Global oil and gas resources are declining continuously, and sustainable development has become a common challenge worldwide. In terms of environmental protection and economic benefits, the application of microemulsions for enhanced oil recovery often requires fewer chemical agents, showing distinct advantages.
- middle-phase microemulsion
- enhanced oil recovery
- microscopic mechanism
- field tests
1. Introduction
2. Formation Mechanisms of Microemulsions
2.1. Interface Adsorption Film Theory
Schulman and Prince, based on the concept of a third phase [28][18], proposed that the interfacial film is an intermediate phase located between the water phase and the oil phase. Furthermore, the curvature direction of this film depends on the relative magnitudes of different interfacial tensions. In a microemulsion system, this interfacial film is composed of surfactants and co-surfactants, and based on different component ratios, it can bend towards either the water phase or the oil phase, resulting in the formation of either O/W-type or W/O-type microemulsions. When the interfacial tension γos between the interfacial film and the oil phase is greater than the interfacial tension γws between the interfacial film and the water phase, the interfacial film curves towards the oil phase, giving rise to O/W-type microemulsions. Conversely, when γos is less than γws, the interfacial film bends towards the water phase, leading to the formation of W/O-type microemulsions.2.2. Instantaneous Negative Interfacial Tension Theory
As the number of active components in the system increases, more molecules may adsorb and mix at the interface, resulting in a continuous reduction in interfacial tension (IFT) [29][19]. Therefore, under the combined action of surfactants and cosolvents, ultra-low or instantaneous negative interfacial tension (IFT < 0) may be produced. The interfacial tension can approach zero with positive or negative values; the relaxation of the colloidal suspension towards a uniform fluid takes place via an interface of low or of high curvature [30,31][20][21]. This spontaneous interface expansion promotes the formation of microemulsions. However, this theory has some limitations. Firstly, negative tensions are not measurable, making this theory suitable only for theoretical research. Secondly, this theory only explains the formation mechanism of middle-phase microemulsions and cannot account for the formation of O/W and W/O microemulsions. Thus, further validation is still required.2.3. R Ratio Theory
The R ratio theory is primarily used to investigate the interactions between molecules. As surfactants are amphiphilic molecules, their interactions with both oil and water determine the preferred bending direction of the interfacial film. The R ratio reflects the strength of the hydrophobic and hydrophilic properties of the interfacial layer and the variations in microemulsion structure. Researchers can adjust the R value by increasing the branching of hydrophobic groups or the chain length in surfactants [32][22]. The cohesive energy ratio R can describe the relationships mentioned above through Equation (1):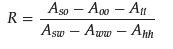 where Aso and Asw represent the binding energies between the oil/water and surfactant, respectively. Aoo and Aww are the cohesive energies of oil and water; Aii stands for the binding energy of the surfactant’s hydrophobic group; and Ahh denotes the binding energy of the surfactant’s head group.
where Aso and Asw represent the binding energies between the oil/water and surfactant, respectively. Aoo and Aww are the cohesive energies of oil and water; Aii stands for the binding energy of the surfactant’s hydrophobic group; and Ahh denotes the binding energy of the surfactant’s head group.
2.4. Hydrophilic–Lipophilic Difference Theory
Researchers initially proposed the hydrophilic–lipophilic balance (HLB) theory to prepare microemulsions by controlling different temperatures (PIT) and different component contents (PIC). Lin proposed the phase inversion composition (PIC) method or the emulsion inversion point method [33][23]. However, this method cannot control the major parameters affecting microemulsion behavior, such as salinity, oil properties, pressure, and surfactant and co-surfactant parameters. Subsequently, Salager put forward the hydrophilic–lipophilic difference (HLD) theory and the HLD equation to describe microemulsion systems. In contrast with HLB values, which only consider formulation and equilibrium, the HLD theory reflects the Winsor model but with significantly different formulaic forms for various types of surfactants. Negative, positive, and zero values of HLD represent Type I, Type II, and Type III microemulsion systems, respectively. Anionic surfactants, which are the most commonly used for enhanced oil recovery, have the HLD equation as shown in Equation (2) [34][24].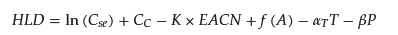 where 𝐶𝑠𝑒 represents salinity, 𝐶𝑐 and K are surfactant properties, EACN represents the oil equivalent alkane number, 𝛽 represents the ethanol constant, T and 𝛼𝑇 represent temperature constants, and P represents the pressure constant.
