Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Karolina Kryczka.
Peripartum cardiomyopathy (PPCM) is a form of heart failure, often severe, that occurs in previously healthy women at the end of their pregnancy or in the first few months after delivery. In PPCM, the recovery of heart muscle function reaches 45–50%.
- peripartum cardiomyopathy
- heart disease in pregnancy
- heart failure
- cardiac biomarkers
1. Introduction
Peripartum cardiomyopathy (PPCM) is a life-threatening disease leading to a deterioration in the systolic function of the left ventricle (LV) associated with pregnancy. The onset of heart failure (HF) is usually observed a few weeks before or in the first few months after delivery in women with no previous cardiac history [1]. PPCM is almost always characterized by a left ventricular ejection fraction (LVEF) under 45% on echocardiography with or without LV enlargement [2,3][2][3].
Cardiomyopathies are diseases of the heart muscle that include dilated, hypertrophic, restrictive, and arrhythmogenic cardiomyopathies and PPCM [4]. Recently, a new type of cardiomyopathy with a preserved LVEF has been distinguished [4]. The pathophysiology and morphology of different types of cardiomyopathies have been elucidated elsewhere [5]. Most types of cardiomyopathies differ morphologically and are easy to differentiate through echocardiography or cardiac magnetic resonance imaging (CMRI). PPCM may mimic dilated cardiomyopathy (DCM) in the case of a reduced LVEF with dilated LV. However, DCM usually occurs later in life due to the slow and irreversible process of heart muscle damage [4]. In the case of genetic mutations, an overlapping phenomenon may be observed, which means that different cardiomyopathies may share mutations in the same gene [5]. This phenomenon is observed in PPCM and DCM, most frequently in the titin gene (TTN) [5]. PPCM is characterized by an approximately 45–50% recovery rate, depending on the studied population, and a wide range of phenotypes and courses of the disease [1,2,6][1][2][6]. However, the mortality from PPCM remains high in long-term observation, and in some registries even reached 24% within three years of observation [2,7][2][7]. PPCM may relapse in future pregnancies, especially in patients who did not improve an LVEF of ≥50% [2,7][2][7]. Even in patients who recovered from PPCM, the subsequent pregnancies may again decrease LV contractility. Although the deterioration of LV function associated with subsequent pregnancy is greater in PPCM patients who do not show improved LV function, the mortality rate during eight years of observation is similar, reaching 20% irrespective of the value of the LVEF before subsequent pregnancies [8].
The etiology of the disease is complex and not fully recognized, including unbalanced oxidative stress leading to the formation of 16-kDa prolactin (PRL) with antiangiogenic and cardiotoxic properties. PPCM pathophysiology includes also extracellular matrix dysfunction, fibrosis, and genetic mutations. Bromocriptine, which blocks PRL being released from the pituitary gland, is currently the most specific PPCM treatment. However, not all patients respond to this treatment. This may be due to other mechanisms beyond 16-kDa PRL or delayed diagnosis. Therefore, novel pathophysiological pathways and biomarkers need further examination, particularly those engaged in microcirculatory, cardiac muscle, and extracellular matrix dysfunction. Currently, there is a deficiency in specific diagnostic and prognostic biomarkers that can be widely used in clinical practice to distinguish the symptoms observed in physiological pregnancy and puerperium from those pathological signs associated with PPCM. According to the International Programme on Chemical Safety, on behalf of the World Health Organization, biomarkers are defined as “any substance, structure, or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease” [9]. This definition indicates the investigation of a broad range of body tissues and genes. Most of the biomarkers already known to be associated with PPCM may be classified according to their role in the pathophysiology of the disease or their diagnostic and prognostic utility (Figure 1). Their role in PPCM pathophysiology, diagnosis and prognosis is discussed in this review.
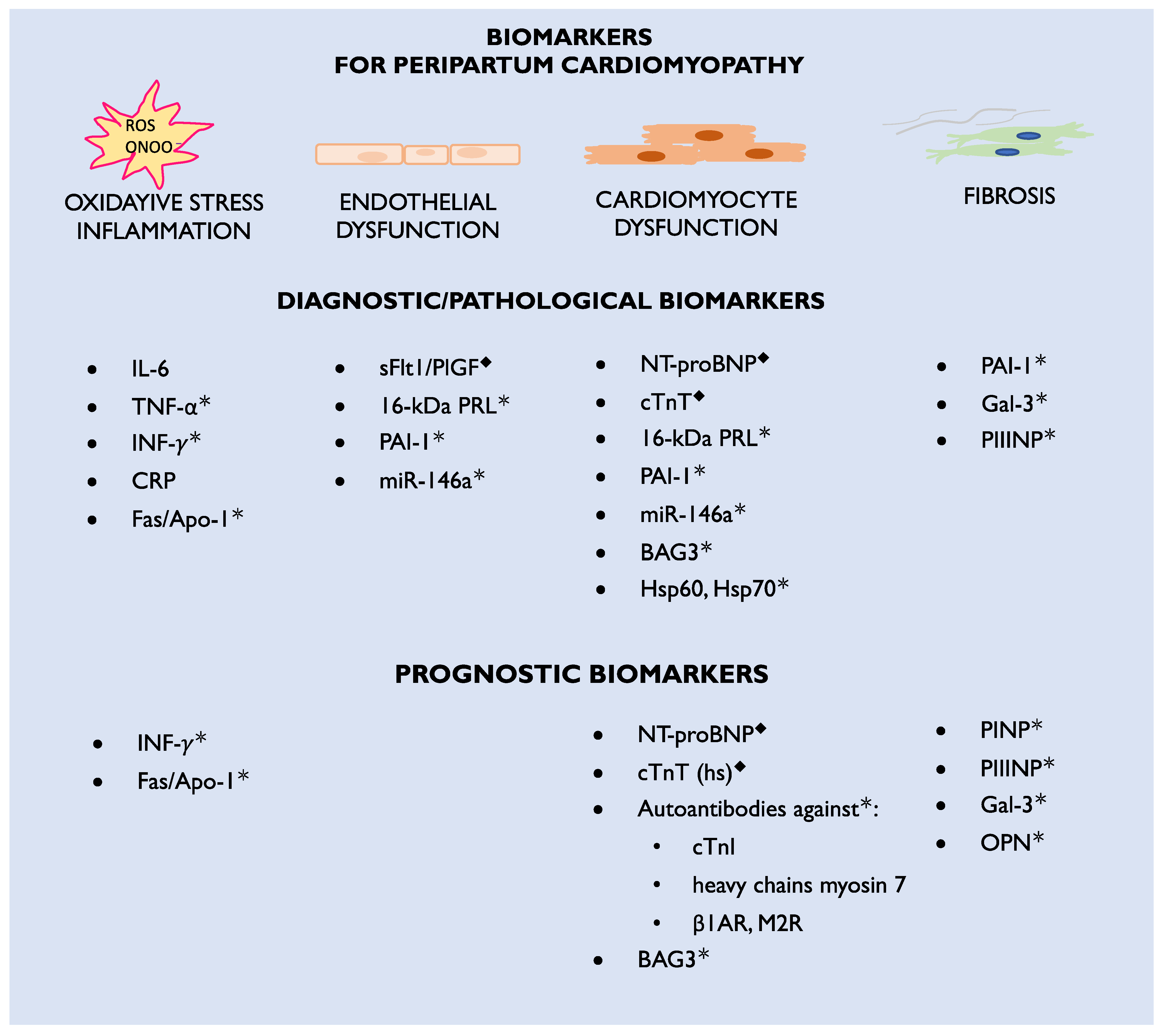
Figure 1. Diagnostic and prognostic biomarkers for peripartum cardiomyopathy; β1AR—beta 1-adrenergic receptors; CRP—C-reactive protein; cTnI—cardiac troponin I; cTnT (hs)—cardiac troponin T (high specific); Fas/Apo1—apoptosis antigen-1; Gal-3—galectin-3; Hsp—heat shock protein; IL-6—interleukin-6; INF-γ—interferon gamma; M2R—M2-muscarinic receptors; miR—microRNA; NT-proBNP—N-terminal pro-Brain-type natriuretic peptide; ONOO•—peroxynitrite; OPN—osteopontin; PAI-1—plasminogen activator inhibitor-1; PINP—procollagen type-I N-terminal propeptide; PIIINP—procollagen type-III N-terminal propeptide; PlGF—placental growth factor; PRL—prolactin; ROS—reactive oxygen species; sFlt1—soluble Fms-like tyrosine kinase-1; TNF-α—tumor necrosis factor alpha; ◆—biomarkers currently used in clinical practice; *—biomarkers which are candidates for future clinical practice.
