík, J. Physicochemical Properties of Biobutanol as an Advanced Biofuel. Materials 2021, 14, 914.
- Zhen, X.; Wang, Y.; Liu, D. Bio-butanol as a new generation of clean alternative fuel for SI (spark ignition) and CI (compression ignition) engines. Renew. Energy 2020, 147, 2494–2521.
- Li, R.; Teng, W.; Li, Y.; Liu, E. Liquefaction of Sewage Sludge To Produce Bio-oil in Different Organic Solvents with In Situ Hydrogenation. Energy Fuels 2019, 33, 7415–7423.
- Liu, H.; Lee, C.F.; Liu, Y.; Huo, M.; Yao, M. Spray and combustion characteristics of n-butanol in a constant volume combustion chamber at different oxygen concentrations. In Proceedings of the SAE 2011 World Congress and Exhibition, Detroit, MI, USA, 12–14 April 2011.
- Jin, C.; Yao, M.; Liu, H.; Lee, C.-F.L.; Ji, J. Progress in the production and application of n-butanol as a biofuel. Renew. Sustain. Energy Rev. 2011, 15, 4080–4106.
- Yusri, I.; Mamat, R.; Najafi, G.; Razman, A.; Awad, O.I.; Azmi, W.; Ishak, W.; Shaiful, A. Alcohol based automotive fuels from first four alcohol family in compression and spark ignition engine: A review on engine performance and exhaust emissions. Renew. Sustain. Energy Rev. 2017, 77, 169–181.
- The Emergency Response Safety and Health Database: Methanol. Available online: https://www.cdc.gov/niosh/ershdb/EmergencyResponseCard_29750029.html (accessed on 20 November 2022).
- Verhelst, S.; Turner, J.W.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88.
- Pandey, S. A critical review: Application of methanol as a fuel for internal combustion engines and effects of blending methanol with diesel/biodiesel/ethanol on performance, emission, and combustion characteristics of engines. Heat Transf. 2022, 51, 3334–3352.
- Kumar, T.S.; Ashok, B. Material compatibility of SI engine components towards corrosive effects on methanol-gasoline blends for flex fuel applications. Mater. Chem. Phys. 2023, 296, 127344.
- Choi, B.; Jiang, X.; Kim, Y.K.; Jung, G.; Lee, C.; Choi, I.; Song, C.S. Effect of diesel fuel blend with n-butanol on the emission of a turbocharged common rail direct injection diesel engine. Appl. Energy 2015, 146, 20–28.
- Ethanol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/ethanol (accessed on 17 November 2022).
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 2008, 451, 86–89.
- Kumar, V.; Kumar, A.; Ashutosh, A.; Ram, J.; Upadhyay, K. Energy, Environment, and Sustainability Series Editor: Avinash Kumar Agarwal Advances in Engine Tribology. Available online: https://link.springer.com/bookseries/15901 (accessed on 20 November 2022).
- Niculescu, R.; Clenci, A. Diesel Fuels. Physico-Chemical Properties. Development of a Test Method for Distillation of Diesel-Biodiesel-Alcohols Mixtures at Reduced Pressure Cold Starting of Biodiesel Fuelled Compression Ignition Engines View Project Variable Compression Ratio View Project. 2018. Available online: https://www.researchgate.net/publication/323538581 (accessed on 20 November 2022).
- Pan, J.; Yang, W.; Chou, S.; Li, D.; Xue, H.; Zhao, J.; Tang, A. Spray and combustion visualization of bio-diesel in a direct injection diesel engine. Therm. Sci. 2013, 17, 279–289.
- WWFC_19_gasoline_diesel. Available online: https://www.acea.auto/files/WWFC_19_gasoline_diesel.pdf (accessed on 20 November 2022).
- Tesfa, B.; Gu, F.; Mishra, R.; Ball, A. LHV Predication Models and LHV Effect on the Performance of CI Engine Running with Biodiesel Blends. 2013. Available online: http://eprints.hud.ac.uk/id/eprint/17195/http://eprints.hud.ac.uk/ (accessed on 20 November 2022).
- García, M.; Gonzalo, A.; Sánchez, J.L.; Arauzo, J.; Peña, J. Prediction of normalized biodiesel properties by simulation of multiple feedstock blends. Bioresour. Technol. 2010, 101, 4431–4439.
