Mitochondria are double-membrane organelles within eukaryotic cells that act as cellular power houses owing to their ability to efficiently generate the ATP required to sustain normal cell function. Also, they represent a “hub” for the regulation of a plethora of processes, including cellular homeostasis, metabolism, the defense against oxidative stress, and cell death. Mitochondrial dysfunctions are associated with a wide range of human diseases with complex pathologies, including metabolic diseases, neurodegenerative disorders, and cancer. Therefore, regulating dysfunctional mitochondria represents a pivotal therapeutic opportunity in biomedicine. Marine ecosystems are biologically very diversified and harbor a broad range of organisms, providing both novel bioactive substances and molecules with meaningful biomedical and pharmacological applications. Many mitochondria-targeting marine-derived molecules have been described to regulate mitochondrial biology, thus exerting therapeutic effects by inhibiting mitochondrial abnormalities, both in vitro and in vivo, through different mechanisms of action.
- mitochondria
- disease
- therapy
- marine natural products
- marine organisms
1. Introduction
| Compound(s) | Marine Organism |
|---|
| Compound(s) | Marine Organism | Mechanism of Action Regarding Mitochondria |
Cell Line or Model of Disease Used in Preclinical Studies | ||||||
|---|---|---|---|---|---|---|---|---|---|
| In Vitro/In Vivo Models | Mechanism of Action Regarding Mitochondria | Disease Area | Mitochondrial Biogenesis | ||||||
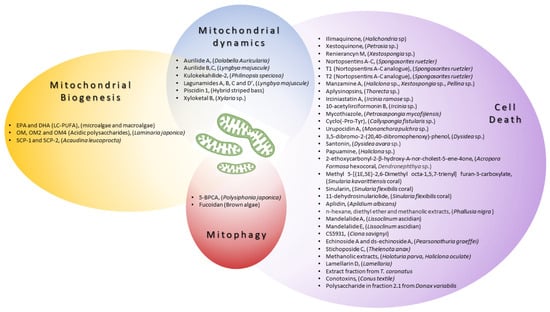
| n-3 PUFA eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) | Microalgae and macroalgae | ↑PGC1-α, ↑NRF1, ↑mitochondrial biogenesis | |||||||
| Ilimaquinone | Halichondria sp. | MCF-7, MDA-MB-231 | C57BL/6J epididymal fat [1][24] | ||||||
| caspase activation, ↑ROS, ↓Δψm | Breast cancer | [ | 15 | ][38] | oligomannuronate (OM) and OM-chromium (III) complexes (OM2 and OM4) | Laminaria japonica | ↑PGC1-α, ↑mitochondrial function, ↑mitochondrial biogenesis | C2C12, 3T3-L1 [2][25] | |
| Xestoquinone | Petrosia sp. | Molt-4, K562, Sup-T1 | ↑ROS, ↓HSP90 | Leukemia [16][39 | SCP-1 and SCP-2 | Acaudina leucoprocta | ↑AMPK/PGC1-α, ↑NRF2, ↑mitochondrial biogenesis, ↓oxidative stress | Fatigue test in ICR mice [ | |
| Renieranycin M | Xestospongia sp. | H460 | 3][26] | ||||||
| ↑BAX, ↓MCL1, ↓BCL2, caspase activation | Lung cancer | [ | 17 | ][18][40,41] | Mitochondrial dynamics | ||||
| Nortopsentins A-C | Spongosorites ruetzler | P388 cells | caspase activation | Leukemia [19][42] | Aurilide A | Dolabella auricularia | |||
| T1 (Nortopsentins A-C analogue) | Accelerate OPA-1 processing, mitochondrial fragmentation, and the release of CytC | [ | Spongosorites ruetzleri | 4][27] | HeLa S3, NCI60 panel [ | HCT-116 colorectal cancer cells5][28] | |||
| caspase activation, ↑mitochondrial trans-membrane potential | Colon cancer | [ | 20 | ][43] | Aurilide B, C | Lyngbya majuscule | |||
| T2 (Nortopsentins A-C analogue) | HCT-8, P388, A549, SK-OV-3, PC-3 | Spongosorites ruetzleri[6][29] | |||||||
| HCT-116 colorectal cancer cells | caspase activation, ↑mitochondrial trans-membrane potential | Colon cancer | [ | 19][42] | Kulokekahilide-2 | Philinopsis speciosa | P388, SK-OV-3, MDA-MB-435 [7] | ||
| Manzamine A | Haliclona sp., Xestospongia sp., Pellina sp. | [ | HCT116 cells | ↓BCL2, Δψm loss, ↑caspase activation, CytC release, | Colon cancer [21][44] | Lagunamides A, B, C and D | L. majuscule | P388, A549, PC3, HCT8, SK-OV3, HCT8, MCF7 [8][9][31,32] | |