1.1. Epidemiology, Risk Factors, and Outcomes
As PPCM is a rare disease, the sources of data on different biomarkers are limited. Moreover, the study population’s number of participants did not exceeded 151 patients. It is not uncommon for a certain biomarker to be investigated in only one study with a limited number of patients. For this review, sStudies available from online medical databases on the topic of biomarkers in PPCM were used. In addition, some representative case reports were presented to highlight new ideas.
The exact and up-to-date statistics on PPCM epidemiology are limited. So far, it has been reported that the disease is most frequent in Nigeria (1:100 deliveries), Haiti (1:300 deliveries), and South Africa (1:1000 deliveries). In the United States, among Caucasians, the frequency increased from 1:2500 in 2004 to 1:1316 deliveries in 2011 [10,11,12,13,14,15][10][11][12][13][14][15]. This process is associated with the older age of and concomitant diseases affecting mothers, such as hypertension or diabetes [1,16][1][16]. The data from the six-month observation of PPCM women in the EuroObservationRegistry Project indicate that PPCM occurs globally, and the frequency in Europe may be comparable to that in Africa [17].
The risk factors for PPCM include the mother’s older or younger age (>30 years old and <18 years old, respectively), multiparity, twin pregnancies, hypertension, preeclampsia, smoking, diabetes, and race [1,2][1][2]. The disease may be underdiagnosed, since signs of HF usually mimic those associated with normal pregnancy and puerperium, such as fatigue or leg edema. Data from the registry validated the main risk factors for PPCM, with preeclampsia being observed in almost one-fourth of patients. The registry highlighted the importance of not only relying on physical examination, as over 40% of PPCM patients did not present with peripheral edema or pulmonary congestion [6,17][6][17].
In most patients, the onset of PPCM was mainly observed in the first month postpartum with sever impairment of LVEF, <35%. The mortality of mothers and neonates was high, reaching 6% and 5%, respectively. In mothers, sudden cardiac death was the main cause of death. The frequency of thrombosis and rehospitalization reached 7 and 10%, respectively. LVEF recovery was observed in less than half of the patients, calling for the improvement of treatment [6].
1.2. Different Phenotypes and Courses of Peripartum Cardiomyopathy
The first case concerns the acute onset of HF in a 26-year-old woman on the third day after delivery of her first pregnancy. Figure 2 demonstrates an enlarged LV with an LV end-diastolic diameter of 68 mm and a severe decrease in LVEF, up to 17%, assessed by CMRI. Apart from pharmacological treatment, also with bromocriptine, the patient required interventional treatment with a biventricular assist device (BiVAD). As a result, the LVEF increased to 35% with BiVAD treatment (Figure 2B) and further to 40–45% in the six-month follow-up period [18]. The effect of the treatment was monitored with biomarkers. NT-proBNP decreased approximately 10 times during treatment, from baseline 10,275 pg/mL (N < 125) to 1019 pg/mL at the six-month observation. Cardiac troponin T was 52.78 ng/L (N < 14.00) at baseline and 7.18 after six months.
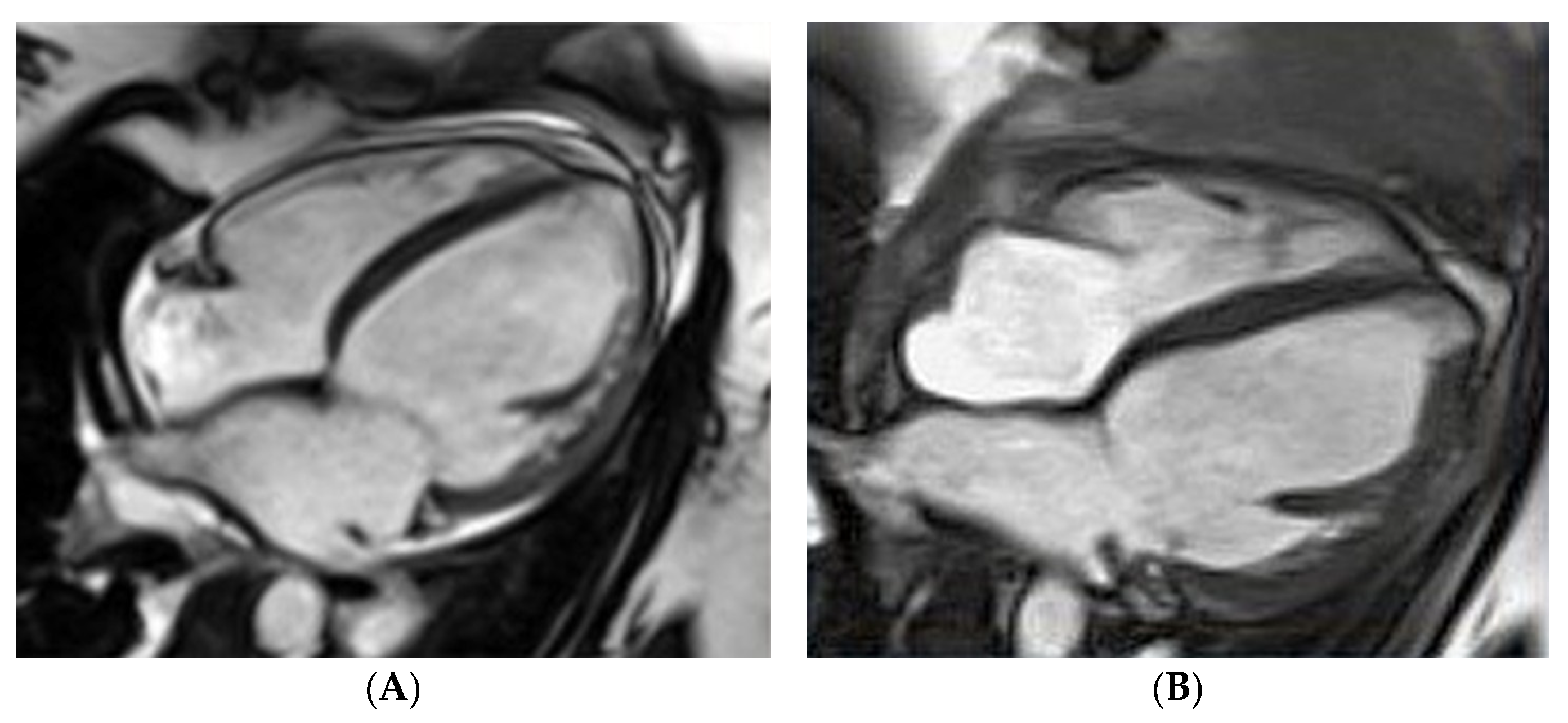
Figure 2. Peripartum cardiomyopathy in a gravida para26-year-old woman in cardiac magnetic resonance imaging: (A) fourth day postpartum, left ventricular ejection fraction (LVEF) of 17%, (B) after bi-ventricle assist device treatment, LVEF of 35%.
By contrast, wresearchers hospitalized a 35-year-old woman on the fourth day after her third cesarean section, who presented with atrial fibrillation, a severe decrease in LVEF to 20%, an enlarged LV end-diastolic diameter of 62 mm, and severe mitral insufficiency. The global longitudinal strain (GLS) was impaired up to −11.5% (norm from −26% to −18%) (Figure 3). According to the literature, a GLS > −11.4% is recognized as a predictor of increased cardiovascular events, death, and a lack of LVEF improvement ≥50% [19]. The LV insufficiency was still present after successful cardioversion. The pharmacological treatment of HF with bromocriptine was introduced, and the patient showed an improved LVEF of over 50% at the six-month observation. The biomarkers were significantly elevated: NT-proBNP equaled 6776.00 pg/mL (N < 125.00) and cardiac troponin T 33.38 ng/L (N < 14.00). The biomarkers’ levels decreased during the six-month follow-up to 170.40 pg/mL for NT-proBNP and to 4.99 for TnT.