- Gonçalves, H.L.; Fregolente, P.B.L.; Maciel, M.R.W.; Fregolente, L.V. Formulation of hydrogels for water removal from diesel and biodiesel. Sep. Sci. Technol. 2021, 56, 374–388.
- Uppar, R.; Dinesha, P.; Kumar, S. A critical review on vegetable oil-based bio-lubricants: Preparation, characterization, and challenges. Environ. Dev. Sustain. 2022, 25, 9011–9046.
- Singh, Y.; Farooq, A.; Raza, A.; Mahmood, M.A.; Jain, S. Sustainability of a non-edible vegetable oil based bio-lubricant for automotive applications: A review. Process Saf. Environ. Prot. 2017, 111, 701–713.
- Neale, M.J. Chapter C1—Viscosity of Lubricants. In Lubrication and Reliability Handbook; Newnes: Oxford, UK, 2001; pp. 1–4.
- Stachowiak, G.W.; Batchelor, A.W. Chapter 2—Physical Properties of Lubricants. In Engineering Tribology Book, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 11–50.
- Sander, D.E.; Knauder, C.; Allmaier, H.; Baleur, S.D.-L.; Mallet, P. Friction Reduction Tested for a Downsized Diesel Engine with Low-Viscosity Lubricants Including a Novel Polyalkylene Glycol. Lubricants 2017, 5, 9.
- Parekh, K.; Radadiya, R.; Gaur, R.; Shahabuddin, S.; Ahmad, I. A cost-effective approach for decontamination of used lubricant oil: Enhanced recovery of base oil using different adsorbents. Int. J. Environ. Sci. Technol. 2022, 20, 12323–12342.
- Yash, M. Re-refining of used lubricating oil. Int. J. Sci. Eng. Res. 2015, 6, 329–332. Available online: http://www.ijser.org (accessed on 20 November 2022).
- Pirro, D.M.; Webster, M.; Daschner, E. Lubrication Fundamentals, Revised and Expanded, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016.
- Shafi, W.K.; Raina, A.; Haq, M.I.U. Friction and wear characteristics of vegetable oils using nanoparticles for sustainable lubrication. Tribol. Mater. Surfaces Interfaces 2018, 12, 27–43.
- Kadirgama, G.; Kamarulzaman, M.K.; Ramasamy, D.; Kadirgama, K.; Hisham, S. Classification of Lubricants Base Oils for Nanolubricants Applications—A Review. In Lecture Notes in Mechanical Engineering; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2023; pp. 205–213.
- Sadriwala, M.; Singh, Y.; Sharma, A.; Singla, A.; Mishra, S. Friction and wear behavior of jojoba oil based biolubricant-Taguchi method approach. Mater. Today Proc. 2019, 25, 704–709.
- Afifah, A.; Syahrullail, S.; Azlee, N.I.W.; Sidik, N.A.C.; Yahya, W.; Rahim, E.A. Biolubricant production from palm stearin through enzymatic transesterification method. Biochem. Eng. J. 2019, 148, 178–184.
- do Valle, C.P.; Rodrigues, J.S.; Fechine, L.M.U.D.; Cunha, A.P.; Malveira, J.Q.; Luna, F.M.T.; Ricardo, N.M.P.S. Chemical modification of Tilapia oil for biolubricant applications. J. Clean. Prod. 2018, 191, 158–166.
- Cavalcanti, E.D.; Aguieiras, C.; da Silva, P.R.; Duarte, J.G.; Cipolatti, E.P.; Fernandez-Lafuente, R.; da Silva, J.A.C.; Freire, D.M. Improved production of biolubricants from soybean oil and different polyols via esterification reaction catalyzed by immobilized lipase from Candida rugosa. Fuel 2018, 215, 705–713.
- Salimon, J.; Salih, N.; Yousif, E. Biolubricants: Raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol. 2010, 112, 519–530.
- Panchal, T.M.; Patel, A.; Chauhan, D.; Thomas, M.; Patel, J.V. A methodological review on bio-lubricants from vegetable oil based resources. Renew. Sustain. Energy Rev. 2017, 70, 65–70.
- Liew Yun Hsien, W. Utilization of Vegetable Oil as Bio-lubricant and Additive. In Towards Green Lubrication in Machining. Springer Briefs in Molecular Science; Springer: Singapore, 2015; pp. 7–17.
- Shashidhara, Y.; Jayaram, S. Vegetable oils as a potential cutting fluid—An evolution. Tribol. Int. 2010, 43, 1073–1081.
- Alves, S.M.; Barros, B.S.; Trajano, M.F.; Ribeiro, K.S.B.; Moura, E. Tribological behavior of vegetable oil-based lubricants with nanoparticles of oxides in boundary lubrication conditions. Tribol. Int. 2013, 65, 28–36.
- Mobarak, H.; Mohamad, E.N.; Masjuki, H.; Kalam, M.; Al Mahmud, K.; Habibullah, M.; Ashraful, A. The prospects of biolubricants as alternatives in automotive applications. Renew. Sustain. Energy Rev. 2014, 33, 34–43.
- Singh, Y.; Garg, R.; Kumar, S. Aspects of Non-edible Vegetable Oil-Based Bio-lubricants in the Automotive Sector. Green 2015, 5, 59–72.
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016.
- Chan, C.-H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological behavior of biolubricant base stocks and additives. Renew. Sustain. Energy Rev. 2018, 93, 145–157.
- Shah, R.; Woydt, M.; Zhang, S. The Economic and Environmental Significance of Sustainable Lubricants. Lubricants 2021, 9, 21.
- Arumugam, S.; Sriram, G. Effect of Bio-Lubricant and Biodiesel-Contaminated Lubricant on Tribological Behavior of Cylinder Liner–Piston Ring Combination. Tribol. Trans. 2012, 55, 438–445.
- Salih, N.; Salimon, J.; Yousif, E. Synthetic biolubricant basestocks based on environmentally friendly raw materials. J. King Saud Univ. Sci. 2012, 24, 221–226.
- Mofijur, M.; Masjuki, H.; Kalam, M.; Shahabuddin, M.; Hazrat, M.; Liaquat, A. Palm Oil Methyl Ester and Its Emulsions Effect on Lubricant Performance and Engine Components Wear. Energy Procedia 2012, 14, 1748–1753.
- Nagendramma, P.; Kaul, S. Development of ecofriendly/biodegradable lubricants: An overview. Renew. Sustain. Energy Rev. 2012, 16, 764–774.
- Quinchia, L.A.; Delgado, M.A.; Valencia, C.; Franco, J.M.; Gallegos, C. Viscosity Modification of High-Oleic Sunflower Oil with Polymeric Additives for the Design of New Biolubricant Formulations. Environ. Sci. Technol. 2009, 43, 2060–2065.
- Shahabuddin, M.; Masjuki, H.; Kalam, M.; Bhuiya, M.; Mehat, H. Comparative tribological investigation of bio-lubricant formulated from a non-edible oil source (Jatropha oil). Ind. Crop. Prod. 2013, 47, 323–330.
- Kaminski, P. Experimental Investigation into the Effects of Fuel Dilution on the Change in Chemical Properties of Lubricating Oil Used in Fuel Injection Pump of Pielstick PA4 V185 Marine Diesel Engine. Lubricants 2022, 10, 162.
- Santos, J.C.O.; Santos, I.M.G.; Souza, A.G. Thermal degradation of synthetic lubricating oils: Part II—Rheological study. Pet. Sci. Technol. 2017, 35, 535–539.
- Fernández-Feal, M.; Sánchez-Fernández, L.R.; Pérez-Prado, J.R. Study of Metal Concentration in Lubricating Oil with Predictive Purposes. Curr. J. Appl. Sci. Technol. 2018, 27, 1–12.
- Ting, C.-C.; Chen, C.-C. Viscosity and working efficiency analysis of soybean oil based bio-lubricants. Measurement 2011, 44, 1337–1341.
- Rodrigues, J.d.A.; Cardoso, F.d.P.; Lachter, E.R.; Estevão, L.R.M.; Lima, E.; Nascimento, R.S.V. Correlating chemical structure and physical properties of vegetable oil esters. J. Am. Oil Chem. Soc. 2006, 83, 353–357.
- Kalam, M.; Masjuki, H.; Cho, H.M.; Mosarof, M.; Mahmud, I.; Chowdhury, M.A.; Zulkifli, N. Influences of thermal stability, and lubrication performance of biodegradable oil as an engine oil for improving the efficiency of heavy duty diesel engine. Fuel 2017, 196, 36–46.