| ] | 30 | ] | |||||||
| Aplysinopsins | Thorecta sp. | K562 cells | ↓BCL2, Δψm loss | Leukemia [22][45] | Piscidin-1 | Hybrid striped bass | |||
| Irciniastatin A | ↓MFN1, ↓MFN2, ↓OPA1, ↓OXPHOS, ↑DRP1, ↑FIS1, ↑mtROS, mitochondrial dysfunction, apoptosis | Ircinia ramose sp. | MG63 [10][11][33,34] | ||||||
| Jurkat cells | ↑ROS, ↑JNK, ↑p38, apoptosis | Leukemia | [ | 23][46] | Xyloketal B | Xylaria sp. | ↑Drp1, ↓mitochondrial fregmentation, ↓mitochondrial superoxide production |
In vitro model of ischemic stroke in PC12 [12][35] | |
| 10-acetylirciformonin B | Ircinia sp. | HL 60 cells | ↓BCL2, ↓Bcl-xL, ↑BAX, ↑ROS, CytC release, apoptosis | Mitophagy | |||||
| Leukemia | [ | 23 | ] | [46] | |||||
| Mycothiazole | Petrosaspongia mycofijiensis | T47D cells | ↓HIF-1 signaling, ↓mitochondrial function |
Breast tumor [24][47] | 5-BPCA | Polysiphonia japonica | The preservation of PARKIN expression and stabilization of mitochondrial morphology | Model of palmitate (PA)-induced lipotoxicity in a rat pancreatic β-cell line (Ins-1 cells) [13][36 | |
| Cyclo(-Pro-Tyr) | Callyspongia fistularis sp. | HepG2 cell | ] | ↓BCL2, ↑BAX, ↑ROS, apoptosis | Hepatocellular carcinoma [25][48 | Fucoidan: treatment with Fucoidan nanoparticles loaded with proanthocyanidins | Brown algae | ↑PINK1, ↑PARKIN, ↓mtDNA release | A model of cisplatin-induced damage in vitro (HK-2 cells) and in vivo (Kunming mice) [14][37] |
| ] | ||||
| Urupocidin A | ||||
| Monanchora pulchra | ||||
| sp. | ||||
| PCa cells | ||||
| Δψm loss, ↑ROS, CytC release, apoptosis | ||||
| Prostate cancer | ||||
| [ | 26 | ] | [ | 49] |
| 3,5-dibromo-2-(20,40-dibromophenoxy)-phenol | Dysidea sp. | PANC-1 | Complex II inhibition | Pancreatic carcinoma [27][50] |
| Santonin | Dysidea avara sp. | ALL B-lymphocytes | ↓Δψm, ↑ROS, CytC release, apoptosis | Acute lymphoblastic leukemia [28][51] |
| Papuamine | Haliclona sp. | MCF-7 | mitochondrial damage and JNK activation | Breast cancer [29][52] |
| 2-ethoxycarbonyl-2-β-hydroxy-A-nor-cholest-5-ene-4one | Acropora Formosa hexocoral, Dendronephthya sp. | A549 | ↓ TNF-α, ↓IL-8, ↓Bcl2, ↓MMP2, ↓MMP9, ↑ROS, ↑ BAX, ↑p21, CytC release | Lung cancer [30][53] |
| Methyl 5-[(1E,5E)-2,6-Dimethyl octa-1,5,7-trienyl] furan-3-carboxylate | Sinularia kavarittiensis coral | THP-1 | ↓Bcl-xL, ↑BAX, ↑ROS, ↓Δψm, CytC release, apoptosis |
Leukemia [31][54] |
| Sinularin | Sinularia flexibilis coral | SK-HEP-1 | ↑ROS, ↓Δψm,↓OXPHOS, apoptosis |
Liver cancer [32][55[33],56] |
| 11-dehydro-sinulariolide | Sinularia flexibilis coral | Ca9-22 | ∆Ψm loss, ↑caspase-3/-9 ↑Bax, ↓Bcl-2/Bcl-Xl, CytC release, apoptosis |
Melanoma [32][55] |
| Aplidin | Aplidium albicans | MOLT-4, NIH3T3 | ↑ROS, ↓Δψm, ↓ATP, apoptosis | Leukemia, Lymphoma [34][35][57,58] |
| n-hexane, diethyl ether and methanolic extracts | Phallusia nigra | Isolated mitochondria from skin tissue of melanoma induced albino/Wistar rats | mitochondrial swelling, ↑ROS, ↓Δψm, CytC release, apoptosis | Melanoma [36][59] |
| Mandelalide A | Lissoclinum ascidian | NCI-H460, Neuro-2A, HeLa cells | complex V inhibition, apoptosis | Lung cancer, Neuroblastoma [37][60] |
| Mandelalide E | Lissoclinum ascidian | NCI-H460, HeLa, U87-MG, HCT116 | apoptosis | Lung cancer, Glioblastoma [38][61] |
| CS5931 | Ciona savignyi | HCT-8 | ↑caspase-3, ↑caspase-9, ↑Bax, ↓Δψm, CytC release, apoptosis | Colon cancer [39][62] |