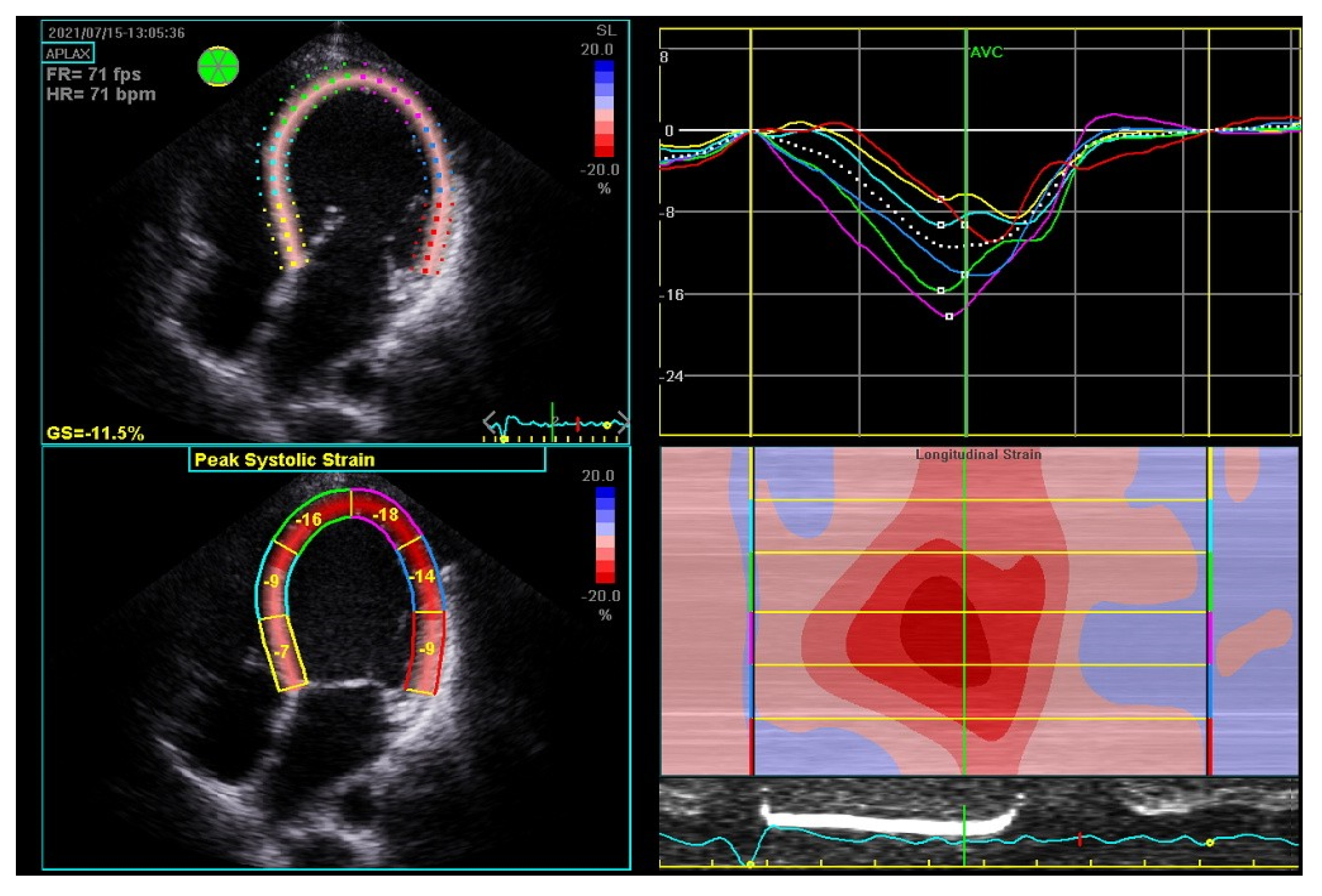
Figure 3. Global strain in echocardiography of 35-year-old woman with peripartum cardiomyopathy with severe impairment of left ventricular (LV) heart muscle functions. Four-chamber apical view: diastolic (upper left); systolic (bottom left); LV systolic and level and asynchrony of maximal contractility of LV segments illustrated by lines of different colors (upper right); longitudinal strain map of LV (bottom right); FR—frame rate, fps—frames per second; GS—global strain longitudinal; HR—heart rate; bpm—beats per minute.
2. Therapy for Peripartum Cardiomyopathy
Currently, wresearchers are lacking a specifically targeted therapy for PPCM. Bromocriptine, the D2 receptor agonist that inhibits the secretion of PRL from the pituitary gland, in addition to standard HF pharmacotherapy, currently appears to be the most specific drug for PPCM [1,42][1][20]. The pathophysiology of PPCM and the effect of bromocriptine treatment were first validated on a STAT 3 CKO mouse model that developed PPCM during pregnancy and postpartum [21]. Mice with PPCM were characterized by increased cathepsin D levels, the presence of 16 kDa PRL, decreased levels of capillaries and cardiomyocytes in the heart, an increased level of MMP3, and fibrosis, which resulted in decreased survival correlated with an increased number of pregnancies [21].
Bromocriptine treatment was associated with an increased number of capillaries and cardiomyocytes in the heart, a decreased MMP3 level, and fibrosis [21]. The bromocriptine-treated mice had a normal shortening fraction and LV end-diastolic and end-systolic diameters in contrast to the untreated mice with PPCM [21]. Currently, PPCM treatment is based on the BOARD (Bromocriptine, Oral heart failure therapies, Anticoagulants, vaso-Relaxing agents, and Diuretics) concept that recommends using bromocriptine and anticoagulants on top of standard HF treatment (Figure 4) [60][22]. This concept originates from two randomized studies that suggested the beneficial role of bromocriptine treatment [42,103][20][23]. The outcome of the last randomized study highlights the necessity for further studies on bromocriptine, other pathophysiological aspects of the disease, and new drug targets.
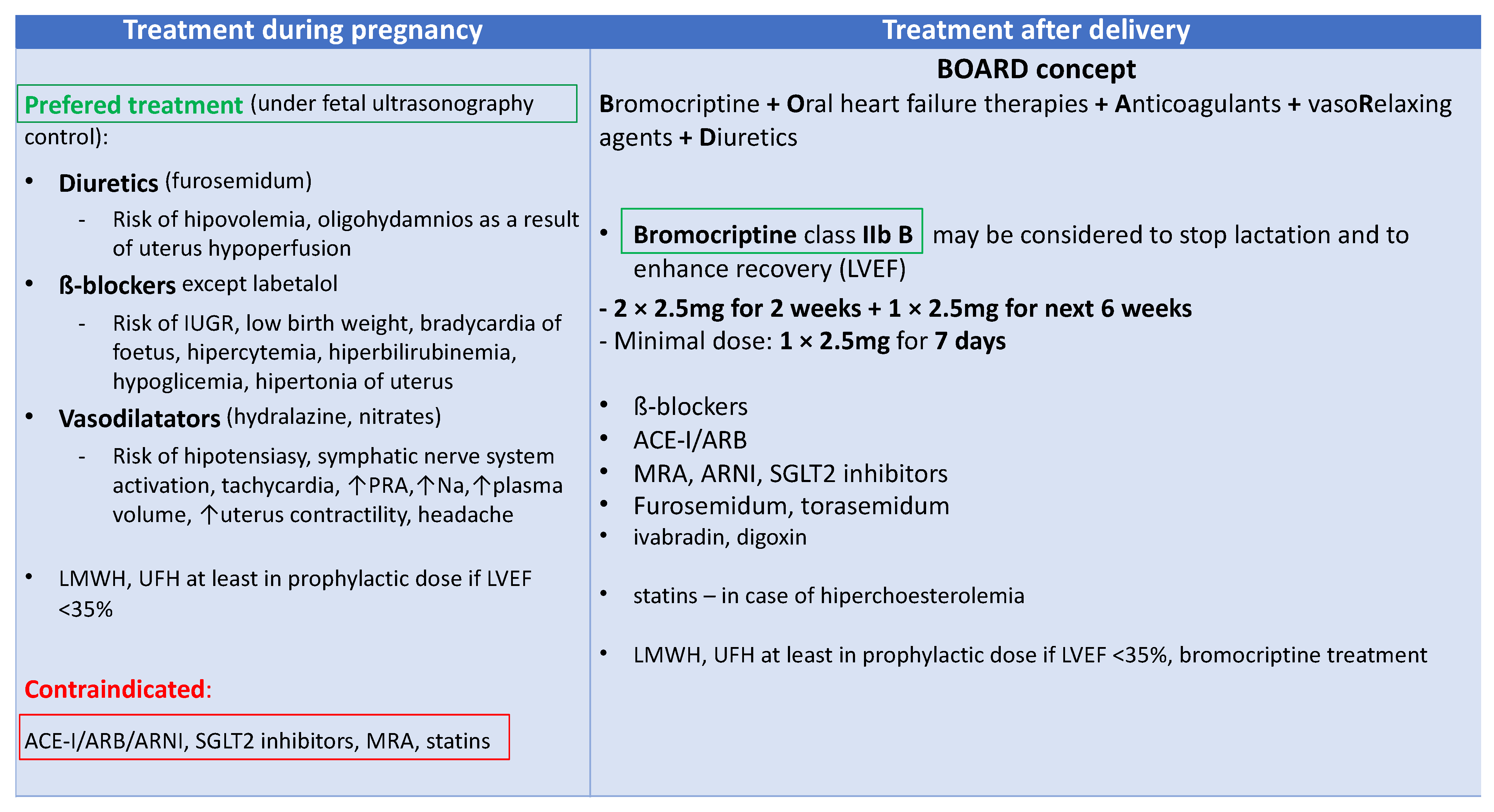
Figure 4. Peripartum cardiomyopathy (PPCM) treatment; ACE-I—angiotensin convertase enzyme inhibitor; ARB—angiotensin receptor blockers; ARNI—angiotensin receptor neprilysin inhibitor; IUGR—intrauterine growth retardation; LMWH—low molecular weight heparin; LVEF—left ventricular ejection fraction; MRA—mineral corticosteroid receptor agonists; Na—natrium; PRA—plasma renin activity; SGLT2—sodium-glucose cotransporter-2; UFH—unfractionated heparin.