Since there are many parameters and they are not easy to obtain, scholars often use the net average curvature equation (HLD-NAC) to simplify them. Jin [39][25] proposed HLD-NAC equation modeling with a non-iterative algorithm. By modeling the solubilization ratio curves and phase volume fractions of different microemulsion systems, the HLD values can still be calculated using the natural logarithm of salinity for optimal salinity in the absence of information on surfactant characteristic curvature.
where 𝐶𝑠𝑒 represents salinity, 𝐶𝑐 and K are surfactant properties, EACN represents the oil equivalent alkane number, 𝛽 represents the ethanol constant, T and 𝛼𝑇 represent temperature constants, and P represents the pressure constant.
Since there are many parameters and they are not easy to obtain, scholars often use the net average curvature equation (HLD-NAC) to simplify them. Jin [39][25] proposed HLD-NAC equation modeling with a non-iterative algorithm. By modeling the solubilization ratio curves and phase volume fractions of different microemulsion systems, the HLD values can still be calculated using the natural logarithm of salinity for optimal salinity in the absence of information on surfactant characteristic curvature.
3. Phase Behaviors of Microemulsions
Phase behavior control is one of the crucial parameters in the preparation of microemulsions. Traditional phase behavior experiments involving salt scans to find the optimal middle-phase system are time-consuming, especially since different surfactant concentrations may result in various salt concentrations for achieving the best middle-phase system. When using the HLD equation modeling approach, parametric design lacks intuitive expression. Therefore, there is a need for a more straightforward and intuitive method to construct models that quantitatively describe the formation regions and stability of microemulsions, optimize microemulsion formulations, and define the formation boundaries and stability limits of microemulsions. Additionally, phase diagrams can be utilized to explore changes in the properties and interactions of microemulsions. By studying the phase behaviors of microemulsions under different conditions, such as phase transitions and phase separations, researchers can gain insights into the stability mechanisms, interfacial properties, and interaction patterns of microemulsions.
3.1. Winsor Phase Diagrams
Winsor phase diagrams are typically employed to depict changes in the phase behavior of a system. These diagrams illustrate the volume or volume fraction of each phase, allowing for the determination of the width of the alcohol region and the salt region. Winsor phase diagrams can aid in selecting the most suitable salt for a given system. For example, Yao [41][26] compared the ranking of different emulsification degrees of three petroleum sulfonates and found that the solubilization of the middle phase is the best when describing the hydrophilic–lipophilic balance with salt region width. Yin [42][27] used the Winsor phase diagram approach to study the influence of NaCl and n-butanol dosage on the phase behavior of dodecyl dimethyl betaine/n-butanol/n-hexane/NaCl microemulsion systems, as well as the effects of changes in phase volumes on the solubilization ability and interfacial tension of the microemulsions, ultimately determining that the NaCl salt width for the middle-phase microemulsions is 3.75%. By using Winsor phase diagrams, Luo [43,44][28][29] discovered that as the carbon chain of the alcohol increases, the required NaCl concentration for the formation and disappearance of middle-phase microemulsions also increases. In this context, the salt width and optimal salt concentration both decrease.3.2. Fish-like Diagrams
The ε-β fish-like diagrams can compensate for some of the shortcomings of Winsor phase diagrams, providing information about the composition and solubilization capability of the system when middle-phase and single-phase microemulsions begin to form. In this context, β represents the mass fraction of surfactant in the total composition, and ε represents the mass fraction of cosurfactant in the total composition. One of its advantages is that when the system contains cosurfactants such as alcohols, it can efficiently screen alcohols. Yang [46][30] investigated the effects of different oil and water media on phase behavior, interfacial layer composition, and solubilization capability. The results showed that oils with smaller molecular volumes enhanced the solubilization capability. By using the ε-β diagrams, Zhang [47][31] studied the influence of different XSDS values (molar fraction of SDS in a mixture of CTAB and SDS) and screened for microemulsion systems with lower alcohol solubility at the interface but higher solubilization capability than microemulsion systems containing only a single surfactant SDS or CTAB. By using the ε-β diagrams, Hou [48][32] investigated the solubilization effects of non-aqueous liquids, including trichloroethylene, carbon tetrachloride, 1,2-dichloroethane, and tetrachloroethylene, in microemulsions containing a mixed surfactant, 1-dodecyl-3-methylimidazolium bromide, and sodium dodecyl sulfate.3.3. Quasi-Ternary Phase Diagrams
The quasi-ternary phase diagram illustrates the changes in microemulsion phases with various components. It allows for a more detailed prediction of the formation process of middle-phase microemulsions and enables the precise identification of the optimal positions for their formation. A quasi-ternary phase diagram typically includes a surfactant, oil, water and salt, as well as alcohol as a cosurfactant. One corner of the phase diagram often represents the surfactant/cosurfactant or the surfactant/salt [49][33].4. Oil Displacement Mechanisms of Middle-Phase Microemulsions
4.1. Tension
An alkali can react with organic acids naturally existing in crude oil to form soap surfactants. These soap surfactants work synergistically with added surfactants to achieve ultra-low interfacial tension (IFT) [58,59][34][35]. This process increases the number of capillary tubes and reduces the capillary pressure, allowing the water phase to mobilize the remaining oil and thereby improving oil recovery [60,61,62][36][37][38]. The Winsor III-type middle-phase microemulsions are the ideal condition for enhancing oil recovery. Due to the ultra-low IFT, these microemulsions can produce mixed-phase solubilization and expand the sweep coefficient. Different types of microemulsions can maintain low IFT, with Winsor I type microemulsions still having very low IFT values [63][39]. In spontaneous emulsification experiments, both -II-type and +II-type microemulsions display strong emulsification capabilities and low IFT [64][40]. The addition of alkalis can lower the IFT of surfactants to the range of 10−3 to 10−4 mN/m [65,66][41][42]. Therefore, interfacial tension testing is a necessary step in screening middle-phase microemulsion EOR systems.4.2. Microscopic Mechanism
When IFT is extremely low, since the dynamic contact angle increases with an increase in the number of capillary tubes, there are noticeable differences in microscopic displacement dynamics [67][43]. Therefore, researchers often use custom-made glass etching models for microfluidic chip experiments. These chips are used to create a single-layer flow model on a glass slide, allowing for microscopic visualization. Microfluidic chips, with their precise channel structures and control capabilities, can simulate rock pore structures. By accurately controlling fluid injection and displacement processes, they enable researchers to observe fluid movement and interactions within rock models. This experimental method helps researchers gain a deeper understanding of fluid behavior within rock pores, reveals flow mechanisms and displacement effects, and provides guidance at the microscopic level for optimizing oil recovery processes [68,69,70,71][44][45][46][47]. Through glass etching model experiments, fluid flow and interface changes within the rock model can be intuitively observed. In research on using microemulsions for enhanced oil recovery, researchers need to observe the process of emulsion formation and study how to achieve separation and oil mobilization after emulsion formation, as well as analyze the type of residual oil.4.3. CT
Micro-CT scanning is an advanced technique for characterizing and observing microemulsions for enhanced oil recovery. It can be used to measure physical properties and perform non-destructive scanning to determine the distribution of oil and water phases and the position of phase interfaces. Additionally, it helps in calculating and analyzing saturation, tracking phase transition processes, studying phase transition mechanisms, visualizing flow channels, revealing the permeability distribution of microemulsions in reservoirs, evaluating displacement effects, and analyzing displacement extent and zoning effects. This technology deeply aids in understanding and optimizing the oil displacement process of microemulsions. Typically, normal heptane and iodododecane are used as emulsifying contrast agents, and the addition of sodium iodide can enhance image contrast during CT scanning. A three-dimensional structure of porous media is reconstructed from an image stack, which allows for measuring average porosity and pore throat connectivity [53][48]. CT technology can also be used to characterize the miscible behavior of middle-phase microemulsions. She [76][49] observed the dispersion of solvent-type emulsions into oil, while the oil was simultaneously dissolved into the emulsions. This shows that although there is no obvious interface between the emulsions and the oil phase, there is a component gradient in the three-dimensional pore space, demonstrating the elimination of capillary forces during the oil displacement process. During the displacement process of the middle-phase microemulsion system, CT technology can be used to observe the distribution and changes in the content of each component, as well as the locations and advancing process of emulsification. In the study conducted by She [76][49], from images taken at 1.0–3.0 PV in oil-wet cores, it can be observed that the color of the oil on top changes from green to dark red, indicating that trapped residual oil gradually dissolves into the emulsified phase. The gradient change in oil color suggests that under the influence of viscous forces, emulsions disperse into the oil phase, leading to miscible displacement behavior.5. Application of Middle-Phase Microemulsions in EOR
5.1. Development of Microemulsion EOR
In the early stages when Winsor proposed the three models of microemulsions, researchers typically used organically synthesized alkyl/aryl sulfonates to prepare microemulsions [5,78,79,80][5][50][51][52]. Large oil companies like Marathon [81][53], Shell [82][54], and Exxon [83][55] applied these microemulsions in field tests, with them achieving significant improvements in crude oil recovery rates. Currently, microemulsion-based enhanced oil recovery (microemulsion EOR) technology is advancing in multiple directions. These include exploring the formation and action mechanisms of middle-phase microemulsions, designing surfactant molecular groups, and carrying out simulation and prediction through mathematical models. The purpose of these studies is to explain and design microemulsions from a microscopic scale and reveal how microemulsions mobilize crude oil during the oil displacement process from a smaller scale. Through studying the phase behaviors of microemulsions, researchers have developed mathematical models that can predict the optimal phase behavior of microemulsions for given surfactant and cosurfactant molecular structures, as well as oil phase types [84,85,86,87,88][56][57][58][59][60].5.2. Practical Application of Microemulsion EOR
In recent years, great progress has been made in the indoor research and practical applications of middle-phase microemulsions. Researchers have improved the system for their respective target oil reservoirs, achieving considerable success [52,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77]. In general, both direct injection and in situ methods are preferred choices for indoor research. In practical applications, however, considering the transition from SP to ASP flooding and the impact of economic benefits, the in situ method is still favored. In the injection way of the oil drive system, the negative salinity gradient method is becoming more and more popular, which can effectively reduce the adsorption of the surface activator by the reservoir, prolong the action time of middle-phase microemulsion, and expand the action range of the slug. The surfactant concentration in the aqueous effluent was intermediate when a negative salinity gradient was applied. This implied that the formulation was closer to hydrophobic–hydrophilic balance during surfactant propagation in the reservoir which prohibited excessive partition of the surfactant in either the oil phase or the aqueous phase. The concentration and type of surfactant and the amount of surfactant used tends to decrease in the displacing system. The selection of environmentally friendly anionic and non-ionic surfactants tends to increase in surfactant types. Lignin-type surfactants are beneficial to environmental sustainability. In the selection of additives for surfactants, researchers tend not to use additives or use low flash point alcohols as surface activators, which is conducive to enhancing the fluidity of the interface film and ensuring the safety of the production site. In terms of injection pressure, researchers have found that injection pressure, capillary force, and chemical osmotic pressure are mechanisms for pressure transmission. On the one hand, microemulsions have smaller particle sizes of 80–100 nm due to their low IFT, which makes it easier for them to enter smaller pore throats compared to conventional microemulsions. On the other hand, the ultra-low IFT and the wettability inversion cause the crude oil on the rock surface to roll up so that the oil was easier to separate from the rock surface in a low permeability reservoir in the field [14,110][14][78].6. Conclusions
- (1)
-
As a low-concentration surfactant chemical flooding technique, middle-phase microemulsions offer significant advantages in terms of low dosage and high effectiveness. This is mainly reflected in three aspects of mechanism, namely, the expansion of two additional types based on the Winsor model, the quantitative modeling of the HLD equation to control different parameters for various types of surfactants, and the experimental methods linking quasi-ternary phase diagrams with the HLD equation and multiple phase diagrams. These improvements make phase diagrams more intuitive and more efficient for the screening of middle-phase microemulsion systems, which can then guide quantitative parameter-based studies on middle-phase emulsions as the next step.
- (2)
-
Middle-phase microemulsion systems are environmentally friendly for enhanced oil recovery, as the use of mildly alkaline chemicals in these systems poses minimal harm to reservoirs. The application of middle-phase microemulsion flooding in practice offers enhanced safety and reliability. Microscopic mechanism studies and CT technology enable quantitative and visual representation of wettability reversal, capillary pressure reduction, and emulsification, providing strong support for the efficacy of middle-phase microemulsion flooding.
- (3)
-
Middle-phase microemulsion flooding systems exhibit a wide range of applications, particularly in more complex reservoirs. In low-permeability tight reservoirs characterized by low permeability, low porosity, and poor reservoir properties, middle-phase microemulsion flooding systems can control the profile, emulsify, and reduce interfacial tension. For high-temperature, high-salinity reservoirs, middle-phase microemulsion flooding systems can enhance permeability, inhibit the formation of salt and mineral scales in the oil layer, stabilize and disperse oil droplets, and maintain stability under high-temperature, high-salinity conditions, and they can be continuously used in enhancing oil recovery.
References
- Nelson, R.C.; Pope, G.A. Phase Relationships in Chemical Flooding. Soc. Pet. Eng. J. 1978, 18, 325–338.
- Wasan, D.T.; McNamara, J.J.; Shah, S.M.; Sampath, K.; Aderangi, N. The Role of Coalescence Phenomena and Interfacial Rheological Properties in Enhanced Oil Recovery: An Overview. J. Rheol. 1979, 23, 181–207.
- Doscher, T.M.; Wise, F.A. Enhanced Crude Oil Recovery Potential-An Estimate. J. Pet. Technol. 1976, 28, 575–585.
- Atanase, L.I.; Riess, G. Block Copolymer Stabilized Nonaqueous Biocompatible Sub-Micron Emulsions for Topical Applications. Int. J. Pharm. 2013, 448, 339–345.
- Gogarty, W.B. Oil Recovery with Surfactants: History and a Current Appraisal, in Improved Oil Recovery by Surfactant and Polymer Flooding. Acad. Press 1977, 37, 27–54.