- Santos, J.C.O.; Lima, L.N.; Santos, I.M.G.; Souza, A.G. Thermal, Spectroscopic and Rheological Study of Mineral Base Lubricating Oils. J. Therm. Anal. Calorim. 2007, 87, 639–643.
- Von, G.H.; Diamond, H. Oxidation Characteristics of Lubricating Oils Relation between Stability and Chemical Composition. Available online: https://pubs.acs.org/sharingguidelines (accessed on 20 November 2022).
- Yilmaz, N.; Ileri, E.; Atmanli, A. Performance of biodiesel/higher alcohols blends in a diesel engine. Int. J. Energy Res. 2016, 40, 1134–1143.
- Wilson, R.W.; Lyon, S.B. Corrosion in lubricants/fuels. In Shreir’s Corrosion; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1299–1307.
- Wang, J.; Hu, W.; Li, J. Lubrication and Anti-Rust Properties of Jeffamine-Triazole Derivative as Water-Based Lubricant Additive. Coatings 2021, 11, 679.
- Prolić, T.Ć.; Lepušić, A. Effect of foaming on the antiwear properties of lubricating oils. Goriva Maz. 2012, 51, 38.
- Mang, T.; Noll, S.; Bartels, T. Lubricants, 1. Fundamentals of Lubricants and Lubrication. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2011.
- Markova, L.; Myshkin, N.; Makarenko, V.M.; Semenyuk, M.S.; Kong, H.; Han, H.; Yun, E.S. The Dry Sliding of Ceramics View Project Ceramic Wear View Project Monitoring of Water Content in Oil as a Method of Tribodiagnostics. 2004. Available online: https://www.researchgate.net/publication/294544148 (accessed on 20 November 2022).
- Li, J.; Tian, H.X.; Sun, Y.L.; Ming, T.F.; Sheng, C.X. Application of FTIR Spectrum in Quantitatively Monitoring Oil Contaminants. Spectrosc. Spectr. Anal. 2019, 39, 3459–3464.
- Abu-Elella, R.; Ossman, M.E.; Farouq, R.; Abd-Elfatah, M. Used Motor Oil Treatment: Turning Waste Oil into Valuable Products. Int. J. Chem. Biochem. Sci. 2015, 7, 57–67.
- Fu, J. Flash points measurements and prediction of biofuels and biofuel blends with aromatic fluids. Fuel 2018, 241, 892–900.
- Inthawatkul, I.; Sriratana, W.; Satthamsakul, S. Measurement of Metal Particles in Oil Lubricant using Hall Effect Sensor Under Temperature Conditions. In Proceedings of the 2017 56th Annual Conference of the Society of Instrument and Control Engineers of Japan (SICE), Kanazawa, Japan, 19–22 September 2017.
- Total Base Number. Available online: https://en.wikipedia.org/wiki/Total_base_number (accessed on 20 November 2022).
- Dong, J.; van de Voort, F.R.; Yaylayan, V.; Ismail, A.A.; Pinchuk, D.; Taghizadeh, A. Determination of total base number (tbn) in lubricating oils by mid-ftir spectroscopy. Lubr. Eng. 2001, 57, 24–30.
- Malik, M.A.I.; Usman, M.; Hayat, N.; Zubair, S.W.H.; Bashir, R.; Ahmed, E. Experimental evaluation of methanol-gasoline fuel blend on performance, emissions and lubricant oil deterioration in SI engine. Adv. Mech. Eng. 2021, 13, 1–17.
- Acid Number: A Comprehensive Guide. Available online: https://www.machinerylubrication.com/Read/1052/acid-number-test (accessed on 20 November 2022).
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116.
- Hoang, A.T.; Tabatabaei, M.; Aghbashlo, M. A review of the effect of biodiesel on the corrosion behavior of metals/alloys in diesel engines. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 42, 2923–2943.
- Celik, I.; Aydin, O. Effects of B100 Biodiesel on Injector and Pump Piston. Tribol. Trans. 2011, 54, 424–431.
- Velasco, R. Application of Biofuel Impurities and Effect on the Hot Corrosion of Yttria-Stabilized Zirconia Thermal Barrier Coatings. Surf. Coat. Technol. 2018, 358, 340–346.