2.1. New Biomarker-Based Therapies
Biomarkers associated with certain diseases may serve as a potential target for new therapies. The first reported target therapy for PPCM involved treating a mother with anti-miRNA-146a. One of the potential disadvantages of this treatment is that it may enable mothers to nurse neonates. However, studies on STAT 3 CKO mice have demonstrated that, in contrast to bromocriptine treatment, despite improvement in LV function, the LV remains dilated, suggesting that other pathological pathways have not been assessed with this treatment [22][24]. Other new therapies for microcirculatory dysfunction include the anti-sFlt-1 monoclonal antibody (mAb), which has been successful in the treatment of bronchopulmonary dysplasia in infants of mothers with preeclampsia in a rat model [104][25]. VEGF-modified RNA encoding VEGF (AZD-8601) was useful for the induction of therapeutic revascularization in the heart. In preclinical studies, it has been shown to regulate endothelial cells and cardiomyocyte survival and proliferation [105][26]. Pro-angiogenic therapy with recombinant VEGF was found to ameliorate PPCM [22][24]. However, VEGF treatment of PGC-1α HKO mice with sFlt-1-induced HF did not cause a full recovery from PPCM [22][24]. Therefore, treatment with anti-sFlt-1 mAb may improve results. The glucose-uptake-enhancing drug Perhexiline was found to decrease the cardiotoxic side effects of β-AR stimulation in CKO mice [106][27]. The cardioprotective property of this drug appears to be promising in patients with PPCM and cardiogenic shock when β-AR stimulation cannot be avoided [106][27].
The targets and biomarkers under investigation include proteins, such as Gal-3, proteoglycans, and miRNAs, which have been reviewed previously [107][28]. miRs that act as upstream regulators or downstream effectors of the fibrotic process may be useful in biomarker profiling for the identification of patients most likely to respond to the treatment with these agents. Some data demonstrate that fibrosis may be a reversible process. Therefore, as fibrosis is associated with an inferior outcome, more effort should be engaged in identifying therapeutic targets and developing new direct therapies [108,109][29][30]. New therapeutic targets in PPCM should include MPO and Gal-3. A novel, covalent, irreversible MPO inhibitor that decreases inflammation and improves microvascular function in preclinical models is currently being tested in a phase II clinical study (NCT03611153). The authors are investigating whether a single dose of 30 mg of AZD4831 given orally influences hemodynamic processes in patients with preserved LVEF ≥ 50% and with elevated filling pressures at rest or during exercise which can be assessed by pulmonary capillary wedge pressure during catheterization of the right heart. This is currently the most advanced clinical study on MPO inhibitors [110][31]. The available clinical data from phases I and II support further clinical development of AZD4831 for patients with HF with preserved ejection fraction. Anti-gal-3 therapy includes novel small-molecule gal-3 inhibitors, successful in the treatment of fibrosis in preclinical models, and modified citrus pectin multibranched polysaccharide, which ameliorated cardiac dysfunction, decreased myocardial injury, and decreased collagen deposition in rat HF models. It is worth mentioning that eplerenone and spironolactone downregulate gal-3 expression and therefore decrease the levels of collagen type I, collagen III, and TNF-α, preventing fibrosis after acute myocardial infarction in rats. Some molecules targeting Hsps are known to have a beneficial effect on improving HF. These include geranylgeranylacetone for increased Hsp70 expression, which was cardioprotective in cardiomyopathy models, as well as functional inhibitors that decrease the inflammatory effects of Hsps on cardiac tissue, such as an anti-Hsp70 antibody, polymixin B, colistin sulfate, and epigallocatechin-3-gallate [102][32].
2.2. Biomarker-Guided Therapy
Guiding HF therapy with biomarkers such as NT-proBNP and cardiac troponins can be helpful in clinical practice [5,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111][5][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111]. However, randomized trials on guiding therapy with natriuretic peptides have shown contradictory results. Some of them demonstrated the superiority of natriuretic peptide-guided HF treatment over traditional treatment based on clinical experience and guidelines. In these studies, the decrease in hospitalizations and mortality was lower in natriuretic peptide-guided therapy [112,113][112][113]. This was particularly true for patients ≤75 years of age [114]. However, some studies demonstrated no benefits from natriuretic peptide-guided therapy compared with clinically guided management, especially in older patients >60 years of age [115,116][115][116]. Metanalyses were found to have beneficial effects on natriuretic peptide-guided therapy according to a decrease in all-cause mortality compared with usual management, especially in younger patients. In addition, one demonstrated benefits such as a decrease in cardiovascular hospitalizations [117,118][117][118].
Studies on biomarker-managed therapy in PPCM are lacking. However, in patients with an improved LVEF in the six-month observation period, the levels of different biomarkers, including NT-proBNP, Fas/Apo1, IFN-γ, and prolactin, decreased more than in patients with no LVEF improvement [60][22]. A published example of one of ourthe PPCM patients demonstrated that monitoring treatment with 23-kDa PRL may be beneficial in treating this disease, as an increase in PRL level after bromocriptine discontinuation was associated with the exacerbation of symptoms. Prolonged bromocriptine treatment for up to 12 months was particularly beneficial for this patient, with an increase in LVEF >50% [34][45].
References
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.C.; Mebazaa, A.; Pieske, B.; Buchmann, E.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; van Veldhuisen, D.J.; et al. Current state of knowledge on aetiology, diagnosis, management and therapy of peripartum cardiomyopathy (PPCM): A position statement from the Heart Failure Association of the European Society of Cardiology Working group on PPCM. Eur. J. Heart Fail. 2010, 12, 767–778.
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines on the management of cardiovascular diseases during pregnancy, the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 3165–3241.
- Hibbard, J.U.; Lindheimer, M.; Lang, R.M. A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstet. Gynecol. 1999, 94, 311–316.
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.; et al. ESC Scientific Document Group 2023 ESC Guidelines for the management of cardiomyopathies, Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626.
- Sliwa, K.; Bauersachs, J.; Arany, Z.; Spracklen, T.F.; Hilfiker-Kleiner, D. Peripartum cardiomyopathy: From genetics to management. Eur. Heart J. 2021, 42, 3094–3102.
- Sliwa, K.; Petrie, M.C.; van der Meer, P.; Mebazaa, A.; Hilfiker-Kleiner, D.; Jackson, A.M.; Maggioni, A.P.; Laroche, C.; Regitz-Zagrosek, V.; Schaufelberger, M.; et al. Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: An ESC EORP registry. Eur. Heart J. 2020, 41, 3787–3797.
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975.
- Pachariyanon, P.; Bogabathina, H.; Jaisingh, K.; Modi, M.; Modi, K. Long-Term Outcomes of Women with Peripartum Cardiomyopathy Having Subsequent Pregnancies. J. Am. Coll. Cardiol. 2023, 82, 16–26.
- WHO International Programme on Chemical Safety Biomarkers in Risk Assessment, Validity and Validation. 2001. Available online: http://www.inchem.org/documents/ehc/ehc/ehc222.htm (accessed on 1 October 2023).
- Isezuo, S.A.; Abubakar, S.A. Epidemiologic profile of peripartum cardiomyopathy in a tertiary care hospital. Ethn. Dis. 2007, 17, 228–233.
- Fet, J.D.; Christie, L.G.; Carraway, R.D.; Murphy, J.G. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin. Proc. 2005, 80, 1602–1606.
- Desai, D.; Moodley, J.; Naidoo, D. Peripartum Cardiomyopathy: Experiences at King Edward VIII Hospital, Durban, South Africa and a Review of the Literature. Trop. Dr. 1995, 25, 118–123.
- Nabbaale, J.; Okello, E.; Kibirige, D.; Ssekitoleko, I.; Isanga, J.; Karungi, P.; Sebatta, E.; Zhu, Z.W.; Nakimuli, A.; Omagino, J.; et al. Burden, predictors and short-term outcomes of peripartum cardiomyopathy in a black African cohort. PLoS ONE 2020, 15, e0240837.
- Isogai, T.; Kamiya, C.A. Worldwide Incidence of Peripartum Cardiomyopathy and Overall Maternal Mortality. Int. Heart J. 2019, 60, 503–511.
- Davis, M.B.; Arany, Z.; McNamara, D.M.; Goland, S.; Elkayam, U. Peripartum Cardiomyopathy. J. Am. Coll. Cardiol. 2020, 75, 207–221.
- Kolte, D.; Khera, S.; Aronow, W.S.; Palaniswamy, C.; Mujib, M.; Ahn, C.; Jain, D.; Gass, A.; Ahmed, A.; Panza, J.A.; et al. Temporal Trends in Incidence and Outcomes of Peripartum Cardiomyopathy in the United States: A Nationwide Population-Based Study. J. Am. Heart. Assoc. 2014, 3, e001056.
- Sliwa, K.; Mebazaa, A.; Hilfiker-Kleiner, D.; Petrie, M.C.; Maggioni, A.P.; Laroche, C.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; van der Meer, P.; et al. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): EURObservational Research Programme in conjunction with the Heart Failure Association of the European Society of Cardiology Study Group on PPCM. Eur. J. Heart Fail. 2017, 19, 1131–1141.
- Petryka-Mazurkiewicz, J.; Kryczka, K.; Marona, M.; Kuriata, J.; Sitkowska-Rysiak, E.; Konopka, A.; Marczak, M.; Kołsut, P.; Kuśmierczyk, M.; Demkow, M.; et al. Cardiovascular magnetic resonance imaging of biventricular assist device-induced recovery from acute heart failure in peripartum cardiomyopathy. Kardiol. Pol. 2020, 78, 1284–1285.
- Sugahara, M.; Kagiyama, N.; Hasselberg, N.E.; Blauwet, L.A.; Briller, J.; Cooper, L.; Fett, J.D.; Hsich, E.; Wells, G.; McNamara, D.; et al. Global Left Ventricular Strain at Presentation is Associated with Subsequent Recovery in Patients with Peripartum Cardiomyopathy. J. Am. Soc. Echocardiogr. 2019, 32, 1565–1573.
- Sliwa, K.; Blauwet, L.; Tibazarwa, K.; Libhaber, E.; Smedema, J.P.; Becker, A.; McMurray, J.; Yamac, H.; Labidi, S.; Struman, I.; et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: A proof-of-concept pilot study. Circulation 2010, 121, 1465–1473.
- Hilfiker-Kleiner, D.; Kaminski, K.; Podewski, E.; Bonda, T.; Schaefer, A.; Sliwa, K.; Forster, O.; Quint, A.; Landmesser, U.; Doerries, C.; et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007, 128, 589–600.
- Bauersachs, J.; Arrigo, M.; Hilfiker-Kleiner, D.; Veltmann, C.; Coats, A.J.; Crespo-Leiro, M.G.; De Boer, R.A.; van der Meer, P.; Maack, C.; Mouquet, F.; et al. Current management of patients with severe acute peripartum cardiomyopathy, practical guidance from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2016, 18, 1096–1105.
- Hilfiker-Kleiner, D.; Haghikia, A.; Berliner, D.; Vogel-Claussen, J.; Schwab, J.; Franke, A.; Schwarzkopf, M.; Ehlermann, P.; Pfister, R.; Michels, G.; et al. Bromocriptine for the treatment of peripartum cardiomyopathy: A multicentre randomized study. Eur. Heart J. 2017, 38, 2671–2679.
- Halkein, J.; Tabruyn, S.P.; Ricke-Hoch, M.; Haghikia, A.; Nguyen, N.Q.; Scherr, M.; Castermans, K.; Malvaux, L.; Lambert, V.; Thiry, M.; et al. MicroRNA-146a is a Therapeutic Target and Biomarker for Peripartum Cardiomyopathy. J. Clin. Investig. 2013, 123, 2143–2154.
- Wallace, B.; Peisl, A.; Seedorf, G.; Kim, C.; Bosco, J.; Kenniston, J.; Keefe, D.; Abman, S.H. Anti-sFlt-1 Therapy Preserves Lung Alveolar and Vascular Growth in Antenatal Models of Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2018, 197, 776–787.
- Anttila, V.; Saraste, A.; Knuuti, J.; Jaakkola, P.; Hedman, M.; Svedlund, S.; Lagerström-Fermér, M.; Kjaer, M.; Jeppsson, A.; Gan, L.M. Synthetic mRNA Encoding VEGF-A in Patients Undergoing Coronary Artery Bypass Grafting: Design of a Phase 2a Clinical Trial. Mol. Ther. Methods Clin. Dev. 2020, 18, 464–472.
- Pfeffer, T.J.; List, M.; Müller, J.H.; Jaakkola, P.; Hedman, M.; Svedlund, S.; Lagerström-Fermér, M.; Kjaer, M.; Jeppsson, A.; Gan, L.M. Perhexiline treatment improves toxic effects of β-adrenergic receptor stimulation in experimental peripartum cardiomyopathy. ESC Heart Fail. 2021, 8, 3375–3381.
- Heymans, S.; González, A.; Pizard, A.; Papageorgiou, A.P.; López-Andrés, N.; Jaisser, F.; Thum, T.; Zannad, F.; Díez, J. Searching for new mechanisms of myocardial fibrosis with diagnostic and/or therapeutic potential. Eur. J. Heart Fail. 2015, 17, 764–771.
- Azevedo, C.F.; Nigri, M.; Higuchi, M.L.; Pomerantzeff, P.M.; Spina, G.S.; Sampaio, R.O.; Tarasoutchi, F.; Grinberg, M.; Rochitte, C.E. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J. Am. Coll. Cardiol. 2010, 56, 278–287.
- Aoki, T.; Fukumoto, Y.; Sugimura, K.; Oikawa, M.; Satoh, K.; Nakano, M.; Nakayama, M.; Shimokawa, H. Prognostic impact of myocardial interstitial fibrosis in non-ischemic heart failure—Comparison between preserved and reduced ejection fraction heart failure. Circ. J. 2011, 75, 2605–2613.
- Siraki, A.G. The many roles of myeloperoxidase: From inflammation and immunity to biomarkers, drug metabolism and drug discovery. Redox Biol. 2021, 46, 102109.
- Chakafana, G.; Spracklen, T.F.; Kamuli, S.; Zininga, T.; Shonhai, A.; Ntusi, N.A.B.; Sliwa, K. Heat Shock Proteins, Potential Modulators and Candidate Biomarkers of Peripartum Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 633013.
