You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Petra Petranović Ovčariček.
Thyroid nodules are commonly detected in daily clinical practice, and their diagnosis and therapy usually involve different specialists and various diagnostic and therapeutic methods. Thyroid nodule management requires the integration of laboratory, imaging, and pathology examinations to achieve a proper diagnosis. It enables the elimination of unnecessary therapeutic procedures in many individuals and the timely identification of patients who require specific therapies. Furthermore, bioinformatics may change the current management of clinical data, enabling more personalized diagnostic approaches for patients with thyroid nodules.
- thyroid
- ultrasonography
- thyroid stimulating hormone
- nuclear medicine
1. Introduction
Thyroid nodules are more common in countries with iodine-deficient populations, and in women compared to men (ratio 4:1), and their prevalence increases with age and body mass index [1][2][3][4][5].
Luckily, most thyroid nodules (90% to 95%) are benign [6]. Risk factors for thyroid cancer include ionizing radiation (e.g., from cancer treatments, occupational exposure, or nuclear fallout, especially when the exposure occurs at a young age), rapid growth, hoarseness, and a family history of thyroid cancer or cancer syndromes (e.g., multiple endocrine neoplasia type 2, familial adenomatous polyposis) [7]. Notably, while thyroid nodules can be detected in up to 10% of healthy subjects by palpation, neck ultrasonography (US) may detect nodules in up to 68% of them, respectively [8][9][10]. Additionally, most thyroid nodules are currently detected incidentally (i.e., thyroid incidentalomas) when imaging procedures (i.e., computed tomography (CT), magnetic resonance imaging (MRI), and vascular Doppler) are performed for different indications [11]. Considering the high prevalence of thyroid nodules compared to the very low prevalence of thyroid malignancies, screening of thyroid cancer with neck US is discouraged as it results in overdiagnosis and overtreatment without improving patient outcomes [12]. Consequently, attending physicians are required to decide which nodules carry a significant risk of malignancy and require further diagnostic workup. Thyroid US scoring systems need to be integrated into daily clinical practice, complemented with the use of thyroid scintigraphy when indicated to avoid FNAC of low-risk and autonomously functioning nodules [13]. Furthermore, molecular imaging with [99mTc]Tc-MIBI and [18F]FDG is not widely used nowadays, although its usefulness is clearly demonstrated in many studies [14][15][16][17]. It is highly recommended in indeterminate cytology findings to spare patients from “diagnostic” surgeries, improve their quality of life, and reduce total hospital costs caused by unnecessary procedures and their potential complications [14][15][16].
2. Thyroid Laboratory, Imaging, and Cytopathology
2.1. Laboratory Medicine
Thyroid function can be accurately assessed by measuring TSH and free thyroid hormones (i.e., free thyroxine, FT4; free tri-iodo-thyronine, fT3). TSH and FT4 have a complex, non-linear, inverse relationship resulting in relatively large changes in TSH compared to small changes in FT4 concentrations, respectively [18][19][20]. Accordingly, except in some rare conditions (i.e., central hypothyroidism, resistance to thyroid hormones, TSH-secreting pituitary adenoma, hyperthyroidism under treatment, and euthyroid sick syndrome), TSH measurement is a sensitive and the most accurate test for thyroid dysfunction [21][22]. As a consequence, different guidelines endorse the measurement of TSH alone at the front line while restricting FT4 (and rarely FT3) measurement in cases with abnormal TSH results (i.e., TSH reflex strategy) [23][24][25][26]. The same strategy is recommended in patients with thyroid nodules where TSH measurement is unanimously recommended as the first-line functional test by available clinical guidelines. In patients with thyroid nodules, low TSH levels may be related to autonomously functioning thyroid nodule(s) and thyroid scintigraphy is indicated. A normal TSH excludes a clinically significant autonomy but, especially in countries with low iodine intake, cannot exclude compensated autonomy: in those regions, thyroid scintigraphy may properly exclude such nodules (frequently suspicious at neck US) from inappropriate FNAC [13][27]. Routine measurement of serum anti-thyroid peroxidase (TPO) antibodies is not necessary for thyroid nodule evaluation [10][28] and routine measurement of serum thyroglobulin (Tg) is strongly discouraged as it may be elevated in different thyroid diseases, including benign ones, and is aspecific and relatively insensitive for thyroid cancer [29]. Calcitonin is the standard biochemical tumor marker for medullary thyroid carcinoma (MTC) diagnosis and follow-up [30]. However, the value of routine testing in patients with thyroid nodules remains questionable due to the low prevalence, which results in a low PPV of basal calcitonin testing. Indeed, whether routine calcitonin testing improves prognosis in MTC patients remains unclear [31].2.2. Thyroid Ultrasound
Since the 1970s, thyroid US has progressively gained a central role in assessing thyroid diseases. High-resolution US examinations are widely used worldwide, being radiation-free, relatively cheap, easy to learn, and versatile compared to other imaging modalities. Ultrasound devices are equipped with transducer probes with variable frequency (i.e., 2–20 Mega Hertz (MHz)). High-resolution linear transducers with a 7–15 MHz frequency are currently employed for thyroid examination. Since the thyroid gland is superficially located with its posterior border generally situated less than 4 cm from the skin, high-resolution (≥12 MHz) probes provide excellent image quality. High-resolution conventional B-mode (i.e., gray-scale ultrasound) evaluation is now integrated with multiparametric ultrasound (MPUS), including vascularization assessment (spectral Doppler, SD; color Doppler, CD; power Doppler ultrasound, PD; superb microvascular imaging, SMI; contrast-enhanced ultrasound, CEUS) and tissue stiffness assessment (sonoelastography), respectively [13]. In clinical practice, US is the first-line imaging method for the examination of thyroid morphology and structure. The main indications of thyroid US are summarized in Table 1.Table 1.
Thyroid ultrasound: clinical indications.
| Indications |
|---|
|
|
|
|
|
Legend: FNAC, fine-needle aspiration cytology; PEI, percutaneous ethanol injection; TA, thermal ablation.
Although US is critical for the evaluation of diffuse thyroid disease, differentiating between benign and malignant nodules is the main application area. Despite several ancillary techniques like elastography and Doppler that were proposed to differentiate malignant nodules from benign ones, the most commonly used parameters are the high-resolution B-mode ultrasound characteristics of thyroid nodules. Sonographic findings, including assessment of the nodule echogenicity, internal composition, calcification, and border regularity, are commonly used for differential diagnosis. Briefly, solid, hypoechoic nodules, taller-than-wide shape, and irregular borders with microcalcifications have the highest chance of being malignant [32].
Today, thyroid US allows an accurate evaluation of morphologic features, which have been used to propose a standardized risk stratification for thyroid nodules (i.e., Thyroid Imaging And Data Reporting Systems (TI-RADS)) attempting to reduce the admittedly high inter-operator variability [13]. Among these, the American College of Radiology (ACR)-TI-RADS, European (EU)-TI-RADS, Korean (K)-TI-RADS, British Thyroid Association (BTA), American Thyroid Association (ATA) classification and American Association of Clinical Endocrinologists (AACE), American College of Endocrinology (ACE), and Associazione Medici Endocrinologi (AME) classification systems are commonly used [33][34][35][36][37][38]. The rationale of these classification systems is that the risk of malignancy rises in parallel with the increase in the number of suspicious US features and the lack of benign findings. Risk classification aims to identify the most clinically significant malignancies and decrease the number of unnecessary FNACs on benign nodules.
American College of Radiology (ACR)-TI-RADS [34] is based on the assessment of different US features of thyroid nodules: composition (spongiform, mixed cystic, and solid), echogenicity (anechoic, hyperechoic/isoechoic hypoechoic and very hypoechoic), shape (wider than tall or taller than wide), margin (smooth, ill-defined, lobulated or irregular, extra-thyroidal), and echogenic foci (none or large comet-tail artifacts, macrocalcifications, peripheral (rim) calcification, and punctate echogenic foci). It associates each of these features with a score ranging from 0 to 3 points. The sum of the assigned points defines the risk of malignancies according to five grades, with each grade corresponding to benign (TR1: 0 points), not suspicious (TR2: 2 points), mildly suspicious (TR3: 3 points), moderately suspicious (TR4: 4–6 points), or highly suspicious for malignancy (TR5: ≥7 points). This system does not include subcategories or a TR0 group to indicate a normal thyroid. EU-TIRADS is one of the most commonly used TI-RADS systems across Europe. EU-TIRADS 1 defines a normal thyroid gland without nodules. EU-TIRADS 2 is defined as a benign category, whereas EU-TIRADS 3 defines a nodule with a low risk of malignancy. Nodules with EU-TIRADS 4 have an intermediate risk, and with EU-TIRADS 5 have a high risk of malignancy. Detailed EU-TIRADS categories, risks of malignancy, and recommendations are explained in Table 2.
]. The role of [99mTc]Tc-MIBI thyroid scintigraphy (i.e., molecular imaging) to better assess the risk of malignancy in hypofunctioning and cytologically indeterminate thyroid nodules has been investigated for more than three decades [45]. In 2004, Hurtado-Lopez first compared intranodular [99mTc]Tc-MIBI and Na[99mTc]TcO4 uptake and found a quite absolute NPV for nodules with a matching hypoactive pattern with both tracers [50]. More recently, the [99mTc]Tc-MIBI uptake in the thyroid nodule has been qualitatively (i.e., visual analysis) compared to the uptake in the extranodular (i.e., normal) thyroid parenchyma and classified as hyper-, iso-, and hypointense, with the latter ruling out malignancy with up to 99% NPV [51][52]. Vice versa, an increased [99mTc]Tc-MIBI uptake with respect to surrounding parenchyma or compared to a Na[99mTc]TcO4 image conferred a higher risk of cancer [50][51][53][54][55]. However, a positive pattern (i.e., abnormal [99mTc]Tc-MIBI uptake in the nodule) was found in a significant proportion of histologically benign nodules (especially follicular and oxyphilic adenomas), and an unsatisfactory PPV (i.e., 27%) was reported [56]. To improve the diagnostic performance of molecular imaging in such patients, Saggiorato and colleagues proposed a semiquantitative approach using the so-called Retention Index (RI) method [57]. They concluded that [99mTc]Tc-MIBI-positive nodules with an RI value ≥ −11.94 were suspicious for malignancy, thus suggesting more aggressive clinical management. In addition, they proved a reduced accuracy of [99mTc]Tc-MIBI scintigraphy in oxyphilic nodules and discouraged its use in such a setting. More recently, a new semiquantitative method (i.e., wash-out index—WOind) was proposed, taking into account the [99mTc]Tc-MIBI kinetics within the nodules, and was found able to improve the diagnostic accuracy compared to qualitative evaluation [52][53][55] (Figure 4 and Figure 5).