- Morcos, M.; Parsons, G.; Lauterwasser, F.; Boons, M.; Hartgers, W. Detection methods for accurate measurements of the fame biodiesel content in used crankcase engine oil. In SAE Technical Papers; SAE International: Warrendale, PA, USA, 2009.
- Jayaseelan, G.A.C.; Anderson, A.; Manigandan, S.; Elfasakhany, A.; Dhinakaran, V. Effect of engine parameters, combustion and emission characteristics of diesel engine with dual fuel operation. Fuel 2021, 302, 121152.
- Pranoto, H.; Wahab, A.; Arifin, Z.; Siswanto, I. Fuel filter condition monitoring (ffcm) devices innovation on truck diesel engine to prevent filter blocking due to use of bio diesel: b10-b20-b30. J. Phys. Conf. Ser. 2020, 1700, 012099.
- Canha, N.; Felizardo, P.; Correia, M.J.N. Controlling the oxidative stability of biodiesel using oils or biodiesel blending or antioxidants addition. Environ. Prog. Sustain. Energy 2018, 37, 1031–1040.
- Wang, S.; Sun, X.; Yuan, Q. Strategies for enhancing microbial tolerance to inhibitors for biofuel production: A review. Bioresour. Technol. 2018, 258, 302–309.
- Cazarolli, J.C.; de Quadros, P.D.; Bücker, F.; Santiago, M.R.F.; Piatnicki, C.M.S.; Peralba, M.D.C.R.; Cavalcanti, E.H.d.S.; Bento, F.M. Microbial growth in Acrocomia aculeata pulp oil, Jatropha curcas oil, and their respective biodiesels under simulated storage conditions. Biofuel Res. J. 2016, 3, 514–520.
- Longinos, S.N.; Zannikos, F. The effect of microbial growth on physicochemical properties of biodiesel–diesel mixtures. Braz. J. Chem. Eng. 2022, 39, 345–360.
- Komariah, L.N.; Arita, S.; Rendana, M.; Ramayanti, C.; Suriani, N.L.; Erisna, D. Microbial contamination of diesel-biodiesel blends in storage tank: An analysis of colony morphology. Heliyon 2022, 8, e09264.
- Abdullah, A.Z.; Razali, N.; Mootabadi, H.; Salamatinia, B. Critical technical areas for future improvement in biodiesel technologies. Environ. Res. Lett. 2007, 2, 034001.
- Jeyaseelan, T.; Chacko, N.; Pushyanth, N.; Alexander, J.; Porpatham, E. Partial hydrogenation and hydrogen induction: A comparative study with B20 operation in a turbocharged CRDI diesel engine. Int. J. Hydrogen Energy 2021, 46, 22659–22669.
- Bôas, R.N.V.; Mendes, M.F. A review of biodiesel production from non-edible raw materials using the transesterification process with a focus on influence of feedstock composition and free fatty acids. J. Chil. Chem. Soc. 2022, 67, 5433–5444.
- Schumacher, L.; Borgelt, S.C.; Hires, W.G.; Wetherell, W.; Nevils, A. 100,000 Miles of Fueling 5.9L Cummins Engines with 100% Biodiesel. J. Fuels Lubr. 1996, 105, 2332–2339.
- Mahmudul, H.; Hagos, F.; Mamat, R.; Adam, A.A.; Ishak, W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509.
- Hazrat, M.A.; Rasul, M.G.; Mofijur, M.; Khan, M.M.K.; Djavanroodi, F.; Azad, A.K.; Bhuiya, M.M.K.; Silitonga, A. A Mini Review on the Cold Flow Properties of Biodiesel and its Blends. Front. Energy Res. 2020, 8, 598651.
- Kowalewicz, A.; Wojtyniak, M. Alternative fuels and their application to combustion engines. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2005, 219, 103–125.
- Ghadikolaei, M.A. Effect of alcohol blend and fumigation on regulated and unregulated emissions of IC engines—A review. Renew. Sustain. Energy Rev. 2016, 57, 1440–1495.
- Jindra, P.; Kotek, M.; Mařík, J.; Vojtíšek, M. Effect of different biofuels to particulate matters production. Agron. Res. 2016, 14, 783–789.