- Fidziańska, A.; Walczak, E.; Glinka, Z.; Religa, G.; Sobieszczanska-Malek, M.; Bilinska, Z.T. Ultrastructural evidence of myocardial capillary remodeling in peripartum cardiomyopathy. Med. Sci. Monit. 2010, 16, CS62–CS66.
- Patten, I.S.; Rana, S.; Shahul, S.; Rowe, G.C.; Jang, C.; Liu, L.; Hacker, M.R.; Rhee, J.S.; Mitchell, J.; Mahmood, F.; et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012, 485, 333–338.
- Lenke, L.; de la Escalera, G.M.; Clapp, C.; Bertsch, T.; Triebel, J. A Dysregulation of the Prolactin/Vasoinhibin Axis Appears to Contribute to Preeclampsia. Front. Endocrinol. 2020, 10, 893.
- Ricke-Hoch, M.; Bultmann, I.; Stapel, B.; Condorelli, G.; Rinas, U.; Sliwa, K.; Scherr, M.; Hilfiker-Kleiner, D. Opposing roles of Akt and STAT3 in the protection of the maternal heart from peripartum stress. Cardiovasc. Res. 2014, 101, 587–596.
- Bollen, I.A.E.; Ehler, E.; Fleischanderl, K.; Bouwman, F.; Kempers, L.; Ricke-Hoch, M.; Hilfiker-Kleiner, D.; Dos Remedios, C.G.; Krüger, M.; Vink, A.; et al. Myofilament Remodeling and Function is More Impaired in Peripartum Cardiomyopathy Compared with Dilated Cardiomyopathy and Ischemic Heart Disease. Am. J. Pathol. 2017, 187, 2645–2658.
- Seno, A.; Takeda, Y.; Matsui, M.; Okuda, A.; Nakano, T.; Nakada, Y.; Kumazawa, T.; Nakagawa, H.; Nishida, T.; Onoue, K.; et al. Suppressed Production of Soluble Fms-Like Tyrosine Kinase-1 Contributes to Myocardial Remodeling and Heart Failure. Hypertension 2016, 68, 678–687.
- Petryka-Mazurkiewicz, J.; Kryczka, K.; Mazurkiewicz, Ł.; Miłosz-Wieczorek, B.; Śpiewak, M.; Marczak, M.; Henzel, J.; Grzybowski, J.; Demkow, M.; Dzielińska, Z. Cardiovascular Magnetic Resonance in Peripartum Cardiomyopathy: Comparison with Idiopathic Dilated Cardiomyopathy. Diagnostics 2021, 11, 1752.
- Gyöngyösi, M.; Winkler, J.; Ramos, I. Myocardial fibrosis: Biomedical research from bench to bedside. Eur. J. Heart Fail. 2017, 19, 177–191.
- Azibani, F.; Pfeffer, T.J.; Ricke-Hoch, M.; Dowling, W.; Pietzsch, S.; Briton, O.; Baard, J.; Abou Moulig, V.; König, T.; Berliner, D.; et al. Outcome in German and South African peripartum cardiomyopathy cohorts associates with medical therapy and fibrosis markers. ESC Heart Fail. 2020, 7, 512–522.
- Nikolov, A.; Popovski, N. Extracellular Matrix in Heart Disease, Focus on Circulating Collagen Type I and III Derived Peptides as Biomarkers of Myocardial Fibrosis and Their Potential in the Prognosis of Heart Failure: A Concise Review. Metabolites 2022, 12, 297.
- Ruiz-Ruiz, F.J.; Ruiz-Laiglesia, F.J.; Samperiz-Legarre, P.; Lasierra-Diaz, P.; Flamarique-Pascual, A.; Morales-Rull, J.L.; Perez-Calvo, J.I. Propeptide of procollagen type I (PIP) and outcomes in decompensated heart failure. Eur. J. Intern. Med. 2007, 18, 129–134.
- Spracklen, T.F.; Chakafana, G.; Schwartz, P.J.; Kotta, M.C.; Shaboodien, G.; Ntusi, N.A.B.; Sliwa, K. Genetics of Peripartum Cardiomyopathy: Current Knowledge, Future Directions and Clinical Implications. Genes 2021, 12, 103.
- Kryczka, K.E.; Dzielińska, Z.; Franaszczyk, M.; Kryczka, K.E.; Dzielińska, Z.; Franaszczyk, M.; Wojtkowska, I.; Henzel, J.; Śpiewak, M.; Stępińska, J.; et al. Severe Course of Peripartum Cardiomyopathy and Subsequent Recovery in a Patient with a Novel TTN Gene-Truncating Mutation. Am. J. Case Rep. 2018, 19, 820–824.
- Franaszczyk, M.; Chmielewski, P.; Truszkowska, G.; Stawinski, P.; Michalak, E.; Rydzanicz, M.; Sobieszczanska-Malek, M.; Pollak, A.; Szczygieł, J.; Kosinska, J.; et al. Titin Truncating Variants in Dilated Cardiomyopathy—Prevalence and Genotype-Phenotype Correlations. PLoS ONE 2017, 12, e0169007.
- Felkin, L.E.; Walsh, R.; Ware, J.S.; Yacoub, M.H.; Birks, E.J.; Barton, P.J.; Cook, S.A. Recovery of Cardiac Function in Cardiomyopathy Caused by Titin Truncation. JAMA Cardiol. 2016, 1, 234–235.
- Stöhr, E.J.; Takayama, H.; Ferrari, G. Stretch your heart-but not too far: The role of titin mutations in dilated cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2018, 156, 209–214.
- Fang, X.; Bogomolovas, J.; Wu, T.; Zhang, W.; Liu, C.; Veevers, J.; Stroud, M.J.; Zhang, Z.; Ma, X.; Mu, Y.; et al. Loss-of-function mutations in co-chaperone BAG3 destabilize small HSPs and cause cardiomyopathy. J. Clin. Investig. 2017, 127, 3189–3200.
- Horne, B.D.; Rasmusson, K.D.; Alharethi, R.; Budge, D.; Brunisholz, K.D.; Metz, T.; Carlquist, J.F.; Connolly, J.J.; Porter, T.F.; Lappé, D.L.; et al. Genome-wide significance and replication of the chromosome 12p11.22 locus near the PTHLH gene for peripartum cardiomyopathy. Circ. Cardiovasc. Genet. 2011, 4, 359–366.
- Pfeffer, T.J.; Schlothauer, S.; Pietzsch, S.; Schaufelberger, M.; Auber, B.; Ricke-Hoch, M.; List, M.; Berliner, D.; Abou Moulig, V.; König, T.; et al. Increased cancer prevalence in peripartum cardiomyopathy. JACC Cardio Oncol. 2019, 1, 196–205.
- Mebazaa, A.; Seronde, M.F.; Gayat, E.; Tibazarwa, K.; Anumba, D.O.C.; Akrout, N.; Sadoune, M.; Sarb, J.; Arrigo, M.; Motiejunaite, J.; et al. Imbalanced angiogenesis in peripartum cardiomyopathydiagnostic value of placenta growth factor. Circ. J. 2017, 81, 1654–1661.
- Sarma, A.; Aggarwal, N.; Briller, J.; Briller, J.E.; Davis, M.; Economy, K.E.; Hameed, A.B.; Januzzi, J.L.; Lindley, K.J.; Mattina, D.J.; et al. The Utilization and Interpretation of Cardiac Biomarkers During Pregnancy. JACC Adv. 2022, 1, 100064.
- Weber, M.; Hamm, C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart 2006, 92, 843–849.
- Resnik, J.L.; Hong, C.; Resnik, R.; Kazanegra, R.; Beede, J.; Bhalla, V.; Maisel, A. Evaluation of B-type natriuretic peptide (BNP) levels in normal and preeclamptic women. Am. J. Obstet. Gynecol. 2005, 193, 450–454.
- Kale, A.; Kale, E.; Yalinkaya, A.; Akdeniz, N.; Canoruç, N. The comparison of amino-terminal probrain natriuretic peptide levels in preeclampsia and normotensive pregnancy. J. Perinat. Med. 2005, 33, 121–124.