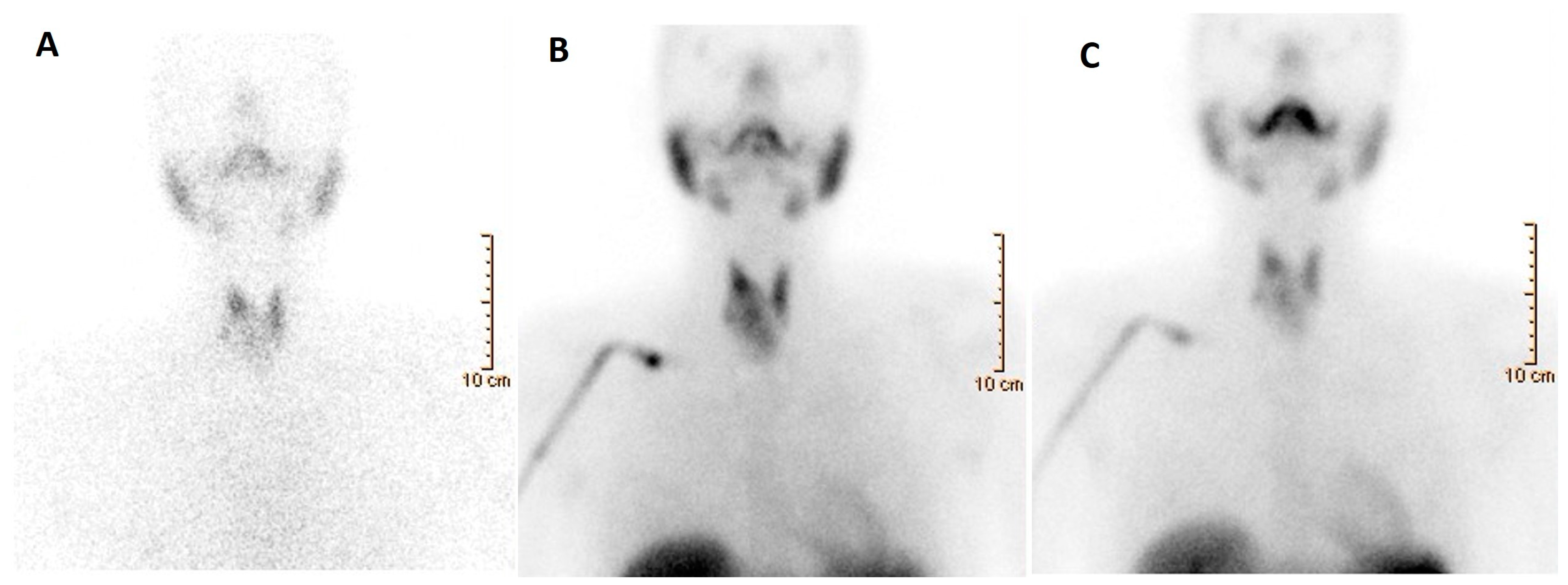
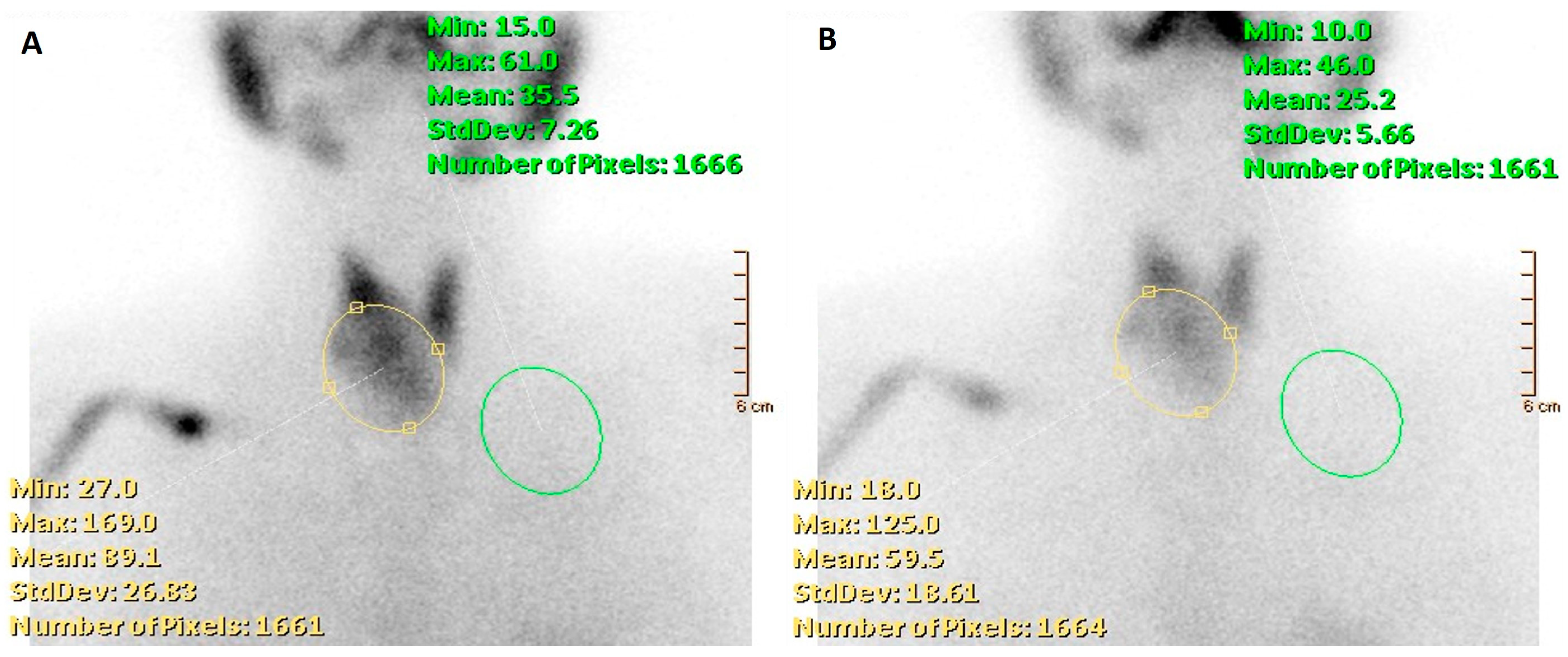 Finally, Schenke and colleagues, in a recent European multicenter study, confirmed that the semiquantitative approach using the WOind method could significantly improve the overall diagnostic performance of [99mTc]Tc-MIBI imaging [54]. In clinical routine, thyroid [99mTc]Tc-MIBI imaging can also be used to differentiate between amiodarone-induced hyperthyroidism (AIT) type 1 (i.e., normal or high uptake) and type 2 (low uptake), respectively [58][59].
Currently, single-photon emission computed tomography (SPECT) associated with computed tomography (SPECT/CT, hybrid imaging) is able to provide a co-registration of anatomic and functional/molecular data results for a better localization and characterization of tracer uptake [54]. [18F]FDG positron emission tomography/computed tomography (PET/CT) is widely used for initial staging, restaging, recurrence detection, and assessment of treatment outcomes in a variety of malignant diseases [60]. [18F]FDG uptake is related to an overexpression of the transmembrane glucose transporter proteins (GLUTs), which move the tracer into the cell, and to the overactivation of hexokinases that phosphorylate [18F]FDG to [18F]FDG-6-phosphate and trap the tracer in the cell [61]. Interestingly, a visually [18F]FDG-negative thyroid nodule with indeterminate cytology carries a negligible risk of malignancy, making [18F]FDG an accurate ruling-out biomarker. Notably, a recent blinded, randomized, controlled multicenter trial in the Netherlands consistently proved that a [18F]FDG PET/CT-driven workup of cytologically indeterminate thyroid nodules may change the clinical management and safely reduce inappropriate surgical interventions by 40%. Notably, the authors warned against the use of [18F]FDG PET/CT in patients with Hurthle cell nodules where a high [18F]FDG is expected, even in benign ones [62].
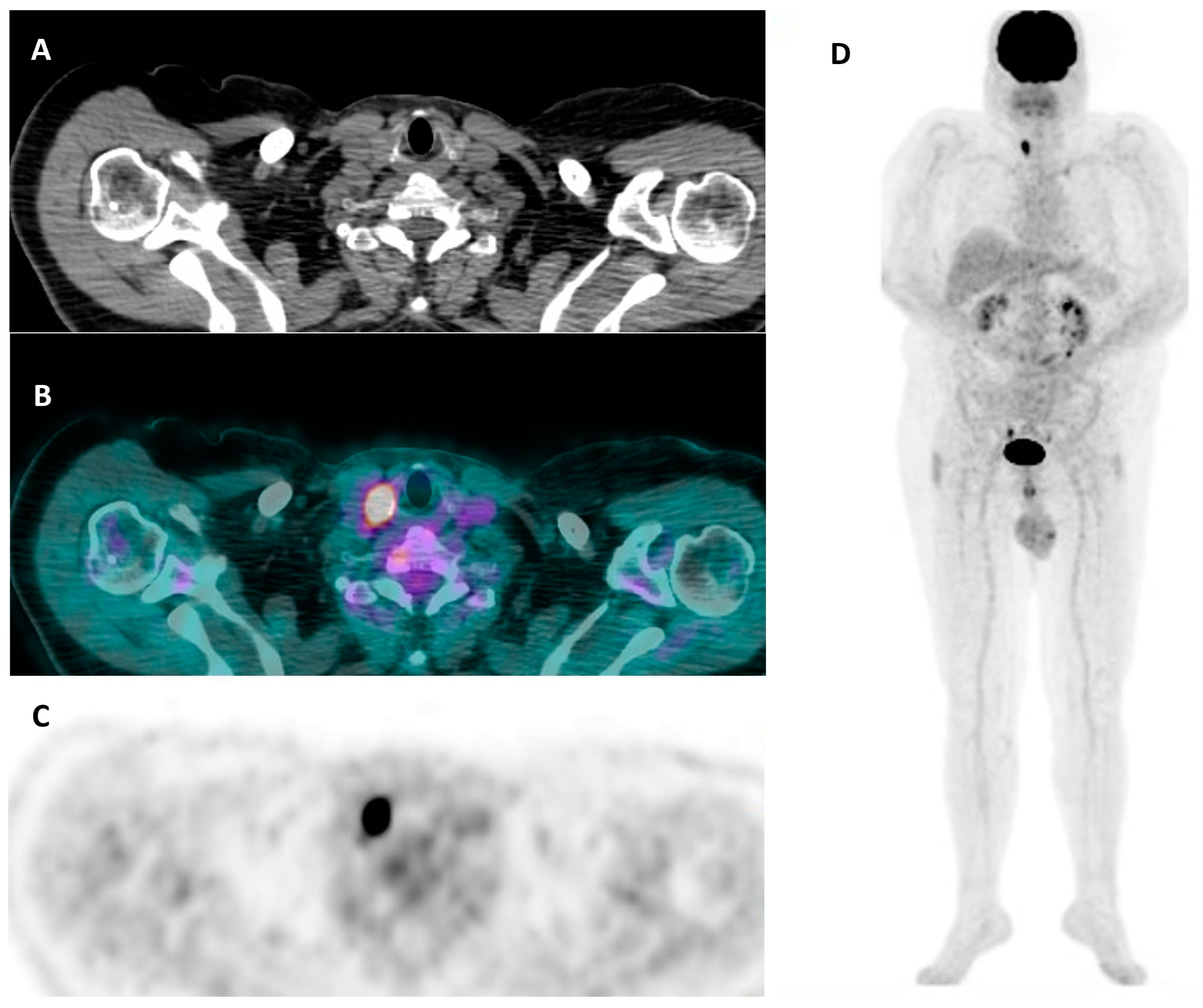
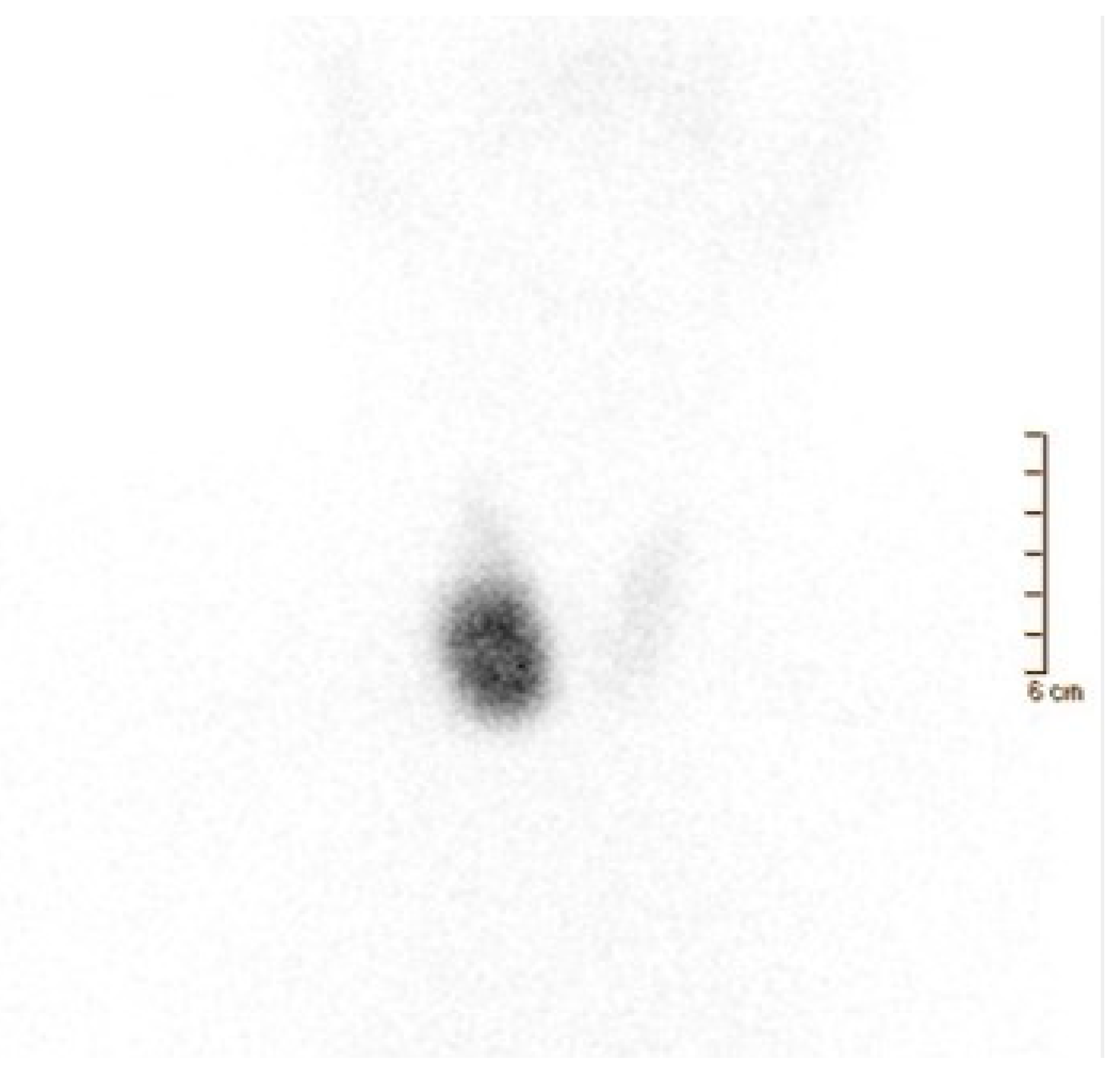
Conversely, a positive [18F]FDG PET/CT is not reliable as a rule-in test since approximately 50% of patients with positive nodules have a benign disease in the final histological report [63][64][65] (Figure 6).
