Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Joao Pinto da Costa.
Although the impacts of plastic pollution have long been recognized, the presence, pervasiveness, and ecotoxicological consequences of microplastic—i.e., plastic particles < 5 mm—contamination have only been explored over the last decade. Far less focus has been attributed to the role of these materials and, particularly, microplastics, as vectors for a multitude of chemicals, including those (un)intentionally added to plastic products, but also organic pollutants already present in the environment.
- microplastics
- additives
- environment
- sorption mechanisms
1. Introduction
Owing to their plasticity, plastics may be molded, extruded, or pressed into solid objects with an endless multitude of shapes. Plastics, primarily derived from petrochemicals, are increasingly used in a variety of products due to their inherent properties such as affordability, water resistance, and resilience to chemicals, temperature, and light. They are particularly prevalent in packaging and single-use items. After usage, plastic waste might be gathered and sorted via official or unofficial waste management systems or by the manufacturers themselves. This waste can then be converted into plastic pellets or flakes, allowing them to rejoin the production cycle. However, a significant portion of these plastics are either incinerated or openly burned, leading to serious environmental and health consequences [1]; disposed of in landfills/dumpsites; or escape into the environment [2]. In fact, concurring estimates highlight that, considering all plastic products manufactured since the 1950s, less than 10% have been recycled or re-used, with subsequent economic and environmental consequences. However, environmental contamination with plastics occurs not only at the end-of-life of these materials, but throughout their life cycle. For example, virgin plastic resin pellets, commonly referred to as “nurdles”, disc-shaped plastic particles which are typically 3–5 mm in diameter and used as the raw material for the production of most thermally molded plastic products [3], are frequently lost during transport and are considered the second-largest source of ocean microplastics [4]. These are particles smaller than 5 mm, and, in the marine environment, although only accounting for 8% of the total mass of the estimated plastic waste, microplastics correspond to over 94% of the estimated 1.8 (1.1–3.6) trillion pieces floating in the area [5], and have been found to be ubiquitous in all spheres of the environment, namely, soil, water, and air, as well as in food [6].
However, plastics do not consist only of polymers. They also include additives that contribute to improved plastic functions that modulate chemical and physical properties. Every plastic item contains additives that determine the properties of the material and influence its cost of production [7], and, according to an estimate by van Oers and colleagues, any random piece of plastic collected in environmental samples will contain at least 20 different additives [8]. The global plastic additives market, in 2020, was valued at USD 48.41 billion, and, at an estimated average annual growth rate of 5.7%, it is projected to surpass the USD 75 billion mark in 2028 [9].
Plastics are composed of chains of polymers, and these different additives may be weakly bound to the polymers or mixed within the polymer matrix. Additionally, because of this weak linkage, additives may leach during use or when disposed of. Additionally, additives themselves, whether still associated with the plastic materials or following their leaching, could degrade and form other toxic compounds, and may persist and bioaccumulate in biota (e.g., [10]). Even when plastic waste is recycled, it is extremely difficult to remove plastic additives found in said waste due to their inherent chemical characteristics. Consequently, it is highly likely that these compounds will incorporated into the newly produced items [11]. This is aggravated by the fact that many industrial manufacturers are not fully transparent regarding the used additives and their concentrations and, consequently, a detailed view across the value chain of the chemical profile of the final products is not available, with concomitant health risks [7]. For example, brominated flame retardants have been inadvertently incorporated into recycled products, including household items [12] and toys [13], posing a significant health risk [14].
These health risks are not minimized when plastic waste is burned, which is particularly problematic in low-technology incinerators or under uncontrolled conditions. When combustion is incomplete, the process causes the emission of hazardous compounds, including persistent organic pollutants (POPs), namely, dioxins, as well as acid gases and ash [5].
2. Migration and Sorption
When present in plastics, chemicals added—whether intentionally or unintentionally—have the potential to migrate to the surrounding matrix, namely, food, water, and sediments, among others, as well as across the material itself, to its surface [21][15]. This migration may be intentional: for example, some mold release agents are added to be gradually and continuously released to the surface of numerous plastic products, which results in improved antistatic, mechanical, or optical properties (e.g., [22,23][16][17]) or prevents the oxidation of silver, yielding a longer shelf-life for foodstuff [24][18]. This is also the case in some medical applications, as the precisely controlled release of drug dosages may greatly improve the quality of life of patients. In most cases, however, and particularly in environmental settings, this observed release is uncontrolled and unplanned, potentially resulting in serious environmental consequences. The overall process of migration can be divided into four essential steps [21][15]:- (1)
-
diffusion through the polymer;
- (2)
-
desorption (from the polymer surface);
- (3)
-
sorption at the plastic–matrix interface and;
- (4)
-
absorption/dispersion in the matrix.
-
J is the diffusion flux. It refers to the amount of substance per unit area per unit time. This parameter measures the amount of substance that flows through a unit area during a unit time interval.
-
D is the diffusion coefficient. It is expressed in area per unit time.
-
φ is concentration, expressed as the amount of substance per unit volume.
-
x is position, the dimension of which is length.
