Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Afsar Naqvi.
The oral cavity is a niche for diverse microbes, including viruses. Members of the Herpesviridae family, comprised of dsDNA viruses, as well as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an ssRNA virus, are among the most prevalent viruses infecting the oral cavity, and they exhibit clinical manifestations unique to oral tissues. Herpesviruses and SARS-CoV-2 are individually associated with oral inflammatory diseases, particularly periodontitis, peri-implantitis, and endodontic disease.
- herpesvirus
- SARS-CoV-2
- oral inflammation
- periodontitis
1. Introduction
Accumulating evidence suggests that the oral cavity is a critical player in SARS-CoV-2 infection and severity [73,74][1][2]. To enter the human body, SARS-CoV-2 uses its spike protein (S protein) to bind to the angiotensin converting enzyme II (ACE2) receptor, which is highly expressed in the salivary glands of the oral cavity, cellular membrane of mucosal tissue cells in the tongue, surrounding buccal, gingival tissues, and pulp [18,75,76][3][4][5]. Recently, SARS-CoV-2 was identified in gingiva and gingival crevicular fluid (GCF) [77][6]. Together, these observations reveal that oral tissues are trophic for the virus. Consistent with this, clinical oral manifestations in COVID-19 patients range from ulcers, