Biomedical devices made from high-modulus and hardness materials play a critical role in enhancing the quality of life for people with bone-related ailments. While these materials have been successfully used in orthopedic applications, concerns including stress-shielding have necessitated the exploration of alternative solutions. An ideal biomedical implant requires a delicate balance of mechanical performance, corrosion resistance, tissue biocompatibility, and other properties such as tribological performance and osseointegration. Materials such as stainless steel, titanium, and cobalt alloys remain non-degradable throughout the implant’s lifespan. In contrast, certain magnesium alloys can be engineered to degrade safely and under controlled conditions within the body.
1. Introduction
Many biomedical devices are constructed from natural or synthetic materials characterized by high modulus and hardness, often serving as implants to enhance the well-being of people afflicted with damaged or missing bone structures
[1,2][1][2]. These sturdy materials find potential applications elsewhere in the human body, such as heart valves and intravascular stents, depending on the specific medical needs
[3,4,5,6,7][3][4][5][6][7]. Elderly people are more likely to experience orthopedic health problems such as arthritis, often necessitating the use of implanted devices to replace compromised biological structures
[8]. Conditions such as osteoporosis, osteoarthritis, and trauma further compound the challenges older patients face, potentially leading to localized pain and physical deterioration in the functionality of hard tissues
[9]. Addressing this problem has involved substantial time and effort invested over several decades in the development of various orthopedic implants
[10]. Metal-based implants crafted from materials such as titanium-based, cobalt-based, or stainless-steel alloys have emerged as prominent choices in load-bearing orthopedic applications due to their high-modulus mechanical properties. However, research has revealed that these implant materials are susceptible to stress-shielding, a side-effect wherein a high-modulus orthopedic implant bears such a substantial load that the adjacent bone, lacking its typical load, undergoes deterioration
[11]. Ideal biomedical implants must possess three fundamental characteristics: mechanical performance, corrosion resistance, and tissue biocompatibility, alongside other essential attributes, including tribological behavior, osseointegration, and non-toxicity
[12].
Introducing a biomedical implant into the body involves multiple interactions at the tissue–implant interface. These interactions define the biocompatibility of an implant, significantly impacting its biological and mechanical performance
[13,14,15][13][14][15]. For instance, if an orthopedic material releases toxic elements, it cannot be considered biocompatible. Therefore, selecting materials for biomedical implants necessitates a paramount focus on non-toxic, biocompatible substances that closely mimic the elastic modulus and strength of human bone
[16,17,18,19,20,21][16][17][18][19][20][21].
Apart from materials designed for permanent implants, there is a growing interest in durable biomaterials that can gradually biodegrade over time, allowing for the regeneration of bone or other tissues to fulfill their original functions. Materials such as stainless steel, titanium, and cobalt alloys remain non-degradable throughout the implant’s lifespan. In contrast, certain magnesium alloys can be engineered to degrade safely and under controlled conditions within the body.
Generally, implants serve as medical devices that facilitate interaction with biological systems
[22]. Their proximity to bodily tissues presents both useful medical applications and significant medical implications. As mentioned earlier, an ideal implant material must exhibit requisite mechanical properties, excellent biocompatibility, and a low corrosion rate suitable for the mechanical and biological demands of the application at hand. For example, titanium alloys find use in joint replacements due to their lower elastic stiffness and excellent compatibility with bone
[10]. Consequently, the design of a safe and reliable implant material necessitates a meticulous blending of mechanical, chemical, physical, and biological properties to ensure prolonged functionality without the need for replacement surgeries.
2. Magnesium Alloys for Biomedical Implants
Achieving successful implant integration requires outstanding biocompatibility; thus, medical implants must meet stringent standards for materials, including being non-toxic, non-carcinogenic, non-pyrogenic, non-allergic, non-inflammatory, and hemocompatible. In the laboratory, it is essential to simulate the physiological conditions when performing in vitro tests, including cytotoxicity assays and cell proliferation studies, to evaluate the potential material effects on the host organism and assess the biocompatibility of the biodegradable implants before implantation
[37][23].