- Pokhriyal, N.K.; Devi, S. Effect of Water Solubility of Monomer on Reaction Kinetics Oil-Water Microemulsion Copolymerisation. Eur. Polym. J. 2000, 36, 333–343.
- Shinoda, K.; Friberc, S. Microemulsions: Colloidal aspects. Adv. Colloid Interface Sci. 1975, 4, 281–300.
- Giang, H.; Shlomovitz, R.; Schick, M. Microemulsions, Modulated Phases and Macroscopic Phase Separation: A Unified Picture of Rafts. Essays Biochem. 2015, 57, 21–32.
- Pei, H.; Zhang, G.; Ge, J.; Jin, L. The Effect of Oil Viscosity, Permeability, and Residual Oil Saturation on the Performance of Alkaline Flooding in the Recovery of Heavy Oil. Energy Sources Part A Recovery Util. Environ. Eff. 2012, 34, 702–710.
- Liu, D.; Zhao, Y.; Zhang, Y.; Wang, Z. The Key to Surfactant-Free Microemulsion Demulsification: CO2 Promotes the Transfer of Amphiphilic Solvent to Aqueous Phase. J. Mol. Liq. 2022, 345, 117000.
- Sottmann, T.; Strey, R. Ultralow Interfacial Tensions in Water–n-Alkane–Surfactant Systems. J. Chem. Phys. 1997, 106, 8606–8615.
- Roger, K.; Cabane, B.; Olsson, U. Formation of 10−100 Nm Size-Controlled Emulsions through a Sub-PIT Cycle. Langmuir 2010, 26, 3860–3867.
- Ahmed, S.; Elraies, K.A. Microemulsion in Enhanced Oil Recovery. In Science and Technology Behind Nanoemulsions; Karakuş, S., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-570-8.
- Liu, D.; Xu, J.; Zhao, H.; Zhang, X.; Zhou, H.; Wu, D.; Liu, Y.; Yu, P.; Xu, Z.; Kang, W.; et al. Nanoemulsions Stabilized by Anionic and Non-Ionic Surfactants for Enhanced Oil Recovery in Ultra-Low Permeability Reservoirs: Performance Evaluation and Mechanism Study. Colloids Surf. Physicochem. Eng. Asp. 2022, 637, 128235.
- Wang, Z.; Pang, R.; Le, X.; Peng, Z.; Hu, Z.; Wang, X. Survey on Injection–Production Status and Optimized Surface Process of ASP Flooding in Industrial Pilot Area. J. Pet. Sci. Eng. 2013, 111, 178–183.
- Bourrel, M.; Schechter, R.S. Microemulsions and Related Systems: Formulation, Solvency, and Physical Properties; Surfactant Science Series; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 1988.
- Rousseau, D.; Le Gallo, C.; Wartenberg, N.; Courtaud, T. Mobility of Microemulsions: A New Method to Improve Understanding and Performances of Surfactant EOR. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 18 April 2022; p. D021S013R001.
- Schulman, J.H.; Stoeckenius, W.; Prince, L.M. Mechanism of Formation and Structure of Micro Emulsions by Electron Microscopy. J. Phys. Chem. 1959, 63, 1677–1680.
- Robbins, M. Microemulsions—Theory and Practice. Abstr. Pap. Am. Chem. Soc. 1976, 172, 48.
- Ostrovsky, M.; Good, R. Mass-Transfer And Dynamic Liquid-Liquid Interfacial-Tension. 3. Theory of Nonequilibrium Pressure Coefficient of Interfacial-Tension. J. Colloid Interface Sci. 1985, 106, 140–145.
- Bier, M. Nonequilibrium Interfacial Tension during Relaxation-All Databases. Phys. Rev. E 2015, 92, 042128.
- Surfactant Science Series Vol. 39. Interfacial Phenomena in Biological Systems—All Databases. Available online: https://www.webofscience.com/wos/alldb/full-record/BIOSIS:PREV199243035081 (accessed on 18 September 2023).
- Lin, T.; Kurihara, H.; Ohta, H. Effects of Phase Inversion and Surfactant Location on Formation of O-W Emulsions. J. Soc. Cosmet. Chem. 1975, 26, 121–139.
- Salager, J.L.; Morgan, J.C.; Schechter, R.S.; Wade, W.H.; Vasquez, E. Optimum Formulation of Surfactant/Water/Oil Systems for Minimum Interfacial Tension or Phase Behavior. Soc. Pet. Eng. J. 1979, 19, 107–115.
- Jin, L.; Jamili, A.; Li, Z.; Lu, J.; Luo, H.; Ben Shiau, B.J.; Delshad, M.; Harwell, J.H. Physics Based HLD–NAC Phase Behavior Model for Surfactant/Crude Oil/Brine Systems. J. Pet. Sci. Eng. 2015, 136, 68–77.