- Aziz, M. Integrated supercritical water gasification and a combined cycle for microalgal utilization. Energy Convers. Manag. 2015, 91, 140–148.
- Chakravarthy, K.; Mcfarlane, J.; Daw, S.; Ra, Y.; Reitz, R.; Griffin, J. Physical Properties of Bio-Diesel and Implications for Use of Bio-Diesel in Diesel Engines. J. Fuels Lubr. 2007, 116, 885–895.
- Enagi, I.I.; Al-Attab, K.A.; Alauddin, Z.A.Z. Combustion Stability Analysis of Liquid Biofuels using Acoustic Signals. J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 76, 145–155.
- Bello, U.; Agu, C.M.; Ajiya, D.A.; Mahmoud, A.A.; Udopia, L.; Lawal, N.M.; Abubakar, A.A.; Muhammad, M. Biodiesel, In a Quest For Sustainable Renewable Energy: A Review on Its Potentials and Production Strategies. J. Chem. Rev. 2022, 4, 272–287.
- Freitas, S.V.D.; Oliveira, M.B.; Lima, S.; Coutinho, J.A.P. Measurement and Prediction of Biodiesel Volatility. Energy Fuels 2012, 26, 3048–3053.
- Agarwal, A.K.; Gupta, J.G.; Dhar, A. Potential and challenges for large-scale application of biodiesel in automotive sector. Prog. Energy Combust. Sci. 2017, 61, 113–149.
- No, S.-Y. Inedible vegetable oils and their derivatives for alternative diesel fuels in CI engines: A review. Renew. Sustain. Energy Rev. 2011, 15, 131–149.
- Gulzar, M.; Masjuki, H.; Varman, M.; Kalam, M.; Zulkifli, N.; Mufti, R.; Liaquat, A.; Zahid, R.; Arslan, A. Effects of biodiesel blends on lubricating oil degradation and piston assembly energy losses. Energy 2016, 111, 713–721.
- Mahapatra, S.; Kumar, D.; Singh, B.; Sachan, P.K. Biofuels and their sources of production: A review on cleaner sustainable alternative against conventional fuel, in the framework of the food and energy nexus. Energy Nexus 2021, 4, 100036.
- Devlin, C.C.; Passut, C.A.; Campbell, R.L.; Jao, T.-C. Biodiesel Fuel Effect on Diesel Engine Lubrication. In SAE Technical Papers; SAE International: Warrendale, PA, USA, 2008.
- Bietresato, M.; Friso, D. Durability test on an agricultural tractor engine fuelled with pure biodiesel (B100). Turk. J. Agric. For. 2014, 38, 214–223.
- Taylor, R.I. Fuel-Lubricant Interactions: Critical Review of Recent Work. Lubricants 2021, 9, 92.
- Maji, N.C.; Rastogi, P.; Krishnasamy, A.; Aidhen, I.S.; Kaisare, N.S.; Basavaraj, M.G. Storage and Temperature Stability of Emulsified Biodiesel–Diesel Blends. ACS Omega 2022, 7, 44762–44771.
- Fang, H.L.; Whitacre, S.D.; Yamaguchi, E.S.; Boons, M. Biodiesel Impact on Wear Protection of Engine Oils. In SAE Technical Papers; SAE International: Warrendale, PA, USA, 2007.
- Sentanuhady, J.; Majid, A.I.; Prasidha, W.; Saputro, W.; Gunawan, N.P.; Raditya, T.Y.; Muflikhun, M.A. Analisis Pengaruh Biodiesel B20 Dan B100 Terhadap Degradasi Viskositas Dan Total Base Number Minyak Pelumas Pada Mesin Diesel Yang Beroperasi Dalam Jangka Panjang Dengan Metode ASTM D2896 Dan ASTM D445-06. TEKNIK 2020, 41, 269–274.
- Cuerva, M.P.; Gonçalves, A.C.; Albuquerque, M.d.C.F.d.; Chavarette, F.R.; Outa, R.; de Almeida, E.F. Analysis of the Influence of Contamination in Lubricant by Biodiesel in a Pin-On-Disk Equipment. Mater. Res. 2022, 25, e20210375.
- Dandu, M.S.R.; Nanthagopal, K. Tribological aspects of biofuels—A review. Fuel 2019, 258, 116066.
More