2.1. Factors Affecting Sorption
2.1.1. Particle Size of (Micro)Plastics
The reduction in size of polymeric particles, as well as their irregularity in shape, is accompanied by an increase in their surface area-to-volume ratio which commonly results in an enhanced sorption capacity [33][25]. By determining the specific surface area through the Brunauer–Emmett–Teller (BET) method, these authors found that the BET area of three polymers—PE, PS, and PVC—showed a positive correlation with the sorption of pyrene. However, although shape and size may play a role in the adsorption process, these factors likely have a less important role in absorption, as the latter does not depend on the availability of sorption sites on the surface [34][26]. It should also be noted that, particularly for smaller particles, such as those commonly referred to nanoplastics (<1 μm in size) [35][27], aggregation phenomena could occur that may result in an effective reduction in the available surface area, as reported by [36][28], who noted that sorption behaviors were altered when microplastics were reduced to the nanoscale and attributed this lower sorption capacity to the aggregation of nano-sized microplastics. Moreover, the presence and size of pores on the surface of microplastics is of the utmost importance, as smaller pores enhance the interaction of the sorbate with the surface of the sorbent, leading to the formation of monolayer adsorption, while larger pores result in both mono- and multilayer adsorption [37][29].2.1.2. Crystallinity of (Micro)Plastics
Plastics and, by extension, smaller plastics (microplastics) are composed of amorphous and crystalline regions. Based on the extent of molecular chain alignment, these materials may be classified as either amorphous, semi-crystalline, or crystalline [29][23]. Therefore, the degree of crystallinity of a given polymer expresses the fraction of crystalline regions, or, in other words, the fraction in which the polymer chains are aligned with each other, and this parameter varies greatly among different polymers, ranging from just over 0% (e.g., atactic PS) to over 90% (e.g., some commercial PE microspheres [38][30]), although no polymer is 100% crystalline. A higher prevalence of amorphous regions will result in higher mobility and polymer accessibility, thus favoring the diffusion of the chemical’s molecules compared to crystalline regions, which require higher energies for their uptake [34][26]. For example, Yao and colleagues studied the sorption of dibutyl phthalate, a member of the phthalate acid ester (PAE) family, which is commonly used in plastic manufacturing to impart flexibility to a wide variety of plastic products [39][31], onto PE microplastics of different morphological characteristics and degrees of crystallinity [38][30]. Not only did they conclude that size was not a decisive factor, they also established a significant decrease in adsorption with increasing crystallinity (r2 = 0.98).2.1.3. Glass Transition Temperature of (Micro)Plastics
Closely associated with crystallinity, glass transition temperature (Tg) also affects MPs and chemical sorption processes. Simply put, Tg is the temperature value at which there is a transition in the amorphous region of a polymer, when heated, from a “glassy” to a “rubbery” state [33][25]. It is, in other words, “the macro-manifestation of a polymer chain’s flexibility” [40][32]. At lower temperatures, the amorphous regions of the polymer are in the so-called “glassy” state, and, as such, they are more rigid and only vibrate; however, when heated, some regions will transition to the noted “rubbery” state, and these segments will show higher flexibility, as molecules will have a greater degree of freedom to move and, therefore, absorption of chemicals in these regions will be enhanced [34][26] owing to higher accessibility to hydrophobic organic compounds. As noted in the previous section, the crystalline regions of the polymer will not favor sorption owing to the high energy needed to destabilize the strongly ordered polymer chains. Hence, it is possible to postulate that the effect of Tg will thus be mostly felt within the amorphous regions, which will be more susceptible to sorption processes, although this requires more detailed research.2.1.4. Functionalization and Cross-Linkage of (Micro)Plastics
Polymers exhibiting higher degrees of cross-linking, i.e., the presence of strong covalent bonds between polymeric chains, commonly show higher structural rigidity and therefore do not favor the internal diffusion of contaminants [29][23]. A typically cited example is that of vulcanized rubber with sulfur; the cross-linking results in an increased Tg, thus increasing the range of temperatures associated with the “glassy” state of the polymer [41][33], ultimately influencing the sorption process as discussed above. Similarly, the presence of functional groups may also affect their sorption behavior. For example, it has been demonstrated that highly aromatic PS exhibited a higher sorption affinity to polychlorinated biphenyl compounds, likely attributable to the hydrophobicity and π–π interactions when compared with PE [42][34]. Conversely, functional groups containing oxygen could also act as H-bond acceptors, thus interacting with water molecules, leading to the formation of water clusters on the surface of the polymeric materials. These three-dimensional water clusters may reduce the accessibility of contaminants to the sorption domains of plastics due to competition, yielding a de facto reduction in sorption affinities [33,43][25][35]. Hence, the type and extent of functionalization will affect the sorption process, though this will not, by itself, enhance or negatively affect the sorption behavior, and attention should be paid to these specific characteristics of the polymers.2.1.5. Surface Polarity of (Micro)Plastics
Carbon is the key constituent of the most commonly used plastics. Having four valence electrons, it achieves stability by sharing four more electrons, allowing it to form a wide range of covalent bonds. Carbon also catenates, forming strong bonds with itself [44][36]. In polymers, the existing functional groups, hydrophobicity, and presence of unsaturated bonds all contribute to the type and strength of the formed secondary bonds [28][22], and these parameters are essential for ascertaining and determining the interactions of the polymers with chemical compounds. Velzeboer and colleagues, for example, showed that planar PCBs exhibited show stronger surface adsorption due to their supposed ability to move closer to the sorption surface than the more bulky nonplanar congeners [45][37], although their findings were not consistent between the two studied matrices, which were freshwater and saltwater (in freshwater, the effects of planarity were inconsistently or not significantly different).2.1.6. Age and Degree of Weathering of (Micro)Plastics
When exposed to the elements, plastics undergo degradation and structural alterations that may lead not only to morphological changes, but also modifications at the level of surface area and polarity [29][23]. These alterations have been demonstrated to enhance the sorption of different compounds, including metals and organic contaminants. For example, Liu and colleagues showed that ciprofloxacin had an elevated sorption capacity towards UV-aged PS when compared to virgin plastic [46][38]. An identical observation was made for the adsorption of oxytetracycline on weathered PS by Zhang and colleagues [47][39]. The authors suggested that these effects may be due to the formation of oxygen-containing groups at the surface of these materials [48][40], as well as to the light-induced surface oxidation of plastics [28][22]. Aging may also result in reductions in the hydrophobicity of plastics, thereby favoring the sorption of hydrophilic contaminants. In fact, it has been shown that non-polar compounds were more likely to sorb onto weathered microplastics than those of polar nature, thus suggesting that weathered or aged plastics may exert a more toxic effect than virgin or pristine plastics due to the higher sorption potential of contaminants [49][41].2.1.7. Color of (Micro)Plastics
Though severely understudied, color constitutes a parameter that may also play a role in the sorption of contaminants by plastics. Frias and colleagues, for example, collected microplastics from two beaches and showed that black pellets had the highest concentrations of POPs, except for PAHs [50][42]. This could be due to the fact that colored and darker plastics typically contain more additives, which may enhance their sorption capacity [29][23], although the nature and type of additives used for obtaining darker colored plastics could also be a factor. Fisner and colleagues [51][43] also concluded that darker colors mirrored a higher concentration of PAHs, although the degree or darkness was associated with weathering. As such, the enhanced sorption capacity could be reflective of weathering and not color itself. This limited set of results highlights the current need to further explore this parameter and how it affects sorption capacity, if it does at all.2.1.8. Hydrophobicity and Planarity of the Sorbate
The properties of the sorbate (contaminant) also constitute a key factor in its uptake and release by microplastics, as reflected by the accumulation capacity and the equilibrium state. Although it has not been thoroughly studied, it has been demonstrated that hydrophobicity/hydrophilicity, surface charge, and the presence of functional groups in the sorbate do exert an effect on the overall sorption behavior. Among these, however, hydrophobicity is considered to be the most important, as the hydrophobic nature of most MPs’ surfaces renders these interactions the main driver of the sorption mechanism of numerous chemicals [28,29,52][22][23][44]. Accordingly, organic contaminants with high hydrophobicity tend to be more readily adsorbed by (micro)plastics [33][25]. In cases in which diffusion is the rate-limiting step of the sorption process, the molecular weight of the molecule should also be considered, as the lower both the molecular weight and the hydrophobicity of the compound (higher hydrophilicity) are, the faster the diffusion mass transfer until reaching equilibrium or steady-state conditions. This is because, in such cases—with diffusion as the rate-limiting process—the molecular weight of the sorbate becomes more relevant than hydrophobicity, as diffusion is more prominently hindered by the increase in the molecular size [34][26]. Planarity also plays a role in the kinetics of the sorption process. In fact, planar molecules, such as PAHs, show higher sorption coefficients when directly compared with other non-planar molecules of identical hydrophobicity. This is due to the fact that higher degrees of planarity will result in higher proximities towards the surface of the plastics, facilitating non-covalent π–π interactions between the sorbent and sorbate [30][24].2.1.9. Speciation/Ionization and Functionalization of the Sorbate
Chemical speciation is also a noteworthy aspect. Simply put, speciation depends on the acid dissociation constant (pKa) of the contaminant and the pH of the solution (pHsol), as these determine the isoelectric charge of the chemical. If pHsol > pKa, the contaminant will predominantly be in an ionized form [53][45]. For organic contaminants, namely, ionic compounds, different ionization states under different conditions could affect the sorption mechanism due to electrostatic interaction [33][25]. For example, when studying the partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) in commercial microplastics, Wang and co-workers showed that FOSA was adsorbed by PS but PFOS was not due to the electrostatic repulsion observed between PS and PFOS, as both were negatively charged [54][46]. Similarly, for metals, chemical speciation could be an important parameter for predicting their sorption behavior, as the process is partly driven by the formation of free cationic species and coordination complexes within the matrix [34][26]. Free cations subsequently have a higher likelihood of interacting with the negatively charged regions of microplastics, which can be generated through the adsorption of organic molecules [55][47] or even due to the presence of other plastic additives. The organo-metallic complexes, however, can interact with areas of the surface of the plastics showing a neutral charge due to hydrophobic interactions. The presence of functional groups in the contaminant/sorbate could also impact the degree and extent of sorption. For example, in an already mentioned study, Wang and colleagues described a higher sorption of FOSA than that of PFOS on PE, which could, at least partially, be ascribed to the presence of a sulfonamide group on FOSA [54][46]. Similarly, when assessing the sorption of three anti-inflammatory drugs to microplastics, Elizalde-Velázquez and colleagues showed that some exhibited an enhanced sorption, likely due to the presence of amino groups in their structure [56][48]. This likely stems from the fact that functional groups may attract the π electron(s) on the surface of plastics and therefore enhance π–π electrostatic interactions between polymers and chemicals.2.1.10. pH of the Medium
External factors of the surrounding medium play an active role in sorption processes, with numerous relevant characteristics and parameters. By far, however, the most studied of these is pH. As previously noted, the pH of the solution contributes to determining the charged state of the contaminants, which affects the sorption affinity through electrostatic interaction. For example, it has been described that the degree of sorption by commercially acquired virgin PS particles increased with increasing pH values when this was kept under the 5. The authors suggested that this was likely due to the fact that, within the studied range of pH values, PS was negatively charged and the sorbent was positively charged; however, the high concentration of H+ inhibited the sorption of the positively charged chemical, and, as such, sorption increased with a decrease in H+ concentration [46][38]. Subsequently, when the pH value was further increased, sorption quickly decreased as the positively charged chemical became negatively charged, resulting in an electrostatic repulsion between the sorbate and sorbent. Fred-Ahmadu and colleagues [28][22] summarized the role of pH as follows:- (i).
-
Electrostatic repulsion will increase with an increase in the solution’s pH, suppressing electrostatic interaction between differently charged sorbates and sorbents;
- (ii).
-
An increase in pH may favor the dissociation of the hydrophobic neutral sorbate molecules into negatively charged, hydrophilic species, resulting in a diminished hydrophobic interaction;
- (iii).
-
High pH may increase the π donor ability of sorbate, thus enhancing π–π interactions.
2.1.11. Salinity of the Medium
Salinity plays an especially important role in marine environments. Salts and, particularly, NaCl promote a “salting-out” effect that increases with molecular weight and decreases with compound polarity [29][23]. This results in a shift of equilibrium towards the organic/polymeric phase. In other words, the presence of salts in a solution where sorption may occur will induce alterations in the partition equilibrium of natural organic solutes towards non-aqueous phases and may end up increasing sorption to microplastics by decreasing the solubility of the chemical contaminant [34][26]. The effects of salinity can also manifest through the rate or extent of agglomeration of sorbent, namely, microplastics, inducing changes at the level of size and available surface area, causing a reduction in the sorption of contaminant ions. In cases in which electrostatic interactions are the predominant driving force, a competition between ion-exchangeable sites can take place, again yielding a de facto decrease in the sorption of chemical compounds [58][50]. However, other authors have found that a wide range of levels of salinity exerted little to no effect on the rate of sorption of different chemicals on a variety of plastics, as in the case of polybrominated diphenyl ethers with four microplastics (PE, PP, PA, and PS) [59][51], suggesting that the sorption mechanisms by microplastics may indeed be contaminant-specific, resulting in sorption processes that are not dependent on the salinity of the surrounding environment. On the other hand, ions available in the surrounding medium may compete with organic compounds for sorption sites on the surface of plastics, thus negatively affecting the sorption processes of these materials. This has been confirmed by Zhang and colleagues, who showed that increasing concentrations of CaCl2 or Na2SO4, for example, decreased the sorption of oxytetracycline in both virgin and weathered plastics [47][39], which was ascribed to the strong competition of Ca2+ and Na+ for cationic exchange sites at the surfaces of the tested microplastics. Similarly, by directly comparing the sorption affinity of PFAs on microplastics in both fresh and seawater, Llorca and co-workers [60][52] found sorption to be weaker in the latter, thus confirming the expected behaviors for these cases. These findings showcase the need to further expand research on the topic, as contamination of the environment and subsequent ecotoxicological implications will likely vary depending on the environmental compartment the contamination refers to. This is especially relevant for metals, namely, copper, lead, and cadmium [61][53], as the sorption mechanisms for these more directly vary depending upon the existing competition for binding sites between ions in solution and contaminants. Indeed, this appears to be the determining factor for the observed effects of ionic strength during sorption [28][22].2.1.12. (Dissolved) Organic Matter in the Medium
Organic matter (OM) is of special importance for metals, as it can react with them and affect the sorption process. Generally, this interaction yields neutral organo-metallic complexes which display higher degrees of hydrophobicity when compared to that of the free ions. Consequently, this favors sorption by the hydrophobic microplastics [33][25]. On the other hand, this complexation also contributes to a decrease in the concentration of the free ions in solution, which may result in a lower degree of sorption. As such, the overall process should be analyzed on a case-by-case basis, as it depends on the specificity of the (dissolved) organic matter, ions, and their concentrations, making difficult to determine or assume a “general” behavior of the sorption process when (D)OM is a present [34][26]. Nonetheless, the size of the plastics also likely plays a role. In fact, Chen and colleagues demonstrated that the interactions of DOM and plastics depended, among other factors, on the size of the particles [62][54] due to their effects on the stability of the dispersions. For organic chemicals, the presence of dissolved organic matter probably results in a decrease in their sorption to microplastics, as it likely binds the chemicals and competes with microplastics [60][52]. However, these effects will possibly vary with the polarity of the chemicals: non-polar chemicals have a higher affinity towards organic matter, resulting in a decreased sorption to plastics, but this influence is likely reduced in the case of polar chemicals. For cases in which absorption is the dominant process, the presence of DOM has been demonstrated to be of less significance owing to the fact that its effects are more pronounced at the surface of polymeric materials, especially non-porous ones, as there is no blockage of pores by the organic matter [63][55].2.1.13. Co-Existing Pollutants in the Medium
