You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Tao Ran and Version 2 by Catherine Yang.
Bacteriophages, a class of viruses that exclusively infect bacteria, share a prolonged evolutionary history with their hosts. There are three life cycle modes including lytic, lysogenic, and chronic infection for bacteriophages. Bacteriophages possess a plethora of applications and potential in human bacterial diseases and enteropathogenic diseases of livestock and poultry, specifically in the direction of antibiotic substitution, which exhibits tremendous potential for practical applications.
- bacteriophage
- antibiotics
- antibiotic resistance genes
- livestock
1. Regulation of Intestinal Microecology
Gastrointestinal microbes play a pivotal role in the digestion and absorption of nutrients and in the construction of intestinal immune barriers; the distribution and function of bacteriophages in the intestine are shown in Figure 1. Countless bacteriophages and their host bacteria exist in the GIT of farm animals, and in the long-term struggle and coexistence state, bacteria and bacteriophages have evolved a variety of mutualistic mechanisms. On the one hand, besides host bacteria, virulent bacteriophages can exert indirect effects on non-host bacteria, thus exerting contact-dependent antagonistic effects on diverse Gram-positive bacteria [1][40]. Virulent bacteriophages are able to cause changes in the structure of intestinal microbiota through a cascade effect, which in turn affects the composition of intestinal metabolites (tryptamines, carbohydrates, nicotinamide mononucleotides, etc.), reduces neurotransmitter production, and alters bile acid metabolism, which finally leads to physiological and metabolic effects on the organism [2][41]. On the other hand, bacteriophages possess strong permeability, dynamic adaptability, and excellent biocompatibility, making them capable of crossing the multi-layered mucosal barrier and regulating the microbial community through the metabolic cycle in the body, thereby overcoming the deficiencies of antibiotics [3][42]. Bacteriophage supplementation in broiler feed restores fecal microbiota composition to normal levels, in contrast to the antibiotic group (reduced relative abundance of Firmicutes at the phylum level and Lactobacillus at the genus level) [4][37]. Similar results are observed in weaned piglets, where bacteriophages significantly affect bacterial diversity and intestinal metabolism by regulating the dominant composition of microbes. Compared with the antibiotic group, the addition of bacteriophages in feed significantly increases the microbial abundance in the cecum of piglets, exerting an environmental selective pressure on intestinal pathogens, which is crucial for maintaining gastrointestinal homeostasis [5][31].
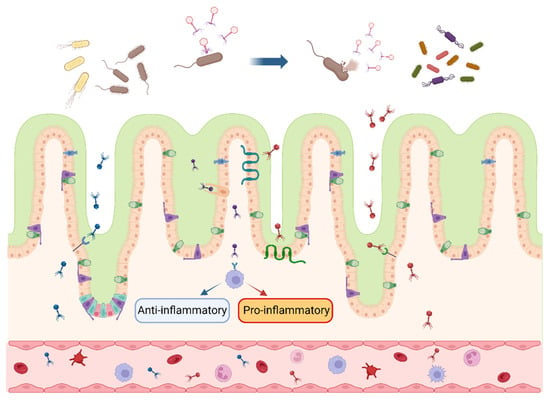
Figure 1. Interactions between bacteriophages and intestinal bacteria and cells. After multiplying and expanding within the intestinal host bacteria, bacteriophages secrete lytic enzymes to lyse the cell wall and release bacteriophage progeny, which continue to inhabit the host bacterial population. Bacteriophages enter the intestinal wall in response to specific receptors and cytophagy in intestinal epithelial cells and cross into the blood circulation, inducing anti-inflammatory (blue arrow) or pro-inflammatory (red arrow) response. The blue arrow at the top of the picture indicates that the bacteriophages infest and lyse the bacteria in the intestinal lumen. Different colors represent various species of bacteriophages and their progeny. (figure created using BioRender, https://biorender.com. accessed on 27 November 2023).
2. Targeted Inhibition of Pathogens
Unlike the broad-spectrum inhibitory approach of antibiotics, bacteriophages exert host specificity for they can recognize specific signal molecules such as polysaccharides and proteins on the bacterial surface [6][43]. Bacteriophages are capable of encoding depolymerase to degrade macromolecules such as capsular polysaccharides, extracellular polysaccharides, and lipopolysaccharides (LPSs) on the outer surface of bacteria, thereby breaking through the bacterial external barrier, which accelerates the injection of bacteriophage nucleic acids into the bacteria to complete the invasion process [7][44].
The reasonabledesign of bacteriophage cocktails can accelerate the advancement of bacteriophages in replacing antibiotics for treating pathogen-induced diseases. Bacteriophage therapy is currently used to treat GIT diseases such as diarrhea induced by E. coli and Salmonella in pigs and poultry. Enterococcus, which has strong antibiotic resistance, is prevalent in the GIT of animals where it maintains a high abundance and aggravates the inflammatory response when treated with antibiotics. Both single bacteriophage and bacteriophage cocktails could be used to inhibit Enterococcus growth. However, a consistent proportion of mutations in the Epa extracellular polysaccharide synthesis gene of Enterococcus is observed when using a single bacteriophage, contributing to the prevention of initial recognition and adsorption of Enterococci by bacteriophages. Whereas co-treatment of E. faecalis with Myoviridae, Siphoviridae, and Podoviridae bacteriophages reduces the abundance of E. faecalis to about 10% of the normal growth state and effectively inhibits the growth of bacteriophage-resistant mutants [8][45]. Similarly, S. typhimurium can adhere to and invade intestinal epithelial cells. In both normal and S. typhimurium-infected intestinal epithelial cells INT-407, bacteriophage P22 significantly reduces the amount of adsorption and infiltration of INT-407 by S. typhimurium [9][46]. Additionally, bacteriophage P22 induces the expression of pro-inflammatory genes in macrophage-like HD11 cells through a non-TLR-mediated recognition pathway, enhancing the immune response to kill S. typhimurium. Saez et al. [10][47] found that direct feeding of microencapsulated bacteriophages significantly reduced the detection of S. typhimurium 2 h and 4 h post challenge, compared with the gavage and control groups. Higher concentrations of anti-Salmonella bacteriophages were detected in the contents of the ileum and cecum in the treatment group than in the control group, suggesting that the bacteriophages successfully colonized the pig intestinal tract and exerted their bactericidal effects. Not coincidentally, Seo et al. [11][48] observed that Salmonella bacteriophage mixtures reduced the level of Salmonella detection in the feces of weaned piglets, demonstrating that bacteriophages offer an alternative to antibiotics for the treatment of Salmonella infections in swine. E. coli, a common Gram-negative pathogen, is susceptible to induce acute mastitis in early lactation of dairy cows and diarrhea in weaned piglets. In a cow model of strongly drug-resistant E. coli-induced mastitis, injection of a combination of bacteriophages (vB_EcoM_SYGD1, vB_EcoP_SYGE1, and vB_EcoM_SYGMH1) into the mammary glands of dairy cows significantly decreased the bacterial and somatic cell counts of the milk and significantly reduced the concentrations of IL-1β and TNF-α concentrations in the blood [12][49]. Similar results have been reported in other animal experiments. When weaned piglets were supplemented with 1 g/kg of a bacteriophage cocktail for 35 days, the abundances of E. coli and C. difficile in the ileum were significantly decreased compared with the control group, and the growth of pathogens was restricted; meanwhile, the bacteriophage cocktail was favorable to the predominance of probiotics such as Lactobacillus, with the digestive and absorptive functions of the intestine on nutrients enhanced, which notably increased the daily weight gain of the weaned piglets [13][32]. In a previous study, a bacteriophage cocktail containing phages B44/1 and B44/2 was reported to treat nearly 93% of calves with enterotoxigenic E. coli strain O9:K30.99-induced enteritis, significantly reducing the morbidity and mortality of the calves [14][50]. The above studies suggest that supplemental bacteriophage cocktails are capable of reversing the pathology induced by the pathogenic bacteria to achieve the equivalent effect of antibiotic treatment without the concern of antibiotic residuals, etc. These pilot studies have greatly strengthened our confidence in the application of bacteriophages as an alternative to antibiotics.