Finally, Schenke and colleagues, in a recent European multicenter study, confirmed that the semiquantitative approach using the WOind method could significantly improve the overall diagnostic performance of [99mTc]Tc-MIBI imaging [54]. In clinical routine, thyroid [99mTc]Tc-MIBI imaging can also be used to differentiate between amiodarone-induced hyperthyroidism (AIT) type 1 (i.e., normal or high uptake) and type 2 (low uptake), respectively [58][59].
Currently, single-photon emission computed tomography (SPECT) associated with computed tomography (SPECT/CT, hybrid imaging) is able to provide a co-registration of anatomic and functional/molecular data results for a better localization and characterization of tracer uptake [54]. [18F]FDG positron emission tomography/computed tomography (PET/CT) is widely used for initial staging, restaging, recurrence detection, and assessment of treatment outcomes in a variety of malignant diseases [60]. [18F]FDG uptake is related to an overexpression of the transmembrane glucose transporter proteins (GLUTs), which move the tracer into the cell, and to the overactivation of hexokinases that phosphorylate [18F]FDG to [18F]FDG-6-phosphate and trap the tracer in the cell [61]. Interestingly, a visually [18F]FDG-negative thyroid nodule with indeterminate cytology carries a negligible risk of malignancy, making [18F]FDG an accurate ruling-out biomarker. Notably, a recent blinded, randomized, controlled multicenter trial in the Netherlands consistently proved that a [18F]FDG PET/CT-driven workup of cytologically indeterminate thyroid nodules may change the clinical management and safely reduce inappropriate surgical interventions by 40%. Notably, the authors warned against the use of [18F]FDG PET/CT in patients with Hurthle cell nodules where a high [18F]FDG is expected, even in benign ones [62].
Conversely, a positive [18F]FDG PET/CT is not reliable as a rule-in test since approximately 50% of patients with positive nodules have a benign disease in the final histological report [63][64][65] (Figure 6).
 In summary, either [99mTc]Tc-MIBI or [18F]FDG PET/CT may safely rule out inappropriate diagnostic surgeries in about half of patients with cytologically indeterminate nodules (non-Hurthle types). When adopted as a rule-out test, both [99mTc]Tc-MIBI scintigraphy and [18F]FDG PET/CT also proved to be cost-effective in comparison with the standard practice (i.e., diagnostic surgery) and the use of Gene Expression Classifiers [14][15][16][17]. Their use, however, should be optimized and restricted to patients with non-Hurtle cell cytological patterns and without additional indications to surgery as multinodular goiters or large-index lesions (i.e., >40–50 mm). However, more specific rule-in biomarkers are still warranted in patients with [99mTc]Tc-MIBI- or [18F]FDG-positive cytologically indeterminate nodules in order to improve the PPV.
Moreover, radiomics analysis of [18F]FDG PET/CT data preliminarily proved to increase specificity and PPV in discriminating benign from malignant cytologically indeterminate nodules [66]. However, currently, available data are contrasting [67], likely due to differences in pretest probabilities and other variables. All in all, the application of radiomic analysis in this setting should be reserved for clinical studies and not performed to make clinical decisions.
In summary, either [99mTc]Tc-MIBI or [18F]FDG PET/CT may safely rule out inappropriate diagnostic surgeries in about half of patients with cytologically indeterminate nodules (non-Hurthle types). When adopted as a rule-out test, both [99mTc]Tc-MIBI scintigraphy and [18F]FDG PET/CT also proved to be cost-effective in comparison with the standard practice (i.e., diagnostic surgery) and the use of Gene Expression Classifiers [14][15][16][17]. Their use, however, should be optimized and restricted to patients with non-Hurtle cell cytological patterns and without additional indications to surgery as multinodular goiters or large-index lesions (i.e., >40–50 mm). However, more specific rule-in biomarkers are still warranted in patients with [99mTc]Tc-MIBI- or [18F]FDG-positive cytologically indeterminate nodules in order to improve the PPV.
Moreover, radiomics analysis of [18F]FDG PET/CT data preliminarily proved to increase specificity and PPV in discriminating benign from malignant cytologically indeterminate nodules [66]. However, currently, available data are contrasting [67], likely due to differences in pretest probabilities and other variables. All in all, the application of radiomic analysis in this setting should be reserved for clinical studies and not performed to make clinical decisions.
The system includes six categories, with the most controversial being III–IV, where repeat FNAC, molecular testing, or lobectomy may be warranted. The cytological characteristics of the indeterminate categories (III–IV) can be divided into four different subgroups: qualitatively unsatisfactory specimen, cytologic atypia (mostly nuclear), architectural atypia (microfollicular), and the combination of the latter two [84]. The first case includes specimens with inadequate preparation and artifacts that may cause the appearance of nuclear enlargements suspicious for atypia. Specimens with atypical cells also belong to this category. Atypia can be epithelial, lymphoid, mesenchymal, or even non-specific and does not suggest any specific tumor by itself (Bethesda III). The second case includes cytological atypia in specimens, no matter the cell architecture. Usually, there are very few atypical cells, so the confirmation/exclusion of malignancy is not possible. The presence of oncocytes, without an adequate clinical picture, e.g., Hashimoto’s thyroiditis, is also considered atypical (Bethesda III). The third case involves specimens with some or abundant microfollicular structures but without atypia of the nucleus (Bethesda III and IV). Also, samples with many oncocytes, but without colloid or lymphocytes, are in this category (Bethesda IV). The fourth case includes scarce cellularity and the presence of microfollicular structures and nuclear atypia (Bethesda III). FNAC in these cases is considered as a screening tool because it cannot confirm or exclude malignancy [84].
Although the morphologic parameters used by these various systems (Table 6) are very similar, there are differences in the criteria for inclusion in cytologically indeterminate categories which may affect the recommendations for the clinical management of patients (Table 7).
[89]. ThyGeNEXT is a next-generation sequencing panel with the purpose of sequencing gene mutations (BRAF, NRAS, HRAS, KRAS, and PIK3CA) and mRNA fusions (RET/PTC1, RET/PTC3, and PPARG/PAX8) [85]. It is combined with a miRNA risk classifier ThyraMIR, which determines the expression of growth-promoting miRNA (miR-31, -146, -222, -375, -551) and growth-suppressing miRNA (miR-29, -138, -139, -155, -204) and increases its accuracy, demonstrating sensitivity, specificity, NPV, and PPV of 90, 93, 95, and 74%, respectively [89].
In terms of prognosis, a recent study demonstrated that tumors with BRAF-like profiles positively correlate with larger tumors, higher initial tumor stage, and presence of lateral neck metastasis, compared with the RAS-like profile tumors and non-BRAF/non-RAS-like tumors which included PAX8::PPARG fusion and DICER1, EIF11AX, PTEN, and IDH1 mutations [90].
In malignant thyroid nodules (Bethesda VI), BRAFV600E mutation is detected in 64–76% of tumors, while TERT promoter mutations are detected in 11% of cases [91]. The presence of both mutations positively correlates with extrathyroidal extension, local and distant metastasis, tumor recurrence, and mortality [89], and predicts the development of radioiodine-refractory DTC in PTC patients [92].
Table 2.
EU-TIRADS categories, risks of malignancy, and recommendations
[35]
.
| Category | US Features | Malignancy Risk, % | Recommendations |
|---|
[33].
| Sonographic Pattern | US Features | Estimated Risk of Malignancy, % | FNA Size Cutoff (Largest Dimension) |
|---|

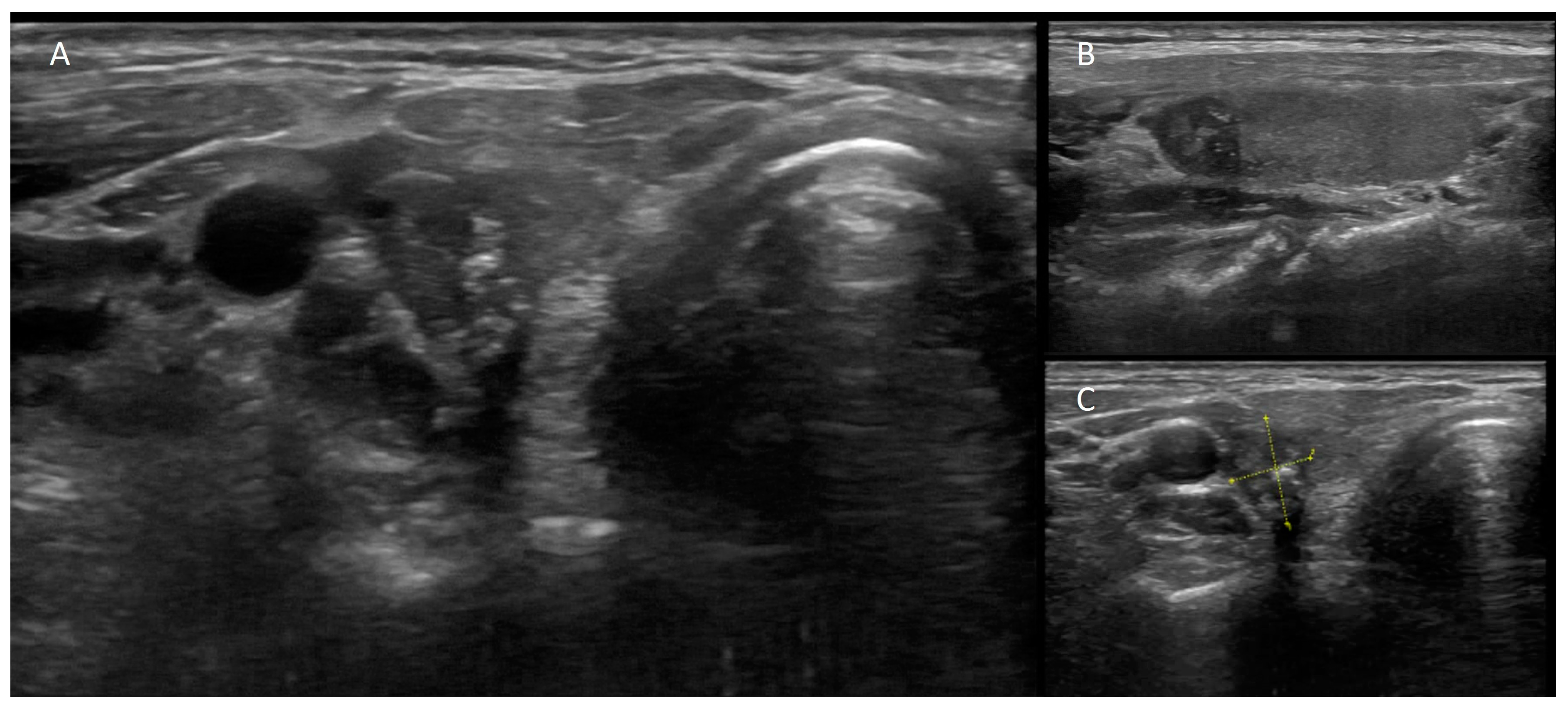
Figure 4. A 52-year-old woman affected by nodular goiter in euthyroid status (TSH 0.60 μIU/mL (0.35–5.50), FT3 3.18 pg/mL (2.30–4.20), FT4 1.17 ng/dL (0.89–1.76)). Thyroid ultrasonography showed a large-sized nodule (39 × 36 × 33 mm) in the right thyroid lobe, irregularly hypoechoic with the presence of isoechoic areas. A moderately increased intra-nodular blood flow was also noted. (A) Thyroid scintigraphy, performed 20 min after Na[99mTc]TcO4 administration (111 MBq; anterior view; magnification: 1.4; matrix: 256 × 256; time frame: 100 Kcs), showed a well-defined hypofunctioning area located in the middle–lower part of the right thyroid lobe. Thus, a nodular goiter was diagnosed while FNAC was conclusive for a benign lesion (Tir2) according to the Italian scoring system. [99mTc]Tc-MIBI scintigraphy was obtained 10 and 120 min (B,C, respectively) after tracer administration (370 MBq). Images were acquired in anterior view, magnification 256 × 256, matrix 1.4, time frame 10 min. Increased [99mTc]Tc-MIBI uptake was noted in the hypofunctioning nodule.

Figure 5. A 52-year-old woman affected by nodular goiter, cytologically benign (TIR2 according to the Italian scoring system). [99mTc]Tc-MIBI scintigraphy was obtained 10 and 120 min after tracer administration (A,B, respectively) using a semiquantitative approach by calculating the wash-out index (WOind). WOind value was <−19% (exactly −37%), thus consistent with a no-malignant lesion. Final histology diagnosis: follicular adenoma.

Figure 6. A 62-year-old man with a hypermetabolic thyroid nodule (SUV max = 18.3) unexpectedly discovered in the right lobe during a [18F]FDG PET/CT study performed for staging in suspected paraneoplastic syndrome. (A) CT – axial view, (B) a [18F]FDG PET/CT axial view, (C) [18F]FDG PET axial view, (D) whole-body [18F]FDG PET. A laboratory test was consistent with euthyroid status (i.e., TSH 2.1 µIU/mL (0.27–4.2), FT3 3.30 pg/mL (2.0–4.4), FT4 18.2 pmol/L (12–22)) with negative anti-thyroid antibodies (i.e., TPOAb, TgAb). Thyroid ultrasound demonstrated a multinodular goiter with a dominant hypoechoic nodule in the right lobe (maximum size: 22 mm), which was then classified as hypofunctioning at thyroid scintigraphy. Accordingly, FNAC was performed: an indeterminate lesion with a high risk of being malignant (i.e., TIR3B according to the Italian scoring system) was diagnosed. The patient underwent total thyroidectomy, and the final histological diagnosis was consistent with Hurtle cell follicular adenoma.
2.4. Fine-Needle Aspiration Cytology and Cytopathology
Thyroid nodules are common in clinical practice, with a prevalence of up to 60% depending on age, sex, etc. [3][4][68]. The majority are benign [69], and the risk of malignancy is 7 to 15% [69], depending on the nodule size, findings of ultrasound and nuclear medicine techniques, and patient characteristics. Several guidelines and RSSs (Table 4) recommend biopsy based on the size and imaging findings.Table 4. Ultrasound-based recommendations for fine-needle aspiration cytology: (a) Biopsy recommendations of EU TI-RADS [35]. (b) Biopsy recommendations of ACR TI-RADS guidelines [34]. (c) Biopsy recommendations of ATA guidelines [33].
| (a) | |||
| ∼10–30 | |||
| Repeat FNA, molecular testing, or lobectomy | |||
| IV. Follicular neoplasm or suspicious for a follicular neoplasm | 10–40 | 25–40 | Molecular testing, lobectomy |
| V. Suspicious for malignancy | 45–60 | 50–75 | Near-total thyroidectomy or lobectomy |
| VI. Malignant | 94–96 | 97–99 | Near-total thyroidectomy or lobectomy |
Table 6. Comparison between 2014 Italian SIAPEC-AIT classification, 2017 Bethesda, and 2016 UK RCPath reporting system for thyroid cytology.
| RCPath | Bethesda | Italian SIAPEC-AIT | |
|---|---|---|---|
| Category | Malignancy Risk, % | Recommendations | |
| EU-TIRADS 1: Normal | None | ||
| Thy1 Non-diagnostic for cytological diagnosis Thy1c Non-diagnostic for cytological diagnosis—cystic lesion |
|
TIR1 Non-diagnostic TIR1c Non-diagnostic cystic |
|
| Thy2 Non-neoplastic Thy2c Non-neoplastic, cystic lesion |
|||
Table 7. “Indeterminate” diagnostic categories: comparison of risk of malignancy (ROM) and clinical management between Italian, Bethesda, and British classifications.
| 2014 Italian SIAPEC-AIT | 2017 Bethesda | 2016 RCPath Classification |
|---|
4. Integrated Diagnostics of Thyroid Nodules
Even if thyroid nodules are very common, randomized clinical trials are scarce, likely due, almost partially, to the good prognosis of thyroid cancer. Instead, recommendations in clinical guidelines are largely based on observational studies and experts’ opinions. Thyroid nodules mostly derive from follicular thyroid cells, and benign nodules (unifocal or multifocal) are the most common thyroid lesions. Additionally, thyroid nodules may also occur and coexist in conditions such as subacute thyroiditis, autoimmune thyroiditis, and Graves’ disease, respectively. Thyroid cancer, however, occurs in 2–5% of thyroid nodules. Finally, infiltrative disorders, lymphoma, metastases from non-thyroid cancers, and paraganglioma can rarely result in a thyroid nodule. Factors related to an increased risk of cancer are summarized in Table 8.Table 8.
Thyroid nodules: factors increasing the risk of cancer.
| History |
|
|||||||
| Clinical examination |
|
|||||||
| Clinical investigations |
| |||||||
| EU-TIRADS 1: Normal | No nodules | None | None | |||||
| High suspicion | Solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, micro-lobulated), microcalcifications, taller-than-wide shape, rim calcifications with small extrusive soft tissue component, evidence of extrathyroidal extension | >70–90 | Recommend FNA at ≥1 cm | |||||
| EU-TIRADS 2: benign | Pure cyst, Entirely spongiform |
No FNA required (unless for therapeutic purposes/to relieve compression) | ||||||
| Intermediate suspicion | Hypoechoic solid nodule with smooth margins without microcalcifications, ETE, or taller-than-wide shape | 0 | 10–20 | Recommend FNA at ≥1 cm | EU-TIRADS 3: low risk | Ovoid, smooth isoechoic/hyperechoic No features of high suspicion |
2–4 | >20 mm FNA |
| Low suspicion | Isoechoic or hyperechoic solid nodule, or partially cystic nodule with eccentric solid areas, without microcalcification, irregular margin or ETE, or taller-than-wide shape | 5–10 | Recommend FNA at ≥1.5 cm | EU-TIRADS 4: intermediate risk | Ovoid, smooth, mildly hypoechoic No features of high suspicion |
6–17 | >15 mm FNA | |
| Very low suspicion | Spongiform or partially cystic nodules without any of the sonographic features described in low-, intermediate-, or high-suspicion patterns | <3 | Consider FNA at ≥2 cm Observation without FNA is also a reasonable option |
EU-TIRADS 5: high risk | At least 1 of the following features of high suspicion:
|
26–87 | >10 mm FNA, <10 mm: consider FNA or active surveillance |
Legend: EU-TIRADS, European Thyroid Imaging Reporting and Data System; US, ultrasound.
The American Thyroid Association (ATA) guidelines for assessing thyroid nodules are meant to improve inter- and intra-reader consistency when reporting thyroid nodules on ultrasound and facilitate communication with referring physicians. The 2015 guideline emphasizes the importance of the sonographic pattern of the nodule for risk stratification. This system does not include scoring but categorizes the risk of malignancy from very low risk to high. The malignancy risk as well as the size of the nodule are the two main criteria for FNA (Table 3).
Table 3. ATA sonographic patterns, estimated risk of malignancy, and fine-needle aspiration guidance for thyroid nodules
| Benign | |
| Purely cystic nodules (no solid component) | |
| <1 | No biopsy |
Legend: US, ultrasound; FNA, fine-needle aspiration; ETE, extrathyroidal extension.
Comparison of thyroid ultrasound RSS is an ongoing debate in the literature. This is understandable; each society deems its RSS to be the preferred system. Endocrinologists from Europe prefer EU-RADS, endocrinologists from the USA uses ATA guidelines, and radiologists and nuclear medicine physicians prefer the ACR-TIRADS system [39][40] (Figure 1).
 In a multicentric German trail, EU-TIRADS was proved to be inferior when compared to other RSSs with diagnostic accuracies of 0.70 vs. 0.79, 0.78, 0.82, and 0.79 for Kwak-TIRADS, ACR-TI-RADS, Korean-TIRADS, and American Thyroid Association (ATA) Guidelines, respectively [41]. In another study, authors found that ACR TI-RADS, American Association of Clinical Endocrinologists/American College of Endocrinology/Associazione Medici Endocrinologi guidelines, European TI-RADS, ATA guidelines, and Korean TI-RADS would have avoided FNA for 34.7%, 31%, 25.7%, 20%, and 6% of nodules with false-negative rates (FNRs) of 24%, 28.5%, 22%, 7.2%, and 1.9%, respectively. In this study, ATA guidelines had the highest area under the curve and a low FNR, whereas ACR TI-RADS would have spared more patients from FNA with a high FNR [42]. So far, no uniform, worldwide accepted RSS has been established because of the controversy in the literature, and the differences in the expertise and preferences of ultrasonographers (Figure 2). Also, there are limitations of RSS like insufficient sensitivity for the diagnosis of follicular thyroid carcinoma and follicular subtypes of PTC and insufficient specificity to rule out autonomously functioning thyroid nodules from FNAC. However, work has recently begun on a new international US-based RSS for thyroid nodules. With the participation of several scientific societies, an International TIRADS (I-TIRADS) will be proposed and established internationally as a uniform evidence-based system. Currently, several working groups are investigating ultrasound criteria. In addition, promising data already exist regarding the use of AI to identify US patterns. This technique could significantly reduce interobserver variability and may be associated with improvements in specificity and accuracy, without significantly sacrificing sensitivity for malignancy detection [43].
In a multicentric German trail, EU-TIRADS was proved to be inferior when compared to other RSSs with diagnostic accuracies of 0.70 vs. 0.79, 0.78, 0.82, and 0.79 for Kwak-TIRADS, ACR-TI-RADS, Korean-TIRADS, and American Thyroid Association (ATA) Guidelines, respectively [41]. In another study, authors found that ACR TI-RADS, American Association of Clinical Endocrinologists/American College of Endocrinology/Associazione Medici Endocrinologi guidelines, European TI-RADS, ATA guidelines, and Korean TI-RADS would have avoided FNA for 34.7%, 31%, 25.7%, 20%, and 6% of nodules with false-negative rates (FNRs) of 24%, 28.5%, 22%, 7.2%, and 1.9%, respectively. In this study, ATA guidelines had the highest area under the curve and a low FNR, whereas ACR TI-RADS would have spared more patients from FNA with a high FNR [42]. So far, no uniform, worldwide accepted RSS has been established because of the controversy in the literature, and the differences in the expertise and preferences of ultrasonographers (Figure 2). Also, there are limitations of RSS like insufficient sensitivity for the diagnosis of follicular thyroid carcinoma and follicular subtypes of PTC and insufficient specificity to rule out autonomously functioning thyroid nodules from FNAC. However, work has recently begun on a new international US-based RSS for thyroid nodules. With the participation of several scientific societies, an International TIRADS (I-TIRADS) will be proposed and established internationally as a uniform evidence-based system. Currently, several working groups are investigating ultrasound criteria. In addition, promising data already exist regarding the use of AI to identify US patterns. This technique could significantly reduce interobserver variability and may be associated with improvements in specificity and accuracy, without significantly sacrificing sensitivity for malignancy detection [43].
 In physiological conditions, NIS activity is positively regulated by TSH and, as a consequence, Na[99mTc]TcO4 uptake in the gland can be properly interpreted in light of TSH values (ideally performed before thyroid scintigraphy). The activity of NIS is inversely regulated by the intrathyroidal iodine pool that reflects the iodine intake.
Although almost all thyroid cancers are non-functioning, most of these nodules are benign (i.e., 90–95%), which greatly reduces the specificity of a thyroid scan. Accordingly, a thyroid scan is generally performed when nodules occur in people with low or low-to-normal TSH levels. The relationship between thyroid autonomy and TSH levels, however, is affected by the degree of iodine sufficiency and varies widely regionally [19][20][22]. Therefore, although autonomous nodules are almost invariably accompanied by decreased TSH levels (i.e., <0.1–0.4 mUI/L) when the iodine supply is adequate, the bulk of autonomous tissue may be insufficient to suppress the TSH level in iodine-depleted thyroids, especially in the early phases of autonomy. As a consequence, different indications are given in current clinical guidelines with a thyroid scan recommended when the TSH level is low or low to normal in the USA (iodine-repleted country), whereas a Na[99mTc]TcO4 scan is recommended in all people with a nodule greater than 10 mm, independently of the TSH level in Germany (iodine-deficient country) [5][27][44][45]
Consequently, high iodine concentrations (e.g., regular use of products rich in iodine) may reduce the quality of a functional image. For this reason, any “excess” iodine intake should be avoided before scintigraphy or it should be postponed for several (i.e., 1–3) to many (i.e., 6 or more) months in patients with severe iodine contamination due to radiological contrast media administration or amiodarone therapy, respectively [44]. [99mTc]Tc-MIBI is a lipophilic cation able to cross the cell membrane, reversibly penetrating the cytoplasm and then irreversibly moving through the membrane of the mitochondria using a different electrical gradient caused by a high negative electric potential of the inner membrane. It is regularly used as a marker for myocardial perfusion and hyperfunctioning parathyroid tissue. All in all, studies reported its abnormal uptake in different tumors such as in the lungs, brain, breast, bone, and thyroid [46][47][48][49
In physiological conditions, NIS activity is positively regulated by TSH and, as a consequence, Na[99mTc]TcO4 uptake in the gland can be properly interpreted in light of TSH values (ideally performed before thyroid scintigraphy). The activity of NIS is inversely regulated by the intrathyroidal iodine pool that reflects the iodine intake.
Although almost all thyroid cancers are non-functioning, most of these nodules are benign (i.e., 90–95%), which greatly reduces the specificity of a thyroid scan. Accordingly, a thyroid scan is generally performed when nodules occur in people with low or low-to-normal TSH levels. The relationship between thyroid autonomy and TSH levels, however, is affected by the degree of iodine sufficiency and varies widely regionally [19][20][22]. Therefore, although autonomous nodules are almost invariably accompanied by decreased TSH levels (i.e., <0.1–0.4 mUI/L) when the iodine supply is adequate, the bulk of autonomous tissue may be insufficient to suppress the TSH level in iodine-depleted thyroids, especially in the early phases of autonomy. As a consequence, different indications are given in current clinical guidelines with a thyroid scan recommended when the TSH level is low or low to normal in the USA (iodine-repleted country), whereas a Na[99mTc]TcO4 scan is recommended in all people with a nodule greater than 10 mm, independently of the TSH level in Germany (iodine-deficient country) [5][27][44][45]
Consequently, high iodine concentrations (e.g., regular use of products rich in iodine) may reduce the quality of a functional image. For this reason, any “excess” iodine intake should be avoided before scintigraphy or it should be postponed for several (i.e., 1–3) to many (i.e., 6 or more) months in patients with severe iodine contamination due to radiological contrast media administration or amiodarone therapy, respectively [44]. [99mTc]Tc-MIBI is a lipophilic cation able to cross the cell membrane, reversibly penetrating the cytoplasm and then irreversibly moving through the membrane of the mitochondria using a different electrical gradient caused by a high negative electric potential of the inner membrane. It is regularly used as a marker for myocardial perfusion and hyperfunctioning parathyroid tissue. All in all, studies reported its abnormal uptake in different tumors such as in the lungs, brain, breast, bone, and thyroid [46][47][48][49
The shape, margin, echogenicity, and presence of calcification are useful criteria for the discrimination of malignant from benign nodules [70].
FNAC Technique:

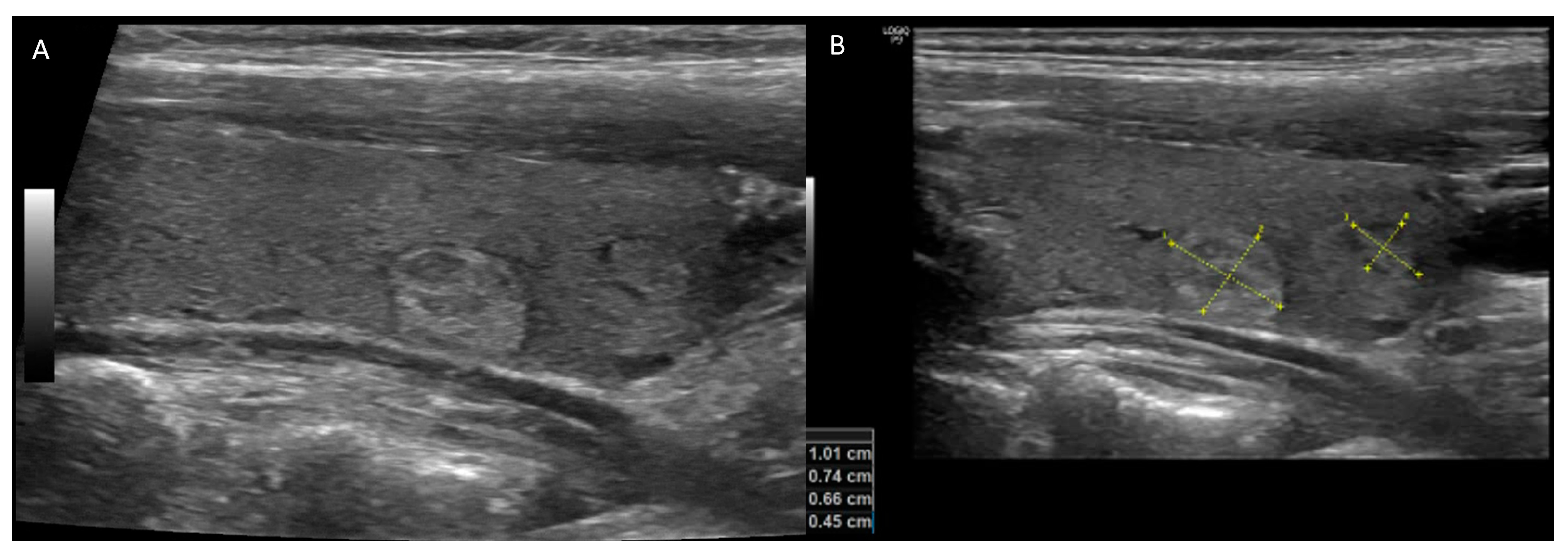
Figure 1. A 56-year-old woman with small hyperechoic and isoechoic nodules with regular borders ((A) longitudinal-plane US image) and size equal to and less than 1 cm ((B) labeled longitudinal-plane image). Thyroid US risk evaluations were reported using ACR TIRADS 3, EU-TIRADS 3, ATA classifications: low suspicion. Considering the size and risk of the nodules, no biopsy was recommended.

Figure 2. A 45-year-old woman with a family history of thyroid cancer. US showed ((A) transverse-plane image, (B) longitudinal-plane image, (C) labeled longitudinal-plane image) hypoechoic nodule with microcalcifications, taller-than-wide shape, irregular borders, and size > 1 cm. Thyroid US risk evaluations were reported using ACR TIRADS 5, EU-TIRADS 5, and ATA classifications: high suspicion. Considering the size and risk of the nodules, FNAC was performed; they were reported to be malignant, and total thyroidectomy revealed papillary thyroid carcinoma.
2.3. Nuclear Medicine
Thyroid scintigraphy performed with functional tracers is used to map the global and regional activity of sodium iodide transporter (NIS) within the thyroid gland. Nowadays, it is commonly performed by using Na[99mTc]TcO4, preferred over iodine tracers (Na[131I]I or Na[123I]I) due to its shorter physical half-life (6 h), pure gamma emission (140 keV), low radiation burden, wide availability, and significantly lower costs [44]. Na[99mTc]TcO4 is caught within thyrocytes through NIS located on the basolateral membrane, like radioiodine tracers. On the contrary, as the main difference, it is not organified and leaves thyrocytes with an effective half-life (6 h) shorter than that of Na[123I]I (about 13 h) and Na[131I]I (about 8 days). However, Na[99mTc]TcO4 uptake is representative of thyroid hormone biosynthesis and provides relevant clinical information on the global and regional function of thyroid cells (Figure 3).
Figure 3. A 65-year-old man affected by overt hyperthyroidism (TSH 0.00 μIU/mL (0.35–4.94), FT3 9.61 pg/mL (1.58–3.91), FT4 2.25 ng/dL (0.70–1.48)) associated with tachycardia, insomnia, and irritability. Thyroid ultrasonography showed a large-sized nodule (38 × 34 × 20 mm) in the right thyroid lobe, isoechoic with a hypoechoic ring. Moderately increased intra-nodular blood flow was also noted. Thyroid scintigraphy was performed 20 min after Na[99mTc]TcO4 administration (111 MBq). Image (anterior view; magnification: 1.4; matrix: 256 × 256; time frame: 100 Kcs) demonstrated a well-defined hyperfunctioning area located in the middle–lower part of the right thyroid lobe. In this latter, tracer uptake/distribution was quite intense and homogeneous, respectively. On the contrary, mild tracer uptake was noted in the remaining right lobe (upper part), isthmus, and left lobe. Thus, a toxic nodular goiter with severe functional inhibition of the remaining thyroid parenchyma was diagnosed.
| |||||
| None | |||||
|
EU-TIRADS 2 | benign, malignancy risk 0%; | No FNA required (unless for therapeutic purposes/to relieve compression) | ||
|
|
EU-TIRADS 3 | low risk, malignancy risk 2–4% | >20 mm FNA | |
| EU-TIRADS 4 | intermediate risk, malignancy risk 6–17% | >15 mm FNA | |||
| EU-TIRADS 5 | high risk, malignancy risk 26–87% | >10 mm FNA, <10 mm: consider FNA or active surveillance | |||
| (b) | |||||
| Category | Points | Malignancy Risk, % | Recommendations | ||
| TR1: | 0 points | benign (aggregate risk level 0.3%); | No FNA required | ||
| TR2: | 2 points | not suspicious (aggregate risk level 1.5%); | No FNA required | ||
| TR3: | 3 points | mildly suspicious (aggregate risk level 4.8%); | ≥25 mm FNA | ||
| TR4: | 4–6 points | moderately suspicious (aggregate risk level 5.9–12.8%); | ≥15 mm FNA | ||
| TR5: | 7 points or more | highly suspicious (aggregate risk level 20.8–68.4% for 10 points). | ≥10 mm FNA, | ||
| (c) | |||||
| Category | Malignancy Risk, % | Recommendations | |||
| Benign | Risk level < 1% | No FNA required | |||
| Very low suspicion | Risk level < 3% | Consider FNA at ≥2 cm Observation without FNA is also a reasonable option |
|||
| Low suspicion | Risk level 5–10% | Recommend FNA at ≥1.5 cm | |||
| Intermediate suspicion | Risk level 10–20% | Recommend FNA at ≥1 cm | |||
| High suspicion | Risk level > 70–90% | Recommend FNA at ≥1 cm | |||
-
Preprocedural
-
Specimen Staining
-
Material Adequacy and False-Negative Results
-
Comparison of Aspiration and Capillary Action
-
Comparison with Core-Needle Biopsy
Table 5. The 2017 Bethesda System for Reporting Thyroid Cytopathology: implied risk of malignancy and recommended clinical management [83].
| Diagnostic Category | Risk of Malignancy if NIFTP ≠ CA (%) | Risk of Malignancy if NIFTP = CA (%) | Usual Management | |||||
|---|---|---|---|---|---|---|---|---|
| I. Non-diagnostic or unsatisfactory | 5–10 | 5–10 | Repeat FNA with ultrasound guidance | |||||
| II. Benign | ||||||||
| Diagnostic category (ROM %) | Management | Diagnostic category (ROM %) | Management | Diagnostic category (ROM %) | Management | |||
| TIR 3A Low-risk indeterminate lesion | 0–3 | 0–3 | Clinical and sonographic follow-up | |||||
| (12–22%) | Consistent with a benign follicular nodule (includes adenomatoid nodule, colloid nodule, etc.). Consistent with lymphocytic (Hashimoto) thyroiditis in the proper clinical context Consistent with granulomatous (subacute) thyroiditis |
TIR2 Non-malignant | ||||||
| Clinical follow | up/Repeat FNA |
III. AUS/FLUS (10–30%) |
Repeat FNA/Molecular testing or lobectomy |
Thy 3a Neoplasm possible – atypia/non-diagnostic (25%) |
Multidisciplinary assessment | III. Atypia of undetermined significance or follicular lesion of undetermined significance | Thy3a Neoplasm possible—atypia/non-diagnostic | |
| TIR 3B High-risk indeterminate lesion | 6–18III. Atypia of undetermined significance or follicular lesion of undetermined significance | TIR3A Low-risk indeterminate lesion (LRIL) | ||||||
(30–55%) |
Surgery | IV. Follicular neoplasm/suspicious follicular neoplasm (25–40%) |
Molecular testing, lobectomy |
Thy3f Neoplasm possible, suggesting follicular neoplasm | IV. Follicular neoplasm or suspicious for a follicular neoplasm Specify if Hürthle cell (oncocytic) type |
TIR3B High-risk indeterminate lesion (HRIL) | ||
| Thy 3f | Thy4 Suspicious of malignancy | V. Suspicious for malignancy | TIR4 Suspicious of malignancy | |||||
| Neoplasm possible, | suggesting follicular | neoplasm (31%) |
Multidisciplinary assessment | Thy5 Malignant | VI. Malignant | TIR5 Malignant |
Legend: ROM: risk of malignancy; AUS: atypia of undetermined significance; FLUS: follicular lesion of undetermined significance.