It is known that multiple contaminants and pollutants are found in the environment and, especially, in aquatic environments. As such, their co-existence could lead to competition for sorption sites on microplastics. Though not many studies have focused on this subject, Bakir and colleagues, for example, studied the sorption of DDT and phenanthrene by unplasticized PVC and ultra-high molecular weight polyethylene in seawater [64][56]. They found that DDT outcompeted phenanthrene for sorption onto plastic, thus illustrating an antagonistic effect. However, as noted by the authors, their work depicted a binary component process, and in the environment a multitude of such contaminants exist. As such, it is necessary to further study these interactions, focusing on an ever-increasing degree of complexity.2.1.14. Temperature of the Medium
The temperature of the medium can induce modifications of the polymers at the structural level, as higher temperatures may result in larger motions of segments of the polymer, thus favoring lower crystallinity, whose effects on the sorption process have been previously described. In spite of the expected role of this parameter in the uptake/release of contaminants by plastics, most of the sorption studies have been conducted at room temperature, except when the experimental design is specifically tailored to determine said effects [28][22]. Some studies have demonstrated that, generally, higher temperatures result in higher sorption rates, which could imply an increase in the number of active sites on the surfaces of the (micro)plastics [31][57]. For example, Lin and colleagues demonstrated that a slight increase in temperature translated into a small increase in the sorption of tetracycline by PS microplastics [65][58]; similarly, desorption of different bisphenols has been shown to increase with increasing temperatures [66][59]. However, broadly speaking, the effects of temperature of the matrix depends on the chemical’s microstructure, meaning that the sorption process varies depending on the degree of crystallinity (as discussed above) and that this, in turn, depends on the temperature. In other words, the sorption rates may increase with increasing temperatures, but decrease with increasing degrees of crystallinity, which increases with increasing temperature. Again, it is therefore likely that the behavior of sorbate–sorbent interactions will have to undergo analysis on a case-by-case basis owing to the inherently different characteristics of contaminants and polymers and their interaction with the environment.2.1.15. Confounding Factors
Multiple factors affect the uptake/release of chemical compounds by polymeric materials, as illustrated in Figure 1. However, how these affect the sorption behavior is, in some cases, insufficiently described and, even for those whose effects are understood, as is the case for weathering, often divergent and contrasting findings are reported, and predictive modelling becomes extremely difficult. For example, some authors have described microplastics found in riverine systems to be less susceptible to weathering phenomena than those found in marine systems [67][60], as the former are less subject to tidal flows and are beached less frequently and for shorter periods of time. Others, however, have claimed that microplastics found in rivers are more exposed to UV radiation, and therefore undergo more intense weathering [68][61].Soil and sediment matrices are highly complex media, and most studies focusing on assessing the sorption behavior of chemicals in the presence of (micro)plastics have been performed in aquatic environments. Nonetheless, both matrices are key reservoirs of chemical contaminants and microplastics [34][26]. Chen and colleagues studied the uptake and release of triclosan, a bacteriostatic agent widely used in numerous consumer products that is intended to reduce or prevent bacterial contamination, by soil and PS and PE particles [69][62]. Interestingly, these authors described different patterns of the release of triclosan, which were highest for soil, followed PE and PS microparticles. This suggests that the typical high rate of uptake of chemicals by plastics [29][23], subsequently accompanied by this lower rate of desorption, could constitute a potential risk for the transfer of pollutants from loaded microplastics to the surrounding environment, whether sediments, water, or even biota. Another potentially confounding factor is microbial activity. In fact, most plastics, and particularly microplastics, are prone to microbial colonization [70][63]. This includes colonization by bacteria, algae, and fungi, which may form a biofilm on the surface of these materials. As discussed by Rodrigues and colleagues [71][64], the presence of biofilms could result in a two-tiered effect on the sorption of contaminants: it may prevent the penetration of UV radiation, and, consequently, actively reduce the weathering of microplastics, with a concomitant decrease in sorption rates, as previously discussed; however, it may also induce higher rates of sorption through modifications of the plastics’ surface morphology. Hence, the overall effect of biofilm formation might depend upon the protective role it confers to the microplastics against weathering, which, in turn, may depend on the type of organism, as well as on the putative alterations these materials undergo in response to the presence of said biofilm.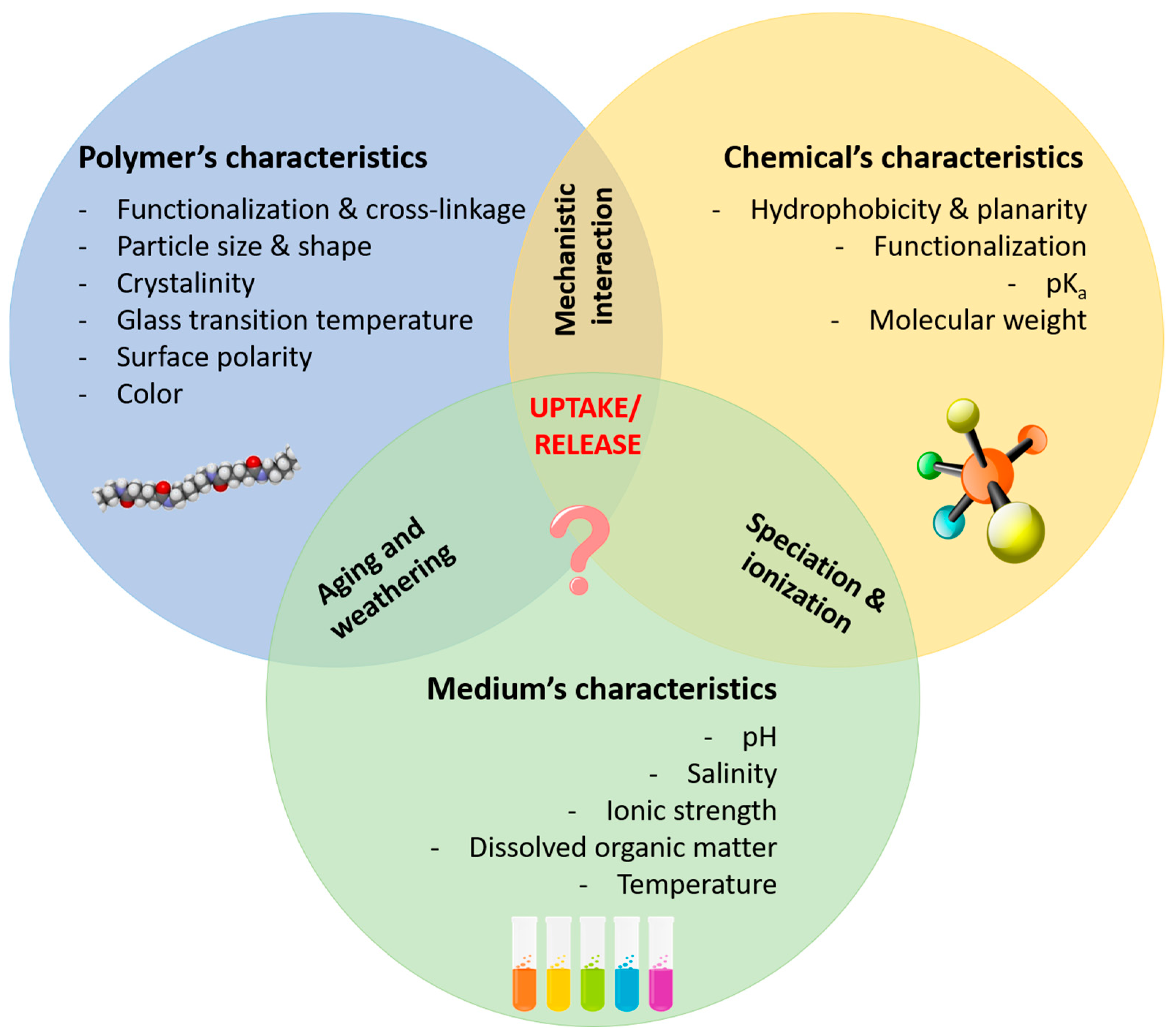
2.2. Sorption Mechanisms
The mechanisms involved in the sorption of contaminants onto (micro)plastics are overviewed in Figure 2 .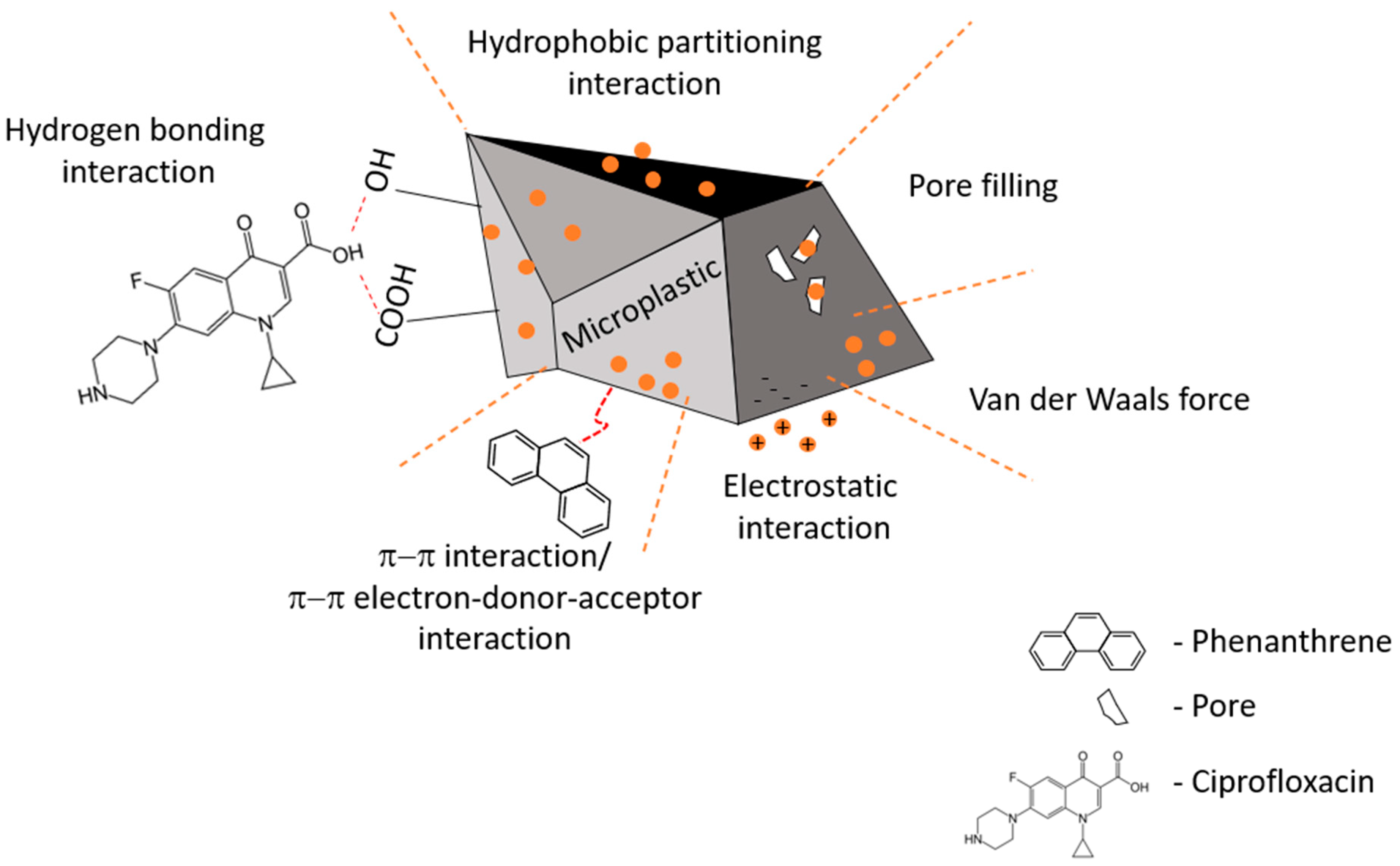 Figure 2. Overview of the sorption mechanisms of organic contaminants in the presence of microplastics. Research suggests that these phenomena may lead to microplastics acting as vectors for these contaminants [30][24]. Phenanthrene and ciprofloxacin are used merely as examples [33][25].
Figure 2. Overview of the sorption mechanisms of organic contaminants in the presence of microplastics. Research suggests that these phenomena may lead to microplastics acting as vectors for these contaminants [30][24]. Phenanthrene and ciprofloxacin are used merely as examples [33][25].2.3. Mechanistic Modelling
Generally, the uptake process of a solute onto a solid particle involves four distinct steps [79][65]:- (1)
-
transport of the solute within the bulk solution/matrix;
- (2)
-
diffusion of the solute through the boundary layer around the sorbent;
- (3)
-
diffusion of the solute inside the solid sorbent and;
- (4)
-
sorption on the active sites on the solid surface.The release process, in turn, results from the reverse sequence of steps, beginning with the desorption from active sites [29][23]. The overall rate of the process is determined by the slowest of these steps, although it should be noted that the contribution of each individual step depends on the properties of the chemicals and the particles themselves, as well as on the environmental characteristics, as discussed in the previous section. Most studies, however, focus on the kinetics of the sole sorption process, and, as such, very few works have delved into the identification of the rate limiting step. One frequently—and understandably—ignored step is the initial step, as the solute transport through the solution may be considered negligible due to the often powerful mechanical stirring used in laboratory studies [80][66]. Generally, Step 4 is considered to be the one controlling the overall sorption process and therefore is the step modeled through simplified empirical equations [29][23] such as the pseudo-first and second order kinetics equations.The overall retention (or release) of a fluid on a solid and their thermodynamic equilibrium are defined as “sorption isotherms” [81][67], a term that accounts for the fact that these equilibria must be determined for a constant temperature, which should be should be specified. Sorption isotherms are, therefore, a graphic representation of the interactions between the sorbent and sorbate per unit weight of the former that also enables the determination of the remainder of the latter when equilibrium is reached. As such, sorption isotherms can be used to predict the total amount of sorbate that can sorb on onto the solid surface of the sorbent, and the kinetic models developed estimate the efficiency of the sorption process [28][22]. Experimental data on equilibria usually fit well into a one parameter linear model, also known as Henry’s Law, or two-parameter linear or non-linear Langmuir or Freundlich isotherms [28][22].In essence, kinetic models serve the purpose of estimating the efficiency of the sorption process, while sorption isotherms can be used to predict the amount of sorbate that can sorb onto a solid matrix.Thus, determining the kinetics and the isotherm models will enable a better understanding of the sorption behavior of different chemicals in the presence of plastics, and multiple studies have focused on this aspect, mostly by modelling such sorption mechanisms based on empirical equations.
References
- Ágnes, N.; Rajmund, K. The environmental impact of plastic waste incineration. AARMS Acad. Appl. Res. Mil. Public Manag. Sci. 2016, 15, 231–237.
- Geyer, R. Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–32.
- Rodrigues, A.; Oliver, D.M.; McCarron, A.; Quilliam, R.S. Colonisation of plastic pellets (nurdles) by E. coli at public bathing beaches. Mar. Pollut. Bull. 2019, 139, 376–380.
- Dhanesha, N. The Massive, Unregulated Source of Plastic Pollution You’ve Probably Never Heard of—Nurdles; Vox, Vox Media: New York, NY, USA, 2022; Available online: https://www.vox.com/recode/23056251/nurdles-plastic-pollution-ocean-microplastics (accessed on 3 December 2023).
- da Costa, J.; Rocha-Santos, T.; Duarte, A.C. The Environmental Impacts of Plastics and Micro-Plastics Use, Waste and Pollution: EU and National Measures; European Parliament, EU: Brussels, Belgium, 2020; Available online: https://www.europarl.europa.eu/RegData/etudes/STUD/2020/658279/IPOL_STU(2020)658279_EN.pdf (accessed on 5 December 2023).
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224.
- Mafuta, C.; Baker, E.; Rucevska, I.; Thygesen, K.; Appelquist, L.R.; Westerveld, L.; Tsakona, M.; Macmillan-Lawler, M.; Harris, P.; Sevaldsen, P. Drowning in Plastics: Marine Litter and Plastic Waste Vital Graphics; United Nations Environment Programme: Nairobi, Kenya, 2021.