It is noteworthy that the LPS mutant evolved in E. coli to evade the bacteriophages show decreased resistance to tetracycline and mucomycin compared with the wild-type parental strain, and this effect persists throughout the 10-day test cycle in which the bacteriophages co-existed with E. coli. Mutated pathogenic bacteria lose their virulence while evading bacteriophages, which provides a novel insight for the application of bacteriophages in the treatment of livestock and poultry diseases [15][51]. The bacteriophages are able to maintain the health of intestinal microecology and promote the normal development of animal intestinal tissues, which is essential for the healthy growth and development of animals. Nevertheless, the complex gene regulatory network among phages, pathogenic bacteria, and organisms in disease states needs to be explored in more intensive in vivo research.
3. Mediation of Immune Response
The effectiveness of bacteriophages on livestock and poultry also relies on the interaction of bacteriophages with the body’s immune cells. Under intestinal homeostasis, bacteriophages in the intestinal lumen can pass through the intestinal epithelium into the lamina propria through specific receptor uptake by epithelial cells or specific cytophagy, and then migrate via the blood into the circulation to interact with the immune cells to induce a pro-inflammatory or anti-inflammatory response. This is accomplished with inhibited synthesis of NF-κB, IL-2, TNF-α, and IL-10 in the bloodstream, which play an indispensable role in the maintenance of immune responses of the animal system [16][52]. Simultaneously, diverse bacteriophages possess rapid and directional transfection ability in the confluent cell layer of the intestine, brain, lung, liver, and kidney, and traverse the epithelial cell layer within 10 min. For instance, the T4 phage maintains the apical-to-basal direction to cross the confluent cell layer for transport under the cytotropic effect of the Golgi apparatus. It is speculated that bacteriophages could enter the metabolic cycle of the organism from the GIT and then be transferred and exchanged among the organs of multiple systems [17][53].
The mucosal immune system is the primary barrier against pathogen invasion. The intestinal mucosal immune system is activated when the intestinal barrier structure is impaired, thereby initiating an immune response. It is well known that bacteriophages are usually enriched at the site of bacterial colonization, and countless bacteria in the gut provide the conditions for bacteriophages to modulate the organism’s immune response. Barr et al. [18][54] demonstrated that the number of bacteriophages adhering to the cell-associated mucus layer and mucin glycoprotein is 4–5 times higher than that of the non-mucosal layer. The bacteriophages’ adherence to the mucus model suggests that the immunoglobulin-like structural domains on the bacteriophage coat protein could interact with the mucin and surface glycoproteins of the epithelial cells, allowing bacteriophages to adhere tightly to the mucus layer, providing immunity from non-host sources and strengthening the mucosal defense barrier [18][54]. Combining metagenomic, meta-transcriptomic, and meta-viromic analyses, Yan et al. [19][55] found that 24.6–40.4% of shared viral populations exist in the mucosal–luminal interface of the colonic lumen versus feces; although there is heterogeneity in the local inflammation in IBD patients (healthy proximal colon versus inflamed distal colon), the distal and proximal colonic mucosal viral differences are significantly less than colon versus feces, suggesting that the colonic mucosa provides a different microecological environment for intestinal viruses compared with feces. It was reconfirmed that the tight binding between the bacteriophage coat protein and mucin promotes bacteriophage colonization in the intestinal mucosal layer and the construction of a microecological environment with a high degree of similarity [19][55]. These findings suggest that the symbiotic relationship between bacteriophages and the organism provides the intestinal mucosa with an invisible protective barrier against pathogens.