Magnesium (Mg) alloys, which are generally biodegradable, have garnered considerable attention in the biomedical field in recent years. In addition to mechanical characteristics such as human bone, Mg shows excellent biocompatibility. As mentioned above, Mg is a vital supplement for keeping the human body healthy and promoting osteo-growth
[2,51,52][2][24][25]. Additionally, during corrosion in the biological environment, Mg
2+ is released, creating non-toxic magnesium hydroxide, hydroxyl ions, and hydrogen gas (as shown above), which are removed from the body via the kidneys
[53,54,55,56,57][26][27][28][29][30]. On the other hand, the main disadvantages of using Mg alloys are the rapid degradation rates and decrease in mechanical properties, which may cause implant failure faster than the healing process
[58][31]. Moreover, hydrogen gas emitted from corrosion may create gas pockets that cause the adjacent tissues to separate, and the OH
− ions may cause surface alkalization and potential cell damage
[59,60][32][33]. Additionally, the mechanical properties of Mg could be improved by alloying. Thus, there are various challenges to tackle in developing Mg alloys. The advantages and disadvantages of Mg material are summarized in
Table 1 and
Table 2.
Table 1. Benefits of Mg alloys in medical implants [24]. Benefits of Mg alloys in medical implants [34].
| Advantages |
Description |
| Reduced density and elastic stiffness |
Density and elastic stiffness are similar to human bone. |
| Higher specific strength |
Strength to weight ratio is roughly 35–260 KNm/kg. |
| Machinability |
Mg has good machining capability to achieve accurate dimensions and processing into complex shapes. |
| Stress shielding effect |
The elastic stiffness of Mg is very close to bone. |
| Biocompatibility |
Mg is shown to have osteogenic functions. |
| Degradability |
Mg naturally degrades in the body, which is favorable to the patients. |
Table 2. Limitations of Mg alloys in medical implants [24]. Limitations of Mg alloys in medical implants [34].
| Disadvantages |
Description |
| Low mechanical properties |
Implants must be able to endure specific loads and deformation. It is difficult for Mg to meet medical demands in strength and plasticity. |
| High degradation rate |
It leads to premature loss of mechanical integrity and implant supports. |
| Hydrogen generation |
Hydrogen release creates air bubbles in the surrounding tissues. |
Even though Mg has many benefits as an implant material, pure Mg is not recommended for biomedical applications due to its higher corrosion rate and insufficient mechanical properties. Moreover, Mg shows low ductility because of the lack of slip characteristic in its hexagonal closest packed (hcp) structure. These problems are averted by alloying, which creates microstructural changes and adjusts surface potential between phases, improving mechanical properties and corrosion resistance [61,62][35][36]. Common alloying elements for Mg are aluminum (Al) and zinc (Zn), as they contribute to hardness, strength, and castability. Lithium provides low density and high solid solubility to Mg alloys. Moreover, Li can change the formability of Mg alloys by changing the crystal structure of Mg from hcp to the body-centered cubic (BCC) [56][29]. Alloying elements refine grains and optimize the type, size, and distribution of the second phase, which reduces the corrosion rate of Mg alloys. Furthermore, alloying elements create passive films to impede further corrosion. Presently, aluminum (Al), zinc (Zn), manganese (Mn), calcium (Ca), strontium (Sr), zirconium (Zr), and neodymium (Nd) are commonly used as alloying elements. The influence of these elements on the Mg alloys is shown in Table 3.
Table 3. Effect of alloying elements on Mg alloy performance [24]. Effect of alloying elements on Mg alloy performance [34].
| Alloying Elements |
Biocompatibility |
Corrosion Resistance |
Mechanical Performance |
| Al |
- ▪
-
Al is neurotoxic. It can cause Alzheimer’s disease and damage muscle fibers.
|
- ▪
-
It is beneficial in providing corrosion resistance.
|
- ▪
-
Increases strength and plasticity.
|
| Zn |
- ▪
-
Non-cytotoxic and good biocompatibility.
|
- ▪
-
Corrosion resistance decreases with higher Zn content.
|
- ▪
-
Zn participates in solid solution strengthening, increasing strength with increasing Zn content.
|
| Mn |
- ▪
-
Cytotoxic and neurotoxic.
|
- ▪
-
Provide good corrosion resistance.
|
- ▪
-
It gives higher yield strength and reduces tensile strength and ductility.
|
| Ca |
- ▪
-
An essential component of human bone.
|
- ▪
-
Corrosion resistance decreases with increasing Ca content.
|
- ▪
-
With increasing Ca content, strength increases, and plasticity decreases.
|
| Sr |
- ▪
-
Sr is a vital component of human bone. It also aids in bone formation.
|
- ▪
-
Corrosion resistance of Mg alloy drops with increasing Sr content.
|
- ▪
-
The strength of the alloy increases with increasing Sr content.
|
| Zr |
- ▪
-
Good biocompatibility and bone-bonding ability.
|
- ▪
-
Corrosion resistance decreases with increasing Zr content.
|
- ▪
-
Grains undergo refinement, increasing strength and plasticity.