- Oers, L.V.; Voet, E.V.D.; Grundmann, V. Additives in the plastics industry. In Global Risk-Based Management of Chemical Additives I; Springer: Berlin/Heidelberg, Germany, 2011; pp. 133–149.
- Fortune. Plastic Additives Market Size, Growth & Forecast Report; Fortune Business Insign: Pune, India, 2022; Available online: https://www.fortunebusinessinsights.com/plastic-additives-market-104448 (accessed on 29 November 2023).
- Peng, X.; Chen, G.; Fan, Y.; Zhu, Z.; Guo, S.; Zhou, J.; Tan, J. Lifetime bioaccumulation, gender difference, tissue distribution, and parental transfer of organophosphorus plastic additives in freshwater fish. Environ. Pollut. 2021, 280, 116948.
- Wagner, S.; Schlummer, M. Legacy additives in a circular economy of plastics: Current dilemma, policy analysis, and emerging countermeasures. Resour. Conserv. Recycl. 2020, 158, 104800.
- Pivnenko, K.; Granby, K.; Eriksson, E.; Astrup, T.F. Recycling of plastic waste: Screening for brominated flame retardants (BFRs). Waste Manag. 2017, 69, 101–109.
- Miller, G.Z.; Tighe, M.E.; Peaslee, G.F.; Peña, K.; Gearhart, J. Evidence of hazardous electronic waste recycled into new consumer products. J. Environ. Prot. 2016, 7, 341.
- Feiteiro, J.; Mariana, M.; Cairrão, E. Health toxicity effects of brominated flame retardants: From environmental to human exposure. Environ. Pollut. 2021, 285, 117475.
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199.
- Chen, S.; Xu, C.; Ma, M.; Shi, Y.; He, H.; Yuan, K.; Xu, R.; Wang, X. Application of solubility parameters in the preparation of PMMA with permanent antistatic, high toughness, and excellent optical properties. Polym. Adv. Technol. 2021, 32, 3750–3758.
- Costa, J.C.; Oliveira, M.; Machado, A.V.; Lanceros-Méndez, S.; Botelho, G. Effect of antistatic additives on mechanical and electrical properties of polyethylene foams. J. Appl. Polym. Sci. 2009, 112, 1595–1600.
- Su, Q.-Z.; Lin, Q.-B.; Chen, C.-F.; Wu, Y.-M.; Wu, L.-B.; Chen, X.-Q.; Wang, Z.-W. Effect of antioxidants and light stabilisers on silver migration from nanosilver-polyethylene composite packaging films into food simulants. Food Addit. Contam. Part A 2015, 32, 1561–1566.
- Davis, P.D.; Parbrook, G.D.; Kenny, G.N.C. (Eds.) CHAPTER 7—Diffusion and Osmosis. In Basic Physics and Measurement in Anaesthesia, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1995; pp. 89–102.
- Conlisk, A.T. Essentials of Micro- and Nanofluidics: With Applications to the Biological and Chemical Sciences; Cambridge University Press: Cambridge, UK, 2013; Available online: https://books.google.pt/books?id=-fWeXMwX1x8C (accessed on 5 December 2023).
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons: Hoboken, NJ, USA, 1984.
- Fred-Ahmadu, O.H.; Bhagwat, G.; Oluyoye, I.; Benson, N.U.; Ayejuyo, O.O.; Palanisami, T. Interaction of chemical contaminants with microplastics: Principles and perspectives. Sci. Total Environ. 2020, 706, 135978.
- Angelucci, D.M.; Tomei, M.C. Uptake/release of organic contaminants by microplastics: A critical review of influencing factors, mechanistic modeling, and thermodynamic prediction methods. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1356–1400.
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493.
- Wang, F.; Zhang, M.; Sha, W.; Wang, Y.; Hao, H.; Dou, Y.; Li, Y. Sorption behavior and mechanisms of organic contaminants to nano and microplastics. Molecules 2020, 25, 1827.
- Tourinho, P.S.; Kočí, V.; Loureiro, S.; van Gestel, C.A.M. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256.
- da Costa, J.P.; Santos, P.S.; Duarte, A.C.; Rocha-Santos, T. (Nano) plastics in the environment–sources, fates and effects. Sci. Total Environ. 2016, 566, 15–26.
- Wang, J.; Liu, X.; Liu, G.; Zhang, Z.; Wu, H.; Cui, B.; Bai, J.; Zhang, W. Size effect of polystyrene microplastics on sorption of phenanthrene and nitrobenzene. Ecotoxicol. Environ. Saf. 2019, 173, 331–338.
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224.
- Yao, S.; Cao, H.; Arp, H.P.H.; Li, J.; Bian, Y.; Xie, Z.; Cherubini, F.; Jiang, X.; Song, Y. The role of crystallinity and particle morphology on the sorption of dibutyl phthalate on polyethylene microplastics: Implications for the behavior of phthalate plastic additives. Environ. Pollut. 2021, 283, 117393.
- Richburg, J.H.; Murphy, C.; Myers, J.L. The Sertoli Cell as a Target for Toxicants. In Comprehensive Toxicology; Elsevier: Amsterdam, The Netherlands, 2018.
- Wang, R.-M.; Zheng, S.-R.; Zheng, Y.-P. Polymer Matrix Composites and Technology; Woodhead Publishing: Amsterdam, The Netherlands, 2011; pp. 101–548. ISBN 9780857092229.
- Bicerano, J.; Sammler, R.L.; Carriere, C.J.; Seitz, J.T. Correlation between glass transition temperature and chain structure for randomly crosslinked high polymers. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 2247–2259.
- Zhang, X.; Zheng, M.; Yin, X.; Wang, L.; Lou, Y.; Qu, L.; Liu, X.; Zhu, H.; Qiu, Y. Sorption of 3,6-dibromocarbazole and 1,3,6,8-tetrabromocarbazole by microplastics. Mar. Pollut. Bull. 2019, 138, 458–463.
- Hüffer, T.; Weniger, A.-K.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225.
- Thrower, P.A. Chemistry & Physics of Carbon; CRC Press: Boca Raton, FL, USA, 2021; Available online: https://books.google.pt/books?id=slu6eaaaqbaj (accessed on 2 December 2023).