| Echinoside A and ds-echinoside A | Pearsonothuria graeffei | HepG2, mice | apoptosis | Hepatocarcinoma [40][63] |
| Stichoposide C | Thelenota anax | HL-60, K562, THP-1, NB4, SNU-C4, HT-29, CT-26; mouse CT-26 subcutaneous tumor and HL-60 leukemia xenograft models | ↑Fas, ↑caspase-3, ↑caspase-8, cleavage of Bid, mitochondrial damage, apoptosis | Leukemia, Colorectal cancer [41][64] |
| Methanolic extracts | Holoturia parva, Haliclona oculate sp. | Mitochondria isolated from a rat model of hepatocellular carcinoma | ↑ROS, ↓Δψm, CytC release, ↑caspase-3, apoptosis | Hepatocellular carcinoma [42][65] |
| Lamellarin D | Lamellaria | p388 | ↓Bcl-2, ↓Δψm, ↑caspase-3, ↑caspase-9, apoptosis | Leukemia [43][44][66,67] |
| Extract fraction of T. coronatus | Turbo coronatus | EOC cells | ↑ROS, ↓Δψm, CytC release, mitochondrial swelling, apoptosis and necrosis | Epithelial ovarian cancer [45][68] |
| Conotoxins | Conus textile | U87MG | ↑ROS, ↓Δψm, CytC release, ↑caspase-3, ↑caspase-9, ↑Bax/Bcl-2 | Glioma [46][69] |
| Polysaccharide in fraction 2.1 | Donax variabilis | A549 | Mitochondrial disfunction, ↓Δψm, CytC release, ↑caspase-3, ↑caspase-9, ↑Bax/Bcl-2, apoptosis | Lung cancer cells [47][70] |
2.Conclusions, Challenges, and Future Perspectives
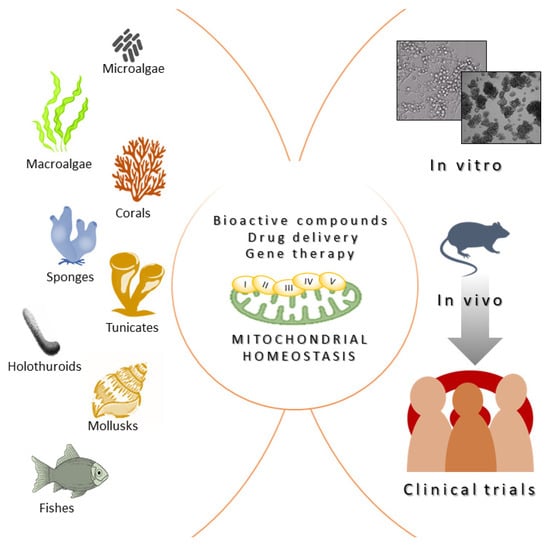
Marine organisms have been used since ancient times as a primary source of therapeutic biomolecules in traditional medicines. As an example, classical texts from the Ancient Greek and Early Byzantine periods describe the therapeutical properties of marine invertebrates, reflecting their common use in medical practice [48][121]. The cellular and molecular bases of these therapeutical actions were not known at that time. Nowadays, we know mitochondria are organelles that act as metabolic hubs of cellular signaling production and transmission [49][122]. They play a pivotal role in the adaptative responses to complex environmental challenges. Marine organisms are an important source of promising multi-target agents that are able to modulate mitochondrial biology and signaling pathways. Here, rwesearchers reviewed molecules obtained from—and strategies inspired by—marine organisms that may be potentially used to mitigate the symptoms of all the disorders with a mitochondrial pathological basis or involving a mitochondrial-related target protein. Importantly, the marine environment still has to offer a huge and unexplored biodiversity, like that present in deep-sea environments or other very extreme/secluded places, which are species-rich habitats that have been less explored compared to more accessible sites. For instance, due to their adaptation to this extreme environment, deep-sea species have the potential to produce a totally novel group of molecules with potent biological activities, bestowing an unexplored trove of novel therapeutically strategies to alleviate or treat many human diseases, including those caused by altered mitochondrial function or dynamics. Fortunately, the interest in natural products derived from deep-sea species is growing and will continue to do so in the next few years [50][123]. Despite yielding great advances in the field, several challenges still remain regarding the identification, characterization, and biomedical translation of novel marine organism-derived compounds acting on mitochondria. It is important to highlight that, often, the molecular mechanisms underlying the beneficial effects of