Despite all these nuances in ultrasound RSS and cytopathology reporting systems, physicians should decide the optimal management with the help of the patient’s history and preferences, the data obtained from nuclear medicine imaging findings, and molecular testing if available.
3. Molecular Biomarkers
Molecular markers may have diagnostic or prognostic purposes. Approximately 15 to 30% of thyroid nodules are classified as indeterminate in the FNAC report [85]. They include Bethesda III (atypia of undetermined significance) and Bethesda IV (follicular neoplasm) lesions, with risks of malignancy (mean, range) of 28 (11–54)% and 50 (28–100)%, respectively [82]. If excluding nodules diagnosed by surgical pathologic examination as non-invasive follicular thyroid neoplasms with papillary-like nuclear features, the risks of malignancy are 6.4 (6–20) and 7.1 (0.2–30) [82]. The principal use of molecular markers in this FNAC categories is diagnostic, i.e., ruling out or ruling in thyroid cancer, with implications for further patient management. The Cancer Genome Atlas Research Network 2014 investigated the molecular profile of 496 papillary thyroid cancers (PTCs), mainly classical and follicular subtypes, and demonstrated two distinct molecular profiles: BRAFV600E-like and RAS-like [86]. The first included the BRAFV600E mutation as well as RET/PTC and BRAF fusions, while the other one included RAS family (HRAS, NRAS, KRAS) mutations, BRAFK601E mutation, EIF1AX mutations, and THADA and PPARG fusions. These two molecular profiles seem to be associated with classical and follicular PTCs but are also noted in other DTC histotypes. Non-invasive follicular neoplasm with papillary-like nuclear features (NIFTP) and follicular thyroid carcinoma (FTC) are also considered RAS-like tumors [85]. Furthermore, benign lesions may also have RAS-like molecular profiles [87]. Molecular panels for indeterminate thyroid nodules range from the 7-gene panel, including BRAF, KRAS, NRAS, HRAS, RET/PTC1, RET/PTC3, and PPARG/PAX8, to 112-gene panels [85]. The small seven-gene panel includes the most common gene alterations in thyroid nodules and has a high specificity. Therefore, it may be useful as a rule-in test. According to a recent prospective study, this test can significantly increase the probability of cancer in mutated undetermined thyroid nodules, with a specificity of 95% and a PPV of 67% [88]. Commercially available panels for molecular testing of indeterminate thyroid nodules include Afirma GSC, Thyroseq v3, and ThyGeNEXT/ThyraMIR. Afirma GSC combines next-generation RNA sequencing and small genomic alterations that are not identifiable with standard methods. It is used mainly as a rule-out test with a high NPV of 96%, while the sensitivity, specificity, and PPV are 68, 91, and 47%, respectively [89]. Thyroseq v3 is a panel of 112 genes with a high NPV of 97%, and the sensitivity, specificity, and PPV are 82, 94, and 66%, respectively, and is also applicable as a rule-out testLegend: TI-RADS, Thyroid Imaging-Reporting and Data System; Na[99mTc]TcO4, 99mTc-pertechnetate; Na[123I]I, iodine-123; PET/CT, positron emission tomography/computed tomography; MTC, medullary thyroid cancer.
As thyroid nodules are very frequent while thyroid cancers are rare and relatively indolent in most cases, the main challenge in approaching patients with thyroid nodules is to identify malignant lesions while avoiding inappropriate excess use of thyroid US, scintigraphy, FNAC, and surgery. Unfortunately, a significant lack of standardization in the preoperative characterization of thyroid nodules is reported in the literature and in guidelines, and, in turn, is commonly observed in clinical practice [93].
To further complicate the problem, a major proportion of nodules are currently detected during imaging examinations for non-thyroid issues or during so-called thyroid nodule screening. Notably, thyroid US examination is discouraged in patients without clinical evidence of thyroid enlargement or nodules, and thyroid incidentaloma, especially when <10 mm, should not be referred for additional examinations [94]. Unfortunately, such concepts are rarely incorporated into clinical practice and a plethora of non-significant nodules are detected, inducing fear and anxiety in patients and a lot of inappropriate additional examinations [95]. Interestingly, Asian thyroid nodule practice has a more conservative approach in general, not only for indeterminate thyroid nodules [96] but also for papillary microcarcinoma [97][98].
Usually, patients experience cancer fears and anxiety during the evaluation of thyroid nodules. Accordingly, a “one-stop-shop” multidisciplinary approach represents an ideal solution. The objectives of a one-stop-shop diagnostic thyroid unit are to concentrate in one place and time all specialists required to provide a correct diagnosis and reach a clinical decision as soon as possible (ideally within 1 day). In any case, patients should be first examined by an experienced clinician (i.e., endocrinologist, nuclear medicine physician, endocrine surgeon) and a blood sample should be obtained for TSH measurement. Based on clinical examination and TSH results, most patients can be properly informed and reassured, and avoid further examinations.
Among patients with clinically relevant nodules and low-to-suppressed TSH levels, a thyroid scintigraphy with either Na[99mTc]TcO4 or Na[123I]I should be ordered to detect autonomously functioning nodules and exclude them from FNAC, as malignancies are exceedingly rare in such cases. In other cases, US examination should be performed and interpreted/reported according to one of the available TI-RADS systems in order to standardize the selection of nodules that need further investigation with FNAC. All in all, FNAC will be necessary for the minority of patients with non-autonomous thyroid nodules and a high-risk TIRADS score. Cytopathology examinations should be reported according to the Bethesda System for Reporting Thyroid Cytopathology and molecular imaging should be considered in selected patients with indeterminate non-Hurtle cell cytology and no additional factors in favor of surgery (i.e., multinodular goiter). Proper patient–physician communication is pivotal before any step in order to clearly explain the pros and cons of a data procedure and its impact on clinical decisions.
Finally, integrating different reports allows the coordinator physician to inform the patient about the diagnosis and formulate a tailored management plan (i.e., surgery, wait and see, thermal ablation). A diagnostic algorithm is proposed in Figure 7.
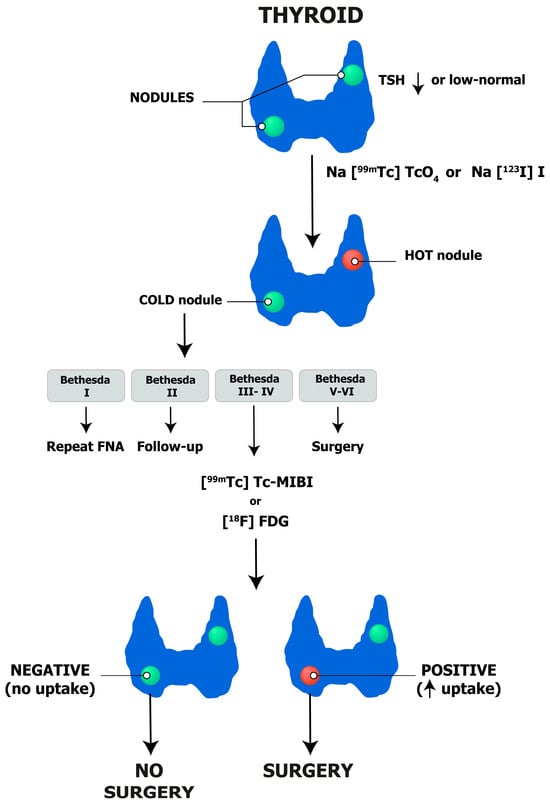 The implementation of this algorithm into clinical practice is mandatory to reassure patients regarding unnecessary therapeutic procedures and to identify those who need specific therapies. The value of thyroid US and its scoring systems, scintigraphy, FNAC, and molecular imaging is proven in many previously mentioned studies and requires further, more extensive, and wider integration into the national guidelines of each country to ensure widespread adoption and accessibility of integrated diagnostics of thyroid nodules.
The implementation of this algorithm into clinical practice is mandatory to reassure patients regarding unnecessary therapeutic procedures and to identify those who need specific therapies. The value of thyroid US and its scoring systems, scintigraphy, FNAC, and molecular imaging is proven in many previously mentioned studies and requires further, more extensive, and wider integration into the national guidelines of each country to ensure widespread adoption and accessibility of integrated diagnostics of thyroid nodules.

Figure 7.
Thyroid nodules: integrated diagnostic flow-chart.
References
- Leung, A.M.; Braverman, L.E.; Pearce, E.N. History of U.S. Iodine Fortification and Supplementation. Nutrients 2012, 4, 1740–1746.
- Popoveniuc, G.; Jonklaas, J. Thyroid Nodules. Med. Clin. N. Am. 2012, 96, 329–349.
- Jolanta, M.D.; Bogsrud, T.V. Nuclear Medicine in Evaluation and Therapy of Nodular Thyroid. In Thyroid Nodules; Springer International Publishing AG: Cham, Switzerland, 2018.
- Alexander, E.K.; Cibas, E.S. Diagnosis of Thyroid Nodules. Lancet Diabetes Endocrinol. 2022, 10, 533–539.
- Schenke, S.A.; Kreissl, M.C.; Grunert, M.; Hach, A.; Haghghi, S.; Kandror, T.; Peppert, E.; Rosenbaum-Krumme, S.; Ruhlmann, V.; Stahl, A.; et al. Distribution of Functional Status of Thyroid Nodules and Malignancy Rates of Hyperfunctioning and Hypofunctioning Thyroid Nodules in Germany. Nuklearmedizin 2022, 61, 376–384.
- Hegedüs, L. Clinical Practice. The Thyroid Nodule. N. Engl. J. Med. 2004, 351, 1764–1771.
- Durante, C.; Costante, G.; Lucisano, G.; Bruno, R.; Meringolo, D.; Paciaroni, A.; Puxeddu, E.; Torlontano, M.; Tumino, S.; Attard, M.; et al. The Natural History of Benign Thyroid Nodules. JAMA 2015, 313, 926–935.
- Fisher, S.B.; Perrier, N.D. The Incidental Thyroid Nodule. CA Cancer J. Clin. 2018, 68, 97–105.
- Guth, S.; Theune, U.; Aberle, J.; Galach, A.; Bamberger, C.M. Very High Prevalence of Thyroid Nodules Detected by High Frequency (13 MHz) Ultrasound Examination. Eur. J. Clin. Investig. 2009, 39, 699–706.
- Li, L.Q.; Hilmi, O.; England, J.; Tolley, N. An Update on the Management of Thyroid Nodules: Rationalising the Guidelines. J. Laryngol. Otol. 2023, 137, 965–970.
- Yousem, D.M.; Huang, T.; Loevner, L.A.; Langlotz, C.P. Clinical and Economic Impact of Incidental Thyroid Lesions Found with CT and MR. Am. J. Neuroradiol. 1997, 18, 1423–1428.