- Dockree, S.; Brook, J.; Shine, B.; James, T.; Vatish, M. Pregnancy-specific Reference Intervals for BNP and NT-pro BNP-Changes in Natriuretic Peptides Related to Pregnancy. J. Endocr. Soc. 2021, 5, bvab091.
- Imran, T.F.; Mohebali, D.; Lopez, D.; Goli, R.R.; DeFilippis, E.M.; Truong, S.; Bello, N.A.; Gaziano, J.M.; Djousse, L.; Coglianese, E.E.; et al. NT-proBNP and predictors of event free survival and left ventricular systolic function recovery in peripartum cardiomyopathy. Inter. J. Cardiol. 2022, 357, 48–54.
- Henderson, C.A.; Gomez, C.G.; Novak, S.M.; Mi-Mi, L.; Gregorio, C.C. Overview of the Muscle Cytoskeleton. Compr. Physiol. 2017, 7, 891–944.
- Communal, C.; Colucci, W.S. The Control of Cardiomyocyte Apoptosis via the Beta-Adrenergic Signaling Pathways. Arch. Mal. Coeur. Vaiss. 2005, 98, 236–241.
- Ricke-Hoch, M.; Hoes, M.F.; Pfeffer, T.J.; Schlothauer, S.; Nonhoff, J.; Haidari, S.; Bomer, N.; Scherr, M.; Stapel, B.; Stelling, E.; et al. In peripartum cardiomyopathy plasminogen activator inhibitor-1 is a potential new biomarker with controversial roles. Cardiovasc. Res. 2019, 116, 1875–1886.
- Hu, C.L.; Li, Y.B.; Zou, Y.G.; Zhang, J.M.; Chen, J.B.; Liu, J.; Tang, Y.H.; Tang, Q.Z.; Huang, C.X. Troponin T measurement can predict persistent left ventricular dysfunction in peripartum cardiomyopathy. Heart 2007, 93, 488–490.
- Peng, B.S.; Frederick, B.; Hidalgo, M.D.; Ryan, J. Peripartum Cardiomyopathy: A Cause of Heart Failure in Young Women. Med. Forum 2017, 18, 15.
- Nikuei, P.; Rajaei, M.; Roozbeh, N.; Mohseni, F.; Poordarvishi, F.; Azad, M.; Haidari, S. Diagnostic accuracy of sFlt1/PlGF ratio as a marker for preeclampsia. BMC Pregnancy Childbirth 2020, 20, 80.
- Rana, S.; Powe, C.E.; Salahuddin, S.; Verlohren, S.; Perschel, F.H.; Levine, R.J.; Lim, K.H.; Wenger, J.B.; Thadhani, R.; Karumanchi, S.A. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012, 125, 911–919.
- Kwiatkowski, S.; Kwiatkowska, E.; Torbe, A. The role of disordered angiogenesis tissue markers (sFlt-1; PlGF) in present day diagnosis of preeclampsia. Ginkol. Pol. 2019, 90, 173–176.
- Bernard, V.; Young, J.; Chanson, P.; Binart, N. New insights in prolactin, pathological implications. Nat. Rev. Endocrinol. 2015, 11, 265–275.
- Brown, R.S.E.; Herbison, A.E.; Grattan, D.R. Effects of Prolactin and Lactation on A15 Dopamine Neurones in the Rostral Preoptic Area of Female Mice. J. Neuroendocrinol. 2015, 27, 708–717.
- Forster, O.; Hilfiker-Kleiner, D.; Ansari, A.A.; Sundstrom, J.B.; Libhaber, E.; Tshani, W.; Becker, A.; Yip, A.; Klein, G.; Sliwa, K. Reversal of IFN-gamma; oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur. J. Heart Fail. 2008, 10, 861–868.
- Lee, S.; Nishino, M.; Mazumdar, T.; Garcia, G.E.; Galfione, M.; Lee, F.L.; Lee, C.L.; Liang, A.; Kim, J.; Feng, L.; et al. 16-kDa Prolactin Down-Regulates Inducible Nitric Oxide Synthase Expression through Inhibition of the Signal Transducer and Activator of Transcription 1/IFN Regulatory Factor-1 Pathway. Cancer Res. 2005, 65, 7984–7992.
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta 2006, 1760, 616–635.
- Sherwi, N.; Merali, S.; Wong, K. Personalizing biomarker strategies in heart failure with galectin-3. Future Cardiol. 2012, 8, 885–894.
- Groh, K.; Alharethi, R.; Ewald, G.; Givertz, M.; Felker, G.M.; Pisarcik, J.; Hanley-Yanez, K.; Halder, I.; McTiernan, C.; McNamara, D. Galectin-3 levels and outcomes in periprartum cardiomyopathy: Results from the multicenter IPAC Investigation. J. Am. Coll. Cardiol. 2016, 67, 1533.
- Yu, L.; Ruifrok, W.P.; Meissner, M.; Bos, E.M.; van Goor, H.; Sanjabi, B.; van der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ. Heart Fail. 2013, 6, 107–117.
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses, cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074.
- Sliwa, K.; Skudicky, D.; Bergmann, A. Peripartum cardiomyopathy, analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/APO-1. J. Am. Coll. Cardiol. 2000, 35, 701–705.
- Sliwa, K.; Forster, O.; Libhaber, E.; Fett, J.D.; Sundstrom, J.B.; Hilfiker-Kleiner, D.; Ansari, A.A. Peripartum cardiomyopathy, inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur. Heart J. 2006, 27, 441–446.
- Wu, Y.; Potempa, L.A.; El Kebir, D.; Filep, J.G. C-reactive protein and inflammation, conformational changes affect function. Biol. Chem. 2015, 396, 1181–1197.
- Ni, C.W.; Hsieh, H.J.; Chao, Y.J.; Wang, D.L. Interleukin-6-induced JAK2/STAT3 signaling pathway in endothelial cells is suppressed by hemodynamic flow. Am. J. Physiol. Cell Physiol. 2004, 287, C771–C780.
- Lecour, S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J. Mol. Cell Cardiol. 2009, 47, 32–40.
- Mann, D.L. Stress-activated cytokines and the heart: From adaptation to maladaptation. Annu. Rev. Physiol. 2003, 65, 81–101.
- Sliwa, K.; Skudicky, D.; Candy, G.; Bergemann, A.; Hopley, M.; Sareli, P. The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur. J. Heart Fail. 2002, 4, 305–309.
- Lasfar, A.; Cook, J.R.; Cohen Solal, K.A.; Reuhl, K.; Kotenko, S.V.; Langer, J.A.; Laskin, D.L. Critical role of the endogenous interferon ligand-receptors in type I and type II interferons response. Immunology 2014, 142, 442–452.
- Weerd, N.A.; Nguyen, T. The interferons and their receptors—Distribution and regulation. Immunol. Cell Biol. 2012, 90, 483–491.
- Hu, X.; Chakravarty, S.D.; Ivashkiv, L.B. Regulation of interferon and toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol. Rev. 2008, 226, 41–56.
- Cesari, M.; Pahor, M.; Incalzi, R.A. Plasminogen activator inhibitor-1 (PAI-1): A key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc. Ther. 2010, 28, e72–e91.
- Zhang, L.; Hu, D.Y.; Li, J.; Wu, Y.F.; Liu, X.L.; Yang, X.C. Autoantibodies against the myocardial β1-adrenergic and M2-muscarinic receptors in patients with congestive heart failure. Chin. Med. J. 2002, 115, 1127–1131.
- Magnusson, Y.; Marullo, S.; Hoyert, S.B.; Waagstein, F.; Andersson, B.; Vahlne, A.; Guillet, J.G. Mapping of a Functional Autoimmune Epitope on the Adrenergic Receptor in Patients with Idiopathic Dilated Cardiomyopathy. J. Clin. Investig. 1990, 86, 1658–1663.
- Duan, X.; Liu, R.; Luo, X.-L.; Gao, X.-J.; Hu, F.-H.; Guo, C.; Wang, J.; Hu, X.-Y.; Chun, Y.-S.; Yuan, J.-S.; et al. The relationship between β1-adrenergic and M2-muscarinic receptor autoantibodies and hypertrophic cardiomyopathy. Exp. Physiol. 2020, 105, 522–530.