- Yao, T.; Li, J. An Experiments Study on Chenmical Flood for Heavy Oil Reservoirs-All Databases. Oilfield Chem. 2010, 27, 84–87+42.
- Yin, D.; Yang, K.; Huang, K. Effect of Salt and Alcohol on the Displacement Performance of Dodecyl Dimethyl Betaine Microemulsion System Using Winsor Phase Diagram Method-All Databases. Oilfield Chem. 2018, 35, 119–124+130.
- Luo, M.; Si, X.; Yang, Z.; Gong, J. Preparation and Performance of Microemulsion for Corrosion Inhibition of Coiled Tubing-All Databases. Fine Chem. 2018, 35, 1758–1764.
- Luo, M.; Liu, J.; Wen, Q.; Liu, H.; Jia, Z. Fracturing Cleanup Effectiveness Improved by Environment-Friendly MES Middle Phase Microemulsion-All Databases. Acta Pet. Sin. Pet. Process. Sect. 2011, 27, 454–460.
- Yang, X.; Zhang, H.; Shi, C.; Chai, J. Middle-Phase Microemulsions Formed by n -Dodecyl Polyglucoside and Lauric-N-Methylglucamide. J. Dispers. Sci. Technol. 2013, 34, 147–152.
- Zhang, Y.; Zhang, X.Y.; Chai, J.L.; Cui, X.C.; Pan, J.; Song, J.W.; Sun, B.; Lu, J.J. The Phase Behavior and Solubilization of Isopropyl Myristate in Microemulsions Containing Hexadecyl Trimethyl Ammonium Bromide and Sodium Dodecyl Sulfate. J. Mol. Liq. 2017, 244, 262–268.
- Hou, N.; Chai, J.-L.; Zhang, J.-Q.; Song, J.-W.; Zhang, Y.; Lu, J.-J. Application of ε-β Fishlike Phase Diagrams on the Microemulsion Solubilizations of Dense Nonaqueous Phase Liquids. Fluid Phase Equilibria 2016, 412, 211–217.
- Javanbakht, G.; Arshadi, M.; Qin, T.; Goual, L. Micro-Scale Displacement of NAPL by Surfactant and Microemulsion in Heterogeneous Porous Media. Adv. Water Resour. 2017, 105, 173–187.
- Al-Sahhaf, T.; Ahmed, A.S.; Elkamel, A. Producing Ultralow Interfacial Tension at the Oil/Water Interface. Pet. Sci. Technol. 2002, 20, 773–788.
- Sun, Q.; Zhou, Z.-H.; Zhang, Q.; Zhang, F.; Ma, G.-Y.; Zhang, L.; Zhang, L. Effect of Electrolyte on Synergism for Reducing Interfacial Tension between Betaine and Petroleum Sulfonate. Energy Fuels 2020, 34, 3188–3198.
- Olajire, A.A. Review of ASP EOR (Alkaline Surfactant Polymer Enhanced Oil Recovery) Technology in the Petroleum Industry: Prospects and Challenges. Energy 2014, 77, 963–982.
- Baek, K.H.; Liu, M.; Argüelles-Vivas, F.J.; Abeykoon, G.A.; Okuno, R. The Effect of Surfactant Partition Coefficient and Interfacial Tension on Oil Displacement in Low-Tension Polymer Flooding. J. Pet. Sci. Eng. 2022, 214, 110487.
- He, W.; Ge, J.; Zhang, G.; Jiang, P.; Jin, L. Effects of Extended Surfactant Structure on the Interfacial Tension and Optimal Salinity of Dilute Solutions. ACS Omega 2019, 4, 12410–12417.
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery Theory and Practice Preface; Gulf Publishing Company: Houston, TX, USA, 2011; p. XIII-+. ISBN 978-0-08-096163-7.
- Afolabi, F.; Mahmood, S.M.; Sharifigaliuk, H.; Bin Kamarozaman, M.I.H.; Mansor, F.N.N.B.M. Investigations on the Enhanced Oil Recovery Capacity of Novel Bio-Based Polymeric Surfactants. J. Mol. Liq. 2022, 368, 120813.
- Latief, F.W.; Setiati, R.; Mardiana, D.A. The Effect of Alkali Type on IFT Value for Surfactant-Alkali Injection. In Proceedings of the 5th Annual Applied Science and Engineering Conference (AASEC 2020), Online, 21–22 April 2020; IoP Publishing Ltd.: Bristol, UK, 2021; Volume 1098, p. 062032.
- Liu, M.; Fang, H.; Jin, Z.; Xu, Z.; Zhang, L.; Zhang, L.; Zhao, S. Interfacial Tensions of Ethoxylated Fatty Acid Methyl Ester Solutions Against Crude Oil. J. Surfactants Deterg. 2017, 20, 961–967.