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Screening for Thyroid Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2017, 317, 1882–1887.
- Schenke, S.A.; Groener, D.; Grunert, M.; Stahl, A.R. Integrated Thyroid Imaging: Ultrasound and Scintigraphy. In Integrated Diagnostics and Theranostics of Thyroid Diseases; Giovanella, L., Ed.; Springer: Cham, Switzerland, 2023; pp. 25–62.
- de Koster, E.J.; Morreau, H.; Bleumink, G.S.; van Engen-van Grunsven, A.C.H.; de Geus-Oei, L.-F.; Links, T.P.; Wakelkamp, I.M.M.J.; Oyen, W.J.G.; Vriens, D. Molecular Diagnostics and FDG-PET/CT in Indeterminate Thyroid Nodules: Complementing Techniques or Waste of Valuable Resources? Thyroid, 2023; online ahead of print.
- de Koster, E.J.; Vriens, D.; van Aken, M.O.; Dijkhorst-Oei, L.T.; Oyen, W.J.G.; Peeters, R.P.; Schepers, A.; de Geus-Oei, L.F.; van den Hout, W.B. FDG-PET/CT in Indeterminate Thyroid Nodules: Cost-Utility Analysis alongside a Randomised Controlled Trial. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3452–3469.
- Wale, A.; Miles, K.A.; Young, B.; Zammit, C.; Williams, A.; Quin, J.; Dizdarevic, S. Combined (99m)Tc-Methoxyisobutylisonitrile Scintigraphy and Fine-Needle Aspiration Cytology Offers an Accurate and Potentially Cost-Effective Investigative Strategy for the Assessment of Solitary or Dominant Thyroid Nodules. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 105–115.
- Heinzel, A.; Müller, D.; Behrendt, F.F.; Giovanella, L.; Mottaghy, F.M.; Verburg, F.A. Thyroid Nodules with Indeterminate Cytology: Molecular Imaging with 99mTc-Methoxyisobutylisonitrile (MIBI) Is More Cost-Effective than the Afirma Gene Expression Classifier. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1497–1500.
- Koulouri, O.; Moran, C.; Halsall, D.; Chatterjee, K.; Gurnell, M. Pitfalls in the Measurement and Interpretation of Thyroid Function Tests. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 745–762.
- D’Aurizio, F.; Kratzsch, J.; Gruson, D.; Petranović Ovčariček, P.; Giovanella, L. Free Thyroxine Measurement in Clinical Practice: How to Optimize Indications, Analytical Procedures, and Interpretation Criteria While Waiting for Global Standardization. Crit. Rev. Clin. Lab. Sci. 2023, 60, 101–140.
- Giovanella, L.; Petranović Ovčariček, P. Functional and Molecular Thyroid Imaging. Q. J. Nucl. Med. Mol. Imaging 2022, 66, 86–92.
- Kronenberg, H.M.; Melmed, S.; Larsen, P.R.; Polonsky, K.S. Principles of Endocrinology. In Williams Textbook of Endocrinology; Melmed, S., Polonsky, K.S., Larsen, P.R., Kronenberg, H.M., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 3–12.
- Giovanella, L.; Avram, A.M.; Ovčariček, P.P.; Clerc, J. Thyroid Functional and Molecular Imaging. Presse Med. 2022, 51, 104116.
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the Treatment of Hypothyroidism: Prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014, 24, 1670–1751.
- Demers, L.M.; Spencer, C.A. Laboratory Medicine Practice Guidelines: Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease. Clin. Endocrinol. 2003, 58, 138–140.
- Plebani, M.; Giovanella, L. Reflex TSH Strategy: The Good, the Bad and the Ugly. Clin. Chem. Lab. Med. 2019, 58, 1–2.
- Sheehan, M.T. Biochemical Testing of the Thyroid: TSH Is the Best and, Oftentimes, Only Test Needed—A Review for Primary Care. Clin. Med. Res. 2016, 14, 83–92.
- Giovanella, L.; D’Aurizio, F.; Campenni’, A.; Ruggeri, R.; Baldari, S.; Verburg, F.; Trimboli, P.; Ceriani, L. Searching For The Most Effective Thyrotropin (TSH) Threshold To Rule-Out Autonomously Functioning Thyroid Nodules In Iodine Deficient Regions. Endocrine 2016, 54, 757–761.
- Repplinger, D.; Bargren, A.; Zhang, Y.W.; Adler, J.T.; Haymart, M.; Chen, H. Is Hashimoto’s Thyroiditis a Risk Factor for Papillary Thyroid Cancer? J. Surg. Res. 2008, 150, 49–52.
- Giovanella, L.; D’Aurizio, F.; Algeciras-Schimnich, A.; Görges, R.; Petranovic Ovcaricek, P.; Tuttle, R.M.; Visser, W.E.; Verburg, F.A. Thyroglobulin and Thyroglobulin Antibody: An Updated Clinical and Laboratory Expert Consensus. Eur. J. Endocrinol. 2023, 189, R11–R27.
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; MacHens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid 2015, 25, 567–610.
- Verbeek, H.H.; de Groot, J.W.B.; Sluiter, W.J.; Kobold, A.C.M.; van den Heuvel, E.R.; Plukker, J.T.; Links, T.P. Calcitonin Testing for Detection of Medullary Thyroid Cancer in People with Thyroid Nodules. Cochrane Database Syst. Rev. 2020, 3, CD010159.
- Rago, T.; Vitti, P. Role of Thyroid Ultrasound in the Diagnostic Evaluation of Thyroid Nodules. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 913–928.
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133.
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595.
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237.
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.H.; Lee, Y.H.; Lim, H.K.; Moon, W.J.; Na, D.G.; Park, J.S.; et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2016, 17, 370–395.
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedüs, L.; Paschke, R.; Valcavi, R.; Vitti, P. American association of clinical endocrinologists, american college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules-2016 update. Endocr. Pract. 2016, 22, 622–639.
- Perros, P.; Boelaert, K.; Colley, S.; Evans, C.; Evans, R.M.; Gerrard Ba, G.; Gilbert, J.; Harrison, B.; Johnson, S.J.; Giles, T.E.; et al. Guidelines for the Management of Thyroid Cancer. Clin. Endocrinol. 2014, 81 (Suppl. 1), 1–122.
- Hoang, J.K.; Asadollahi, S.; Durante, C.; Hegedüs, L.; Papini, E.; Tessler, F.N. An International Survey on Utilization of Five Thyroid Nodule Risk Stratification Systems: A Needs Assessment with Future Implications. Thyroid 2022, 32, 675–681.
- Durante, C.; Hegedüs, L.; Czarniecka, A.; Paschke, R.; Russ, G.; Schmitt, F.; Soares, P.; Solymosi, T.; Papini, E. 2023 European Thyroid Association Clinical Practice Guidelines for Thyroid Nodule Management. Eur. Thyroid J. 2023, 12, e230067.
- Seifert, P.; Schenke, S.; Zimny, M.; Stahl, A.; Grunert, M.; Klemenz, B.; Freesmeyer, M.; Kreissl, M.C.; Herrmann, K.; Görges, R. Diagnostic Performance of Kwak, EU, ACR, and Korean TIRADS as Well as ATA Guidelines for the Ultrasound Risk Stratification of Non-Autonomously Functioning Thyroid Nodules in a Region with Long History of Iodine Deficiency: A German Multicenter Trial. Cancers 2021, 13, 4467.
- Kuru, B.; Kefeli, M.; Danaci, M. Comparison of 5 Thyroid Ultrasound Stratification Systems for Differentiation of Benign and Malignant Nodules and to Avoid Biopsy Using Histology as Reference Standard. Endocr. Pract. 2021, 27, 1093–1099.
- Li, X.; Peng, C.; Liu, Y.; Hu, Y.; Yang, L.; Yu, Y.; Zeng, H.; Huang, W.; Li, Q.; Tao, N.; et al. Modified American College of Radiology Thyroid Imaging Reporting and Data System and Modified Artificial Intelligence Thyroid Imaging Reporting and Data System for Thyroid Nodules: A Multicenter Retrospective Study. Thyroid, 2023; online ahead of print.
- Giovanella, L.; Avram, A.M.; Iakovou, I.; Kwak, J.; Lawson, S.A.; Lulaj, E.; Luster, M.; Piccardo, A.; Schmidt, M.; Tulchinsky, M.; et al. EANM Practice Guideline/SNMMI Procedure Standard for RAIU and Thyroid Scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2514–2525.
- Giovanella, L.; Ceriani, L.; Treglia, G. Role of Isotope Scan, Including Positron Emission Tomography/Computed Tomography, in Nodular Goitre. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 507–518.
- Hetrakul, N.; Civelek, A.C.; Stagg, C.A.; Udelsman, R. In Vitro Accumulation of Technetium-99m-Sestamibi in Human Parathyroid Mitochondria. Surgery 2001, 130, 1011–1018.
- Karamzade-Ziarati, N.; Manafi-Farid, R.; Ataeinia, B.; Langsteger, W.; Pirich, C.; Mottaghy, F.M.; Beheshti, M. Molecular Imaging of Bone Metastases Using Tumor-Targeted Tracers. Q. J. Nucl. Med. Mol. Imaging 2019, 63, 136–149.
- Delmaire, C.; Savatovsky, J.; Boulanger, T.; Dhermain, F.; Le Rhun, E.; Météllus, P.; Gerber, S.; Carsin-Nicole, B.; Petyt, G. Imagerie Des Métastases Cérébrales. Cancer/Radiothérapie 2015, 19, 16–19.
- Liu, H.; Zhan, H.; Sun, D. Comparison of 99mTc-MIBI Scintigraphy, Ultrasound, and Mammography for the Diagnosis of BI-RADS 4 Category Lesions. BMC Cancer 2020, 20, 1–8.
- Hurtado-López, L.M.; Arellano-Montaño, S.; Torres-Acosta, E.M.; Zaldivar-Ramirez, F.R.; Duarte-Torres, R.M.; Alonso-De-Ruiz, P.; Martínez-Duncker, I.; Martínez-Duncker, C. Combined Use of Fine-Needle Aspiration Biopsy, MIBI Scans and Frozen Section Biopsy Offers the Best Diagnostic Accuracy in the Assessment of the Hypofunctioning Solitary Thyroid Nodule. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1273–1279.
- Giovanella, L.; Campenni, A.; Treglia, G.; Verburg, F.A.; Trimboli, P.; Ceriani, L.; Bongiovanni, M. Molecular Imaging with (99m)Tc-MIBI and Molecular Testing for Mutations in Differentiating Benign from Malignant Follicular Neoplasm: A Prospective Comparison. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1018–1026.
- Campennì, A.; Giovanella, L.; Siracusa, M.; Alibrandi, A.; Pignata, S.A.; Giovinazzo, S.; Trimarchi, F.; Ruggeri, R.M.; Baldari, S. (99m)Tc-Methoxy-Isobutyl-Isonitrile Scintigraphy Is a Useful Tool for Assessing the Risk of Malignancy in Thyroid Nodules with Indeterminate Fine-Needle Cytology. Thyroid 2016, 26, 1101–1109.
- Campennì, A.; Siracusa, M.; Ruggeri, R.M.; Laudicella, R.; Pignata, S.A.; Baldari, S.; Giovanella, L. Differentiating Malignant from Benign Thyroid Nodules with Indeterminate Cytology by 99m Tc-MIBI Scan: A New Quantitative Method for Improving Diagnostic Accuracy. Sci. Rep. 2017, 7, 6147.
- Schenke, S.A.; Campennì, A.; Tuncel, M.; Bottoni, G.; Sager, S.; Crncic, T.B.; Rozic, D.; Görges, R.; Özcan, P.P.; Groener, D.; et al. Diagnostic Performance of 99mTc-Methoxy-Isobuty-Isonitrile (MIBI) for Risk Stratification of Hypofunctioning Thyroid Nodules: A European Multicenter Study. Diagnostics 2022, 12, 1358.
- Schenke, S.A.; Campenni, A.; Tuncel, M.; Piccardo, A.; Sager, S.; Bogovic Crncic, T.; Rozic, D.; Goerges, R.; Özcan Kara, P.P.; Groener, D.; et al. A Multicenter Survey of Current Practices of 99mTc-Methoxy-Isobutyl-Isonitrile (MIBI) Imaging for the Diagnosis of Thyroid Nodules: More Standardization Is Essential. Clin. Transl. Imaging 2021, 9, 413–422.