- Velzeboer, I.; Kwadijk, C.J.A.F.; Koelmans, A.A. Strong Sorption of PCBs to Nanoplastics, Microplastics, Carbon Nanotubes, and Fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876.
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26–33.
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Chriestie, P.; Luo, Y. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environ. Pollut. 2018, 243, 1550–1557.
- da Costa, J.P.; Nunes, A.R.; Santos, P.S.M.; Girão, A.V.; Duarte, A.C.; Rocha-Santos, T. Degradation of polyethylene microplastics in seawater: Insights into the environmental degradation of polymers. J. Environ. Sci. Health Part A 2018, 53, 866–875.
- Goedecke, C.; Mülow-Stollin, U.; Hering, S.; Richter, J.; Piechotta, C.; Paul, A.; Braun, U. A first pilot study on the sorption of environmental pollutants on various microplastic materials. J. Environ. Anal. Chem. 2017, 4, 1000191.
- Frias, J.P.G.L.; Sobral, P.; Ferreira, A.M. Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar. Pollut. Bull. 2010, 60, 1988–1992.
- Fisner, M.; Majer, A.; Taniguchi, S.; Bícego, M.; Turra, A.; Gorman, D. Colour spectrum and resin-type determine the concentration and composition of Polycyclic Aromatic Hydrocarbons (PAHs) in plastic pellets. Mar. Pollut. Bull. 2017, 122, 323–330.
- Guo, X.; Chen, C.; Wang, J. Sorption of sulfamethoxazole onto six types of microplastics. Chemosphere 2019, 228, 300–308.
- Seidensticker, S.; Grathwohl, P.; Lamprecht, J.; Zarfl, C. A combined experimental and modeling study to evaluate pH-dependent sorption of polar and non-polar compounds to polyethylene and polystyrene microplastics. Environ. Sci. Eur. 2018, 30, 30.
- Wang, F.; Shih, K.M.; Li, X.Y. The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 2015, 119, 841–847.
- Holmes, L.A.; Turner, A.; Thompson, R.C. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar. Chem. 2014, 167, 25–32.
- Elizalde-Velazquez, A.; Subbiah, S.; Anderson, T.A.; Green, M.J.; Zhao, X.; Cañas-Carrell, J.E. Sorption of three common nonsteroidal anti-inflammatory drugs (NSAIDs) to microplastics. Sci. Total Environ. 2020, 715, 136974.
- Tang, S.; Yang, X.; Zhang, T.; Qin, Y.; Cao, C.; Shi, H.; Zhao, Y. Adsorption mechanisms of metal ions (Pb, Cd, Cu) onto polyamide 6 microplastics: New insight into environmental risks in comparison with natural media in different water matrices. Gondwana Res. 2022, 110, 214–225.
- Jeong, Y.; Schäffer, A.; Smith, K. Equilibrium partitioning of organic compounds to OASIS HLB® as a function of compound concentration, pH, temperature and salinity. Chemosphere 2017, 174, 297–305.
- Xu, P.; Ge, W.; Chai, C.; Zhang, Y.; Jiang, T.; Xia, B. Sorption of polybrominated diphenyl ethers by microplastics. Mar. Pollut. Bull. 2019, 145, 260–269.
- Llorca, M.; Schirinzi, G.; Martínez, M.; Barceló, D.; Farré, M. Adsorption of perfluoroalkyl substances on microplastics under environmental conditions. Environ. Pollut. 2018, 235, 680–691.
- Guo, X.; Pang, J.; Chen, S.; Jia, H. Sorption properties of tylosin on four different microplastics. Chemosphere 2018, 209, 240–245.
- Chen, W.; Ouyang, Z.-Y.; Qian, C.; Yu, H.-Q. Induced structural changes of humic acid by exposure of polystyrene microplastics: A spectroscopic insight. Environ. Pollut. 2018, 233, 1–7.
- Liu, F.-F.; Liu, G.-Z.; Zhu, Z.-L.; Wang, S.-C.; Zhao, F.-F. Interactions between microplastics and phthalate esters as affected by microplastics characteristics and solution chemistry. Chemosphere 2019, 214, 688–694.
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar. Pollut. Bull. 2012, 64, 2782–2789.
- Agboola, O.D.; Benson, N.U. Physisorption and chemisorption mechanisms influencing micro (nano) plastics-organic chemical contaminants interactions: A review. Front. Environ. Sci. 2021, 167, 678574.
- Lin, L.; Tang, S.; Wang, X.; Sun, X.; Liu, Y. Sorption of tetracycline onto hexabromocyclododecane/polystyrene composite and polystyrene microplastics: Statistical physics models, influencing factors, and interaction mechanisms. Environ. Pollut. 2021, 284, 117164.
- Chen, X.; Chen, C.-E.; Guo, X.; Sweetman, A.J. Sorption and desorption of bisphenols on commercial plastics and the effect of UV aging. Chemosphere 2023, 310, 136867.
- Bradney, L.; Wijesekara, H.; Palansooriya, K.N.; Obadamudalige, N.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Kim, K.H.; Kirkham, M.B. Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ. Int. 2019, 131, 104937.
- Nizzetto, L.; Bussi, G.; Futter, M.N.; Butterfield, D.; Whitehead, P.G. A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ. Sci. Process. Impacts 2016, 18, 1050–1059.
- Chen, X.; Gu, X.; Bao, L.; Ma, S.; Mu, Y. Comparison of adsorption and desorption of triclosan between microplastics and soil particles. Chemosphere 2021, 263, 127947.
- da Costa, J.; Duarte, A.; Rocha-Santos, T. Microplastics—Occurrence, Fate and Behaviour in the Environment. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2017.
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC Trends Anal. Chem. 2019, 111, 252–260.
- Chiou, C.T. Partition and Adsorption of Organic Contaminants in Environmental Systems; Wiley: Hoboken, NJ, USA, 2003; Available online: https://books.google.pt/books?id=mTkaj-NaiQsC (accessed on 5 December 2023).
- Plazinski, W.; Dziuba, J.; Rudzinski, W. Modeling of sorption kinetics: The pseudo-second order equation and the sorbate intraparticle diffusivity. Adsorption 2013, 19, 1055–1064.
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275.
More