- Hematpur, H.; Abdollahi, R.; Safari-Beidokhti, M.; Esfandyari, H. Experimental Microemulsion Flooding Study to Increase Low Viscosity Oil Recovery Using Glass Micromodel. Math. Probl. Eng. 2021, 2021, 5021868.
- Bao, B.; Shi, J.; Feng, J. Research Progress of Surfactant Enhanced Oil Recovery Based on Microfluidics Technology. Acta Pet. Sin. 2022, 43, 432–442+452.
- Lapteva, M.; Kalia, Y.N. Microstructured Bicontinuous Phase Formulations: Their Characterization and Application in Dermal and Transdermal Drug Delivery. Expert Opin. Drug Deliv. 2013, 10, 1043–1059.
- Bao, B.; Zhao, S. A Review of Experimental Nanofluidic Studies on Shale Fluid Phase and Transport Behaviors. J. Nat. Gas Sci. Eng. 2021, 86, 103745.
- Zhou, Y.; Yin, D.; Wang, D.; Zhang, C.; Yang, Z. Experiment Investigation of Microemulsion Enhanced Oil Recovery in Low Permeability Reservoir. J. Mater. Res. Technol. 2020, 9, 8306–8313.
- Acosta, E.J.; Kiran, S.K.; Hammond, C.E. The HLD-NAC Model for Extended Surfactant Microemulsions. J. Surfactants Deterg. 2012, 15, 495–504.
- She, Y.; Wang, W.; Hu, Y.; Mahardika, M.A.; Nasir, M.; Zhang, C.; Patmonoaji, A.; Matsushita, S.; Suekane, T. Pore-Scale Investigation on Microemulsion-Based Quasi-Miscible Flooding for EOR in Water-Wet/Oil-Wet Reservoirs: A 3D Study by X-Ray Microtomography. J. Pet. Sci. Eng. 2022, 216, 110788.
- Taber, J. Dynamic and Static Forces Required to Remove a Discontinuous Oil Phase from Porous Media Containing Both Oil and Water. Soc. Pet. Eng. J. 1969, 9, 3–12.
- Holm, L.W. Soluble Oils for Improved Oil Recovery, in Improved Oil Recovery by Surfactant and Polymer Flooding; Academic Press: Cambridge, MA, USA, 1977; pp. 453–485.
- Hirasaki, G.J. Application of the Theory of Multicomponent, Multiphase Displacement to Three-Component, Two-Phase Surfactant Flooding. Soc. Pet. Eng. J. 1981, 21, 191–204.
- Dreher, K.D.; Shoppman, T.D. Separation of Oil and Water Produced by Micellar-Solution/ Polymer Flooding. J. Pet. Technol. 1985, 37, 1459–1465.
- Corsano, A. Field Test of an Aqueous Surfactant System For Oil Recovery, Benton Field, Illinois. J. Pet. Technol. 1973, 25, 195–204.
- Glover, C.J.; Puerto, M.C.; Maerker, J.M.; Sandvik, E.L. Surfactant Phase Behavior and Retention in Porous Media. Soc. Pet. Eng. J. 1979, 19, 183–193.
- Shi, P.; Luo, H.; Tan, X.; Lu, Y.; Zhang, H.; Yang, X. Molecular Dynamics Simulation Study of Adsorption of Anionic-Nonionic Surfactants at Oil/Water Interfaces. RSC Adv. 2022, 12, 27330–27343.
- Witthayapanyanon, A.; Phan, T.T.; Heitmann, T.C.; Harwell, J.H.; Sabatini, D.A. Interfacial Properties of Extended-Surfactant-Based Microemulsions and Related Macroemulsions. J. Surfactants Deterg. 2010, 13, 127–134.
- Sheng, J.J. A Comprehensive Review of Alkaline-Surfactant-Polymer (ASP) Flooding: A Comprehensive Review of Asp Flooding. Asia-Pac. J. Chem. Eng. 2014, 9, 471–489.
- Stoll, W.M. Alkaline/Surfactant/Polymer Flood: From the Laboratory to the Field. SPE Res. Eval. Eng. 2011, 14, 702–712.
- Zhu, Y. Current Developments and Remaining Challenges of Chemical Flooding EOR Techniques in China. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11 August 2015; p. D021S012R003.
- Neuma De Castro Dantas, T.; Viana, F.F.; Thaise Costa De Souza, T.; Dantas Neto, A.A.; Aum, P.T.P. Study of Single-Phase Polymer-Alkaline-Microemulsion Flooding for Enhancing Oil Recovery in Sandstone Reservoirs. Fuel 2021, 302, 121176.