many marine derived molecules that can potentially harness mitochondrial function and act as novel therapeutic entities are still unknown. As an example, a huge number of molecules derived from marine organisms have been described for their antioxidant effect; thus, it is speculated that they are likely acting on mitochondria, but a deeper knowledge on mitochondrial involvement is missing. An exception to this is represented by aurilides—drugs that induce apoptosis by interfering with mitochondrial dynamics and cristae organization—whose molecular mechanism of action has been exhaustively elucidated [4][51][27,85]. However, the molecular-resolution knowledge available for these drugs has not resulted in more applications at the translational level. This issue is further complicated because the experimental data on molecular actors regulating mitochondrial dynamics, as well as data on the strong connection linking mitochondrial dynamics and function, are constantly increasing. Therefore, the identification of specific targets regulating mitochondrial biology is a field characterized by constant growth and remodeling [49][122]. Another important limitation for translational research in the field, shared by all the bioactive molecules derived from marine organisms, regardless of their molecular mechanism of action, is that the costs of production for these molecules is very high. Consequently, the characterization of the beneficial biomedical properties of many natural compounds derived from marine organisms remains stuck in the preliminary stage of in vitro testing, failing to reach the requirements for the sustainable implementation of in vivo pre-clinical and clinical trials (Figure 2). However, the technical and methodological tools and knowledge needed to produce bioactive metabolites from marine sources on an industrial scale started to emerge during the early 2000s, when research on natural, biomedical compounds attracted interest among scientific communities, as well as pharmaceutical companies. Strategies centered around producing natural compounds from marine bacteria, fungi, or microalgae are based on implementing culture, harvesting, and extraction procedures on a large scale [52][124]. This is much more complex for invertebrate sources, where only small amounts of pure compounds are achievable. For these organisms, random sampling directly from the natural environment is not acceptable from an ecological point of view. Moreover, the overall naturally available biomass would be sufficient for industrial demand. Aquaculture of medicine-producing marine invertebrates such as sponges, corals, oysters, or mussels represent a kind of solution to this limitation [53][125]. Importantly, the development of chemical synthesis also supported the strategies of production on an industrial scale, thus overcoming the issue of isolation from biomass. Synthetic strategies include the possibility of modifying the molecule of interest and developing derivatives with less complexity and more manageable properties. Moreover, the recently outlined genome sequencing approaches allow for an understanding of the biochemical mechanisms regulating the biosynthesis of specific compounds, helping their synthetic cloning. Synthetic biology approaches can also facilitate the generation of genetically modified microbial cell factories to produce heterologous bioactive molecules, including marine organism-derived compounds [54][126].