- Giovanella, L.; Suriano, S.; Ricci, R.; Ceriani, L.; Verburg, F.A. Postsurgical Thyroid Remnant Estimation by (99m) Tc-Pertechnetate Scintigraphy Predicts Radioiodine Ablation Effectiveness in Patients with Differentiated Thyroid Carcinoma. Head Neck 2011, 33, 552–556.
- Saggiorato, E.; Angusti, T.; Rosas, R.; Martinese, M.; Finessi, M.; Arecco, F.; Trevisiol, E.; Bergero, N.; Puligheddu, B.; Volante, M.; et al. 99mTc-MIBI Imaging in the Presurgical Characterization of Thyroid Follicular Neoplasms: Relationship to Multidrug Resistance Protein Expression. J. Nucl. Med. 2009, 50, 1785–1793.
- Piga, M.; Cocco, M.C.; Serra, A.; Boi, F.; Loy, M.; Mariotti, S. The Usefulness of 99mTc-SestaMIBI Thyroid Scan in the Differential Diagnosis and Management of Amiodarone-Induced Thyrotoxicosis. Eur. J. Endocrinol. 2008, 159, 423–429.
- Pattison, D.A.; Westcott, J.; Lichtenstein, M.; Toh, H.B.; Gunawardana, D.; Better, N.; Forehan, S.; Sivaratnam, D. Quantitative Assessment of Thyroid-to-Background Ratio Improves the Interobserver Reliability of Technetium-99m Sestamibi Thyroid Scintigraphy for Investigation of Amiodarone-Induced Thyrotoxicosis. Nucl. Med. Commun. 2015, 36, 356–362.
- Hervás Morón, A. PET-CT in Oncology. Clin. Transl. Oncol. 2007, 9, 473–474.
- Meyer, H.J.; Wienke, A.; Surov, A. Associations between GLUT Expression and SUV Values Derived from FDG-PET in Different Tumors-A Systematic Review and Meta Analysis. PLoS ONE 2019, 14, e0217781.
- de Koster, E.J.; de Geus-Oei, L.F.; Brouwers, A.H.; van Dam, E.W.C.M.; Dijkhorst-Oei, L.T.; van Engen-van Grunsven, A.C.H.; van den Hout, W.B.; Klooker, T.K.; Netea-Maier, R.T.; Snel, M.; et al. FDG-PET/CT to Prevent Futile Surgery in Indeterminate Thyroid Nodules: A Blinded, Randomised Controlled Multicentre Trial. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1970–1984.
- Vriens, D.; De Wilt, J.H.W.; Van Der Wilt, G.J.; Netea-Maier, R.T.; Oyen, W.J.G.; De Geus-Oei, L.F. The Role of -2-Fluoro-2-Deoxy-d-Glucose-Positron Emission Tomography in Thyroid Nodules with Indeterminate Fine-Needle Aspiration Biopsy: Systematic Review and Meta-Analysis of the Literature. Cancer 2011, 117, 4582–4594.
- Wang, N.; Zhai, H.; Lu, Y. Is Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography Useful for the Thyroid Nodules with Indeterminate Fine Needle Aspiration Biopsy? A Meta-Analysis of the Literature. J. Otolaryngol. Head Neck Surg. 2013, 42, 38.
- Castellana, M.; Trimboli, P.; Piccardo, A.; Giovanella, L.; Treglia, G. Performance of 18 F-FDG PET/CT in Selecting Thyroid Nodules with Indeterminate Fine-Needle Aspiration Cytology for Surgery. A Systematic Review and a Meta-Analysis. J. Clin. Med. 2019, 8, 1333.
- Giovanella, L.; Milan, L.; Piccardo, A.; Bottoni, G.; Cuzzocrea, M.; Paone, G.; Ceriani, L. Radiomics Analysis Improves 18FDG PET/CT-Based Risk Stratification of Cytologically Indeterminate Thyroid Nodules. Endocrine 2022, 75, 202–210.
- de Koster, E.J.; Noortman, W.A.; Mostert, J.M.; Booij, J.; Brouwer, C.B.; de Keizer, B.; de Klerk, J.M.H.; Oyen, W.J.G.; van Velden, F.H.P.; de Geus-Oei, L.F.; et al. Quantitative Classification and Radiomics of FDG-PET/CT in Indeterminate Thyroid Nodules. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2174–2188.
- Dean, D.S.; Gharib, H. Epidemiology of Thyroid Nodules. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 901–911.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30.
- Moon, W.J.; So, L.J.; Jeong, H.L.; Dong, G.N.; Baek, J.H.; Young, H.L.; Kim, J.; Hyun, S.K.; Jun, S.B.; Dong, H.L. Benign and Malignant Thyroid Nodules: US Differentiation--Multicenter Retrospective Study. Radiology 2008, 247, 762–770.
- Kim, M.J.; Kim, E.K.; Park, S.I.; Kim, B.M.; Kwak, J.Y.; Kim, S.J.; Youk, J.H.; Park, S.H. US-Guided Fine-Needle Aspiration of Thyroid Nodules: Indications, Techniques, Results. Radiographics 2008, 28, 1869–1886.
- Black, J.M. Anticoagulation in Elective Surgery. Plast. Surg. Nurs. 2004, 24, 8–11.
- Oertel, Y.C. Fine-Needle Aspiration of the Thyroid: Technique and Terminology. Endocrinol. Metab. Clin. N. Am. 2007, 36, 737–751.
- Santos, J.E.; Leiman, G. Nonaspiration Fine Needle Cytology. Application of a New Technique to Nodular Thyroid Disease. Acta Cytol. 1988, 32, 353–356.
- Degirmenci, B.; Haktanir, A.; Albayrak, R.; Acar, M.; Sahin, D.A.; Sahin, O.; Yucel, A.; Caliskan, G. Sonographically Guided Fine-Needle Biopsy of Thyroid Nodules: The Effects of Nodule Characteristics, Sampling Technique, and Needle Size on the Adequacy of Cytological Material. Clin. Radiol. 2007, 62, 798–803.
- Titton, R.L.; Gervais, D.A.; Boland, G.W.; Maher, M.M.; Mueller, P.R. Sonography and Sonographically Guided Fine-Needle Aspiration Biopsy of the Thyroid Gland: Indications and Techniques, Pearls and Pitfalls. AJR Am. J. Roentgenol. 2003, 181, 267–271.
- Quinn, S.F.; Nelson, H.A.; Demlow, T.A. Thyroid Biopsies: Fine-Needle Aspiration Biopsy versus Spring-Activated Core Biopsy Needle in 102 Patients. J. Vasc. Interv. Radiol. 1994, 5, 619–623.
- Taki, S.; Kakuda, K.; Kakuma, K.; Annen, Y.; Katada, S.; Yamashita, R.; Kosugi, M.; Michigishi, T.; Tonami, N. Thyroid Nodules: Evaluation with US-Guided Core Biopsy with an Automated Biopsy Gun. Radiology 1997, 202, 874–877.
- Pusztaszeri, M.; Rossi, E.D.; Auger, M.; Baloch, Z.; Bishop, J.; Bongiovanni, M.; Chandra, A.; Cochand-Priollet, B.; Fadda, G.; Hirokawa, M.; et al. The Bethesda System for Reporting Thyroid Cytopathology: Proposed Modifications and Updates for the Second Edition from an International Panel. Acta Cytol. 2016, 60, 399–405.
- Nardi, F.; Basolo, F.; Crescenzi, A.; Fadda, G.; Frasoldati, A.; Orlandi, F.; Palombini, L.; Papini, E.; Zini, M.; Pontecorvi, A.; et al. Italian Consensus for the Classification and Reporting of Thyroid Cytology. J. Endocrinol. Investig. 2014, 37, 593–599.
- Cross, P.; Chandra, A.; Giles, T.; Johnson, S.; Kocjan, G.; Poller, D. Guidance on the Reporting of Thyroid Cytology Specimens; The Royal College of Pathologists: London, UK, 2016.
- Ali, S.Z.; Baloch, Z.W.; Cochand-Priollet, B.; Schmitt, F.C.; Vielh, P.; VanderLaan, P.A. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023, 33, 1039–1044.
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346.
- Bongiovanni, M.; Bellevicine, C.; Troncone, G.; Sykiotis, G.P. Approach to Cytological Indeterminate Thyroid Nodules. Gland Surg. 2019, 8 (Suppl. 2), S98–S104.
- Vignali, P.; Macerola, E.; Poma, A.M.; Sparavelli, R.; Basolo, F. Indeterminate Thyroid Nodules: From Cytology to Molecular Testing. Diagnostics 2023, 13, 3008.
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690.
- Marotta, V.; Bifulco, M.; Vitale, M. Significance of RAS Mutations in Thyroid Benign Nodules and Non-Medullary Thyroid Cancer. Cancers 2021, 13, 3785.
- Bardet, S.; Goardon, N.; Lequesne, J.; Vaur, D.; Ciappuccini, R.; Leconte, A.; Monpeyssen, H.; Saguet-Rysanek, V.; Clarisse, B.; Lasne-Cardon, A.; et al. Diagnostic and Prognostic Value of a 7-Panel Mutation Testing in Thyroid Nodules with Indeterminate Cytology: The SWEETMAC Study. Endocrine 2021, 71, 407–417.
- Alzumaili, B.; Sadow, P.M. Update on Molecular Diagnostics in Thyroid Pathology: A Review. Genes 2023, 14, 1314.
- Yoo, S.K.; Lee, S.; Kim, S.J.; Jee, H.G.; Kim, B.A.; Cho, H.; Song, Y.S.; Cho, S.W.; Won, J.K.; Shin, J.Y.; et al. Comprehensive Analysis of the Transcriptional and Mutational Landscape of Follicular and Papillary Thyroid Cancers. PLoS Genet. 2016, 12, e1006239.
- Labourier, E.; Fahey, T.J. Preoperative Molecular Testing in Thyroid Nodules with Bethesda VI Cytology: Clinical Experience and Review of the Literature. Diagn. Cytopathol. 2021, 49, E175–E180.
- Liu, J.; Liu, R.; Shen, X.; Zhu, G.; Li, B.; Xing, M. The Genetic Duet of BRAF V600E and TERT Promoter Mutations Robustly Predicts Loss of Radioiodine Avidity in Recurrent Papillary Thyroid Cancer. J. Nucl. Med. 2020, 61, 177–182.
- Eloy, C.; Russ, G.; Suciu, V.; Johnson, S.J.; Rossi, E.D.; Pantanowitz, L.; Vielh, P. Preoperative Diagnosis of Thyroid Nodules: An Integrated Multidisciplinary Approach. Cancer Cytopathol. 2022, 130, 320–325.
- Iglesias, P.; Acosta, M.; Sánchez, R.; Fernández-Reyes, M.J.; Mon, C.; Díez, J.J. Ambulatory Blood Pressure Monitoring in Patients with Hyperthyroidism before and after Control of Thyroid Function. Clin. Endocrinol. 2005, 63, 66–72.
- Jansen, T.; Stikkelbroeck, N.; van de Ven, A.; van Engen-van Grunsven, I.; Janssen, M.; Bonenkamp, H.; Gotthardt, M.; Netea-Maier, R.T. Clinical Characteristics, Diagnostic Approach and Outcome of Thyroid Incidental Findings vs. Clinically Overt Thyroid Nodules: An Observational Single-Centre Study. Cancers 2023, 15, 2350.
- Vuong, H.G.; Ngo, H.T.T.; Bychkov, A.; Jung, C.K.; Vu, T.H.; Lu, K.B.; Kakudo, K.; Kondo, T. Differences in Surgical Resection Rate and Risk of Malignancy in Thyroid Cytopathology Practice between Western and Asian Countries: A Systematic Review and Meta-Analysis. Cancer Cytopathol. 2020, 128, 238–249.
- Sakai, T.; Sugitani, I.; Ebina, A.; Fukuoka, O.; Toda, K.; Mitani, H.; Yamada, K. Active Surveillance for T1bN0M0 Papillary Thyroid Carcinoma. Thyroid 2019, 29, 59–63.
- Miyauchi, A.; Kudo, T.; Ito, Y.; Oda, H.; Sasai, H.; Higashiyama, T.; Fukushima, M.; Masuoka, H.; Kihara, M.; Miya, A. Estimation of the Lifetime Probability of Disease Progression of Papillary Microcarcinoma of the Thyroid during Active Surveillance. Surgery 2018, 163, 48–52.
More