- Bardhan, S.; Kundu, K.; Saha, S.K.; Paul, B.K. Physicochemical Investigation of Mixed Surfactant Microemulsions: Water Solubilization, Thermodynamic Properties, Microstructure, and Dynamics. J. Colloid Interface Sci. 2013, 411, 152–161.
- Co, L.; Zhang, Z.; Ma, Q.; Watts, G.; Zhao, L.; Shuler, P.J.; Tang, Y. Evaluation of Functionalized Polymeric Surfactants for EOR Applications in the Illinois Basin. J. Pet. Sci. Eng. 2015, 134, 167–175.
- Nguele, R.; Sasaki, K.; Salim, H.S.-A.; Sugai, Y.; Widiatmojo, A.; Nakano, M. Microemulsion and Phase Behavior Properties of (Dimeric Ammonium Surfactant Salt—Heavy Crude Oil—Connate Water) System. J. Unconv. Oil Gas Resour. 2016, 14, 62–71.
- Chen, Z.; Han, X.; Kurnia, I.; Yu, J.; Zhang, G.; Li, L. Adoption of Phase Behavior Tests and Negative Salinity Gradient Concept to Optimize Daqing Oilfield Alkaline-Surfactant-Polymer Flooding. Fuel 2018, 232, 71–80.
- Ayirala, S.C.; Boqmi, A.; Alghamdi, A.; AlSofi, A. Dilute Surfactants for Wettability Alteration and Enhanced Oil Recovery in Carbonates. J. Mol. Liq. 2019, 285, 707–715.
- Han, X.; Kurnia, I.; Chen, Z.; Yu, J.; Zhang, G. Effect of Oil Reactivity on Salinity Profile Design during Alkaline-Surfactant-Polymer Flooding. Fuel 2019, 254, 115738.
- Li, J.; Niu, L.; Lu, X. Performance of ASP Compound Systems and Effects on Flooding Efficiency. J. Pet. Sci. Eng. 2019, 178, 1178–1193.
- Riswati, S.S.; Bae, W.; Park, C.; Permadi, A.K.; Efriza, I.; Min, B. Experimental Analysis to Design Optimum Phase Type and Salinity Gradient of Alkaline Surfactant Polymer Flooding at Low Saline Reservoir. J. Pet. Sci. Eng. 2019, 173, 1005–1019.
- Han, X.; Chen, Z.; Zhang, G.; Yu, J. Surfactant-Polymer Flooding Formulated with Commercial Surfactants and Enhanced by Negative Salinity Gradient. Fuel 2020, 274, 117874.
- Kurnia, I.; Zhang, G.; Han, X.; Yu, J. Zwitterionic-Anionic Surfactant Mixture for Chemical Enhanced Oil Recovery without Alkali. Fuel 2020, 259, 116236.
- Tianbo, L.; Xurong, Z.; Shuai, Y.; Jiawei, Z. Surfactant-EOR in Tight Oil Reservoirs: Current Status and a Systematic Surfactant Screening Method with Field Experiments-All Databases. J. Pet. Sci. Eng. 2021, 196, 108097.
- Slamet, P.; Ronny Windu, S.; Suherman, S. High-Performance Polymeric Surfactant of Sodium Lignosulfonate-Polyethylene Glycol 4000 (SLS-PEG) for Enhanced Oil Recovery (EOR) Process-All Databases. Period. Polytech. Chem. Eng. 2021, 66, 114–124.
- Sekerbayeva, A.; Pourafshary, P.; Hashmet, M.R. Application of Anionic Surfactant\engineered Water Hybrid EOR in Carbonate Formations: An Experimental Analysis-All Databases. Petroleum 2022, 8, 466–475.
- Panthi, K.; Mohanty, K.K. Chemical Flood with a Single Surfactant. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 18 April 2022; p. D021S013R002.
- Mariam, S.; Aida, S.; Peyman, P.; Muhammad, R.H. Optimization of Low Salinity Water/Surfactant Flooding Design for Oil-Wet Carbonate Reservoirs by Introducing a Negative Salinity Gradient-All Databases. Energies 2023, 15, 9400.
- Sumadi, P.; Yoga, R.; Ivan, K.; Oki, M. Synergy of Surfactant Mixtures and Fe3O4 Nanoparticles for Enhanced Oil Recovery (EOR)-All Databases. Inorg. Chem. Commun. 2023, 155, 111125.
- Zhang, Y.; Yi, Z.; Yang, Z.; Luyu, W. Experimental Study on Characteristics and Mechanisms of Matrix Pressure Transmission near the Fracture Surface during Post-Fracturing Shut-in in Tight Oil Reservoirs-All Databases. J. Pet. Sci. Eng. 2022, 219, 111133.