- Jane-wit, D.; Altuntas, C.Z.; Johnson, J.M.; Yong, S.; Wickley, P.J.; Clark, P.; Wang, Q.; Popović, Z.B.; Penn, M.S.; Damron, D.S.; et al. β1-Adrenergic receptor autoantibodies mediate dilated cardiomyopathy by agonistically inducing cardiomyocyte apoptosis. Circulation 2007, 116, 399–410.
- Stavrakis, S.; Kem, D.C.; Patterson, E.; Lozano, P.; Huang, S.; Szabo, B.; Cunningham, M.W.; Lazzara, R.; Yu, X. Opposing cardiac effect of autoantibody activation of β-adrenergic and M2 muscarinic receptors in cardiac-related diseases. Int. J. Cardiol. 2011, 148, 331–336.
- Vatner, D.E.; Sato, N.; Galper, J.B.; Vatner, S.F. Physiological and biochemical evidence for coordinate increases in muscarinic receptors and Gi during pacing-induced heart failure. Circulation 1996, 94, 102–107.
- Haghikia, A.; Kaya, Z.; Schwab, J.; Westenfeld, R.; Ehlermann, P.; Bachelier, K.; Oettl, R.; von Kaisenberg, C.S.; Katus, H.A.; Bauersachs, J.; et al. Evidence of autoantibodies against cardiac troponin I and sarcomeric myosin in peripartum cardiomyopathy. Basic Res. Cardiol. 2015, 110, 60.
- Liu, J.; Wang, Y.; Chen, M.; Zhao, W.; Wang, X.; Wang, H.; Zhang, Z.; Zhang, J.; Xu, L.; Chen, J.; et al. The Correlation between Peripartum Cardiomyopathy and Autoantibodies against Cardiovascular Receptors. PLoS ONE 2014, 9, e86770.
- Wang, T.L.; Hung, H.F.; Shyu, K.G.; Yeh, J.H.; Chiu, H.C. Successful Treatment of Peripartum Cardiomyopathy with Plasmapheresis. Acta Cardiol. Sin. 2013, 29, 471–474.
- Lau, D.; Baldus, S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol. Ther. 2006, 111, 16–26.
- Fu, X.; Kassim, S.Y.; Parks, W.C.; Heinecke, J.W. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J. Biol. Chem. 2001, 276, 41279–41287.
- Shabani, F.; McNeil, J.; Tippett, L. The oxidative inactivation of tissue inhibitor of metalloproteinase-1 (TIMP-1) by hypochlorous acid (HOCl) is suppressed by anti-rheumatic drugs. Free Radic. Res. 1998, 28, 115–123.
- Reichlin, T.; Socrates, T.; Egli, P.; Potocki, M.; Breidthardt, T.; Arenja, N.; Meissner, J.; Noveanu, M.; Reiter, M.; Twerenbold, R.; et al. Use of myeloperoxidase for risk stratification in acute heart failure. Clin. Chem. 2010, 56, 944–951.
- Serraino, G.F.; Jiritano, F.; Costa, D.; Ielapi, N.; Napolitano, D.; Mastroroberto, P.; Bracale, U.M.; Andreucci, M.; Serra, R. Metalloproteinases and Hypertrophic Cardiomyopathy: A Systematic Review. Biomolecules 2023, 13, 665.
- Münch, J.; Avanesov, M.; Bannas, P.; Säring, D.; Krämer, E.; Mearini, G.; Carrier, L.; Suling, A.; Lund, G.; Patten, M. Serum Matrix Metalloproteinases as Quantitative Biomarkers for Myocardial Fibrosis and Sudden Cardiac Death Risk Stratification in Patients with Hypertrophic Cardiomyopathy. J. Card. Fail. 2016, 2, 845–850.
- Zachariah, J.P.; Colan, S.D.; Lang, P.; Triedman, J.K.; Alexander, M.E.; Walsh, E.P.; Berul, C.I.; Cecchin, F. Circulating Matrix Metalloproteinases in Adolescents with Hypertrophic Cardiomyopathy and Ventricular Arrhythmia. Circ. Heart Fail. 2012, 5, 462–466.
- Yamada, E.; Tobe, T.; Yamada, H.; Okamoto, N.; Zack, D.J.; Werb, Z.; Soloway, P.D.; Campochiaro, P.A. TIMP-1 promotes VEGF-induced neovascularization in the retina. Histol. Histopathol. 2001, 16, 87–97.
- Cui, Q.; Yu, Z.; Pan, Y.; Purisima, E.O.; Wang, E. MicroRNAs preferentially target the genes with high transcriptional regulation complexity. Biochem. Biophys. Res. Commun. 2007, 352, 733–738.
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269.
- Zhou, S.; Jin, J.; Wang, J.; Zhang, Z.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAs in cardiovascular diseases, potential biomarkers: Therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084.
- Kasprzyk-Pawelec, A.; Wojciechowska, A.; Kuc, M.; Zielinski, J.; Parulski, A.; Kusmierczyk, M.; Lutynska, A.; Kozar-Kaminska, K. microRNA expression profile in Smooth Muscle Cells isolated from thoracic aortic aneurysm samples. Adv. Med. Sci. 2019, 64, 331–337.
- Kumar, S.; Kim, C.W.; Simmons, R.D.; Jo, H. Role of Flow-Sensitive microRNAs in Endothelial Dysfunction and Atherosclerosis. Mechanosensitive Athero-miRs. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2206–2216.
- Licholai, S.; Blaż, M.; Kapelak, B.; Sanak, M. Unbiased Profile of MicroRNA Expression in Ascending Aortic Aneurysm Tissue Appoints Molecular Pathways Contributing to the Pathology. Ann. Thorac. Surg. 2016, 102, 1245–1252.
- Staszel, T.; Zapała, B.; Polus, A.; Sadakierska-Chudy, A.; Kieć-Wilk, B.; Stępień, E.; Wybrańska, I.; Chojnacka, M.; Dembińska-Kieć, A. Role of microRNAs in endothelial cell pathophysiology. Pol. Arch. Med. Wewn. 2011, 121, 361–367.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Troughton, R.W.; Frampton, C.M.; Yandle, T.G.; Espiner, E.A.; Nicholls, M.G.; Richards, A.M. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 2000, 355, 1126–1130.
- Jourdain, P.; Jondeau, G.; Funck, F.; Gueffet, P.; Le Helloco, A.; Donal, E.; Aupetit, J.F.; Aumont, M.C.; Galinier, M.; Eicher, J.C.; et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: The STARS-BNP Multicenter Study. J. Am. Coll. Cardiol. 2007, 49, 1733–1739.
- Lainchbury, J.G.; Troughton, R.W.; Strangman, K.M.; Frampton, C.M.; Pilbrow, A.; Yandle, T.G.; Hamid, A.K.; Nicholls, M.G.; Richards, A.M. N-terminal pro-B-type natriuretic peptide-guided treatment for chronic heart failure: Results from the BATTLESCARRED (NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death) trial. J. Am. Coll. Cardiol. 2009, 55, 53–60.
- Felker, G.M.; Anstrom, K.J.; Adams, K.F.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.L., Jr.; Mark, D.B.; Pina, I.L.; Passmore, G.; et al. Effect of natriuretic peptideguided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: A randomized clinical trial. JAMA 2017, 318, 713–720.
- Pfisterer, M.; Buser, P.; Rickli, H.; Gutmann, M.; Erne, P.; Rickenbacher, P.; Vuillomenet, A.; Jeker, U.; Dubach, P.; Beer, H.; et al. BNP-guided vs symptom-guided heart failure therapy: The Trial of Intensified vs Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009, 301, 383–392.
- Porapakkham, P.; Porapakkham, P.; Zimmet, H.; Billah, B.; Krum, H. B-type natriuretic peptide-guided heart failure therapy: A meta-analysis. Arch. Intern. Med. 2010, 170, 507–514.
- Troughton, R.W.; Frampton, C.M.; Brunner-La Rocca, H.P.; Pfisterer, M.; Eurlings, L.W.; Erntell, H.; Persson, H.; O’Connor, C.M.; Moertl, D.; Karlstrom, P.; et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: An individual patient meta-analysis. Eur. Heart J. 2014, 35, 1559–1567.
More