|
| Nd |
- ▪
-
Cytotoxic at high concentrations but has good biosafety at low concentrations.
|
- ▪
-
Improves corrosion resistance.
|
- ▪
-
Forms new phases, grain refinement, and improvement in mechanical performance.
|
Magnesium alloys have attracted substantial attention in the biomedical field owing to their impressive attributes, including remarkable strength, low density, and outstanding osteogenic biocompatibility, as highlighted in
Table 3. Employing pure Magnesium alloys as implant materials comes with specific disadvantages. First, their mechanical properties fail to meet the necessary standards for implant materials, and their rapid resorption results in mechanical instability prior to complete bone healing. Second, magnesium alloys exhibit accelerated degradation rates, particularly in chloride-rich environments such as human bodily fluids. The generation of degradation rates gives rise to problems such as tissue inflammation. As mentioned, a notable challenge arises due to its considerable vulnerability to corrosion, originating from its remarkably low standard electrode potential of −2.37 V. Finally, implants made from pure magnesium alloys experience non-uniform corrosion, leading to premature failure. Among the concerns in the domain of biomedical implants, the corrosion behavior of Mg implants plays a preeminent role. When Mg is immersed in an aqueous milieu, a pivotal electrochemical process occurs, as highlighted in Equations (1) through (3), and underscores the significance of managing corrosion dynamics associated with Mg alloy im-plants for biomedical applications. Tafel plots for Magnesium alloys are shown in
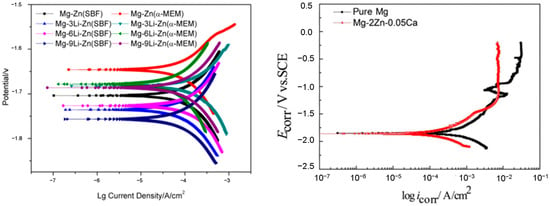
Figure 1 [63][37].
Figure 1. Tafel plots for Magnesium alloys [64,65,66]. Tafel plots for Magnesium alloys [38][39][40].
Because of magnesium’s inherent low strength, alloying becomes the predominant approach for enhancing its mechanical properties. However, introducing alloying elements gives rise to secondary phases that may expedite corrosion. Moreover, the release of alloy ions may play distinct roles in bone healing. Apart from their supporting function, the released ions from magnesium alloys during biodegradation exhibit additional effects, such as antibacterial and antitumor properties. In contrast to non-biodegradable materials, magnesium alloys stand out as a suitable choice for biodegradable implants due to their biocompatibility and the absence of toxicological tissue responses. These magnesium alloys present lower probabilities of releasing cytotoxic ions, thereby preventing stress shielding and local tissue inflammation
[67,68][41][42]. Adding aluminum (Al) in commercial magnesium (Mg) alloys is the most prominent strategy, contributing to increasing the overall corrosion resistance of Mg alloys. AE21, AZ32, and AZ91 are some examples of Al-enriched Mg alloys with remarkable mechanical and corrosion resistance properties that are used for biomedical applications. However, Al’s potential in the development of Alzheimer’s disease raises concerns and underscores the requirement of intricate equilibrium in biomedical applications. Zinc (Zn), on the other hand, is vital for the human body and enhances the material strength of Mg-Al alloys. Mg-Zn alloys are highly biocompatible. Moreover, it forms protective layers that effectively slow down degradation. Mn is an essential trace element for the human body with the potential to form a protective Mn-containing oxide film that inhibits Cl-ion infiltration, rendering it a commonly employed element in Mg alloys for biomedical applications. Mg-Ca alloys favor bone implantation, reducing potential differences and improving corrosion resistance. Furthermore, Strontium (Sr) also shares chemical properties with Mg and Ca; it is found in bones and promotes bone formation. Other elements found in Mg alloys for biomedical applications include Zirconium (Zr), Silicon (Si), and Lithium (Li), which foster bone bonding and corrosion resistance and add ductility to vascular stents in biomedical applications, respectively.
To summarize this section, alloying elements play a crucial role in controlling the degradation rate of magnesium alloys. The composition and concentration can be tailored to achieve the desired balance between sufficient strength for implant functionality and a degradation rate that matches the tissue healing process. The degradation rates of Mg alloys vary and determine their corrosion characteristics. Aluminum and zinc, when alloyed with magnesium, increase the oxidation rate, whereas rare earth elements decrease the oxidation rate of magnesium alloys. Combining aluminum and zinc in specific ratios yields an alloy with an overall improved performance, balanced corrosion resistance, mechanical strength, and biocompatibility
[69,70,71][43][44][45].