The application of bacteriophages enhances animal immunity in livestock and poultry production as well. In weaned piglets, bacteriophages activate the immune system by targeting the TLR-mediated inflammatory response in the intestinal mucosa, significantly increasing the concentrations of sIgA and TGF-α in the ileal mucosa and the mRNA expression of TLR-2, TLR-4, and TLR-9 in the jejunal mucosa [5][31]. Challenging laying hens with Salmonella activates the immune response to produce large amounts of pro-inflammatory cytokines and stimulates compensatory lesions and proliferation in the liver and spleen; however, the addition of 0.1% of bacteriophages to the Salmonella-challenged feed reduces the relative expression of TLR-4 in the jejunum, which is the main receptor for LPSs produced by Gram-negative bacteria, suggesting that bacteriophages effectively inhibit the growth and reproduction of Salmonella and reverse the immune imbalance in laying hens [20][36]. Nevertheless, the induction of pro-inflammatory or anti-inflammatory immune responses in livestock and poultry by bacteriophages and the detailed mechanism of bacteriophages with different bacterial hosts need to be further explored in order to provide a feasible reference for the maintenance of organismal immune homeostasis under pathological conditions.
4. Improvement in Intestinal Morphology
The GIT, as the largest immune organ in mammals, is characterized by microbial communities that determine the development of the immune system. Therefore, direct or indirect interactions between bacteriophages and microbiota in the intestine play an essential role in the health status of the intestine, including the intestinal morphology and structure. Villi height (VH), crypt depth (CD), and the VH/CD ratio have been commonly used to reflect intestinal health.
Pilot studies have shown that supplementation of bacteriophages in livestock and poultry feeds could improve intestinal morphology and structure, thus promoting the healthy development of the intestine. In a trial of bacteriophage supplementation to replace antibiotics in the feed of weaned piglets, Zeng et al. [5][31] demonstrated that the supplementation of bacteriophages in the basal feed at 400 mg/kg significantly increased the VH of the jejunum and ileum, decreased the jejunum and ileum CD, and significantly elevated the relative expression of mRNAs related to tight junction (ZO-1, Claudin-1, and Occludin) in the jejunum. With the effects of probiotic bacterial components and metabolites, the intestinal tissues develop in a homeostatic state, and it is extremely difficult for pathogens to break through the immune barrier composed of intestinal epithelial cells and bacteriophages; therefore the intestinal tract maintains a normal tissue morphology. Lee et al. [21][56] verified that the bacteriophage cocktails added to the feed of weaned piglets increased intestinal secretion of metabolites such as short-chain fatty acids by beneficial bacteria that regulate intestinal morphogenesis and significantly increased VH of the duodenum and jejunum. Similarly, Hosseindoust et al. [22][57] reported that bacteriophage cocktails improve the intestinal organization and microbial communities of weaned piglets, effectively enhancing the efficiency of digestion and absorption to promote overall body health. Zhao et al. [23][58] proved that the supplementation of 1.0 × 109 PFU bacteriophages and 1 mg/mL of amoxicillin in a chick gavage experiment 8–10 days post hatch, respectively, significantly increased the jejunal VH and VH/CD ratio, but bacteriophage supplementation significantly increased the relative mRNA expression of jejunal Occludin and ZO-1. This result suggests that bacteriophages can interrupt the pathways of pathogens through the intestinal epithelium into the bloodstream, which exerts an important effect in maintaining intestinal microbiology and immune homeostasis.
Collectively, the integrity of the intestinal barrier is crucial for maintaining the nutrient absorption and immune function of intestinal epithelial cells. Dietary addition of bacteriophages is available to enhance the physical and microbial barrier function of the GIT and block the pathway for pathogens entering the circulatory system in the intestinal lumen; however, the specific mechanisms of bacteriophages need to be further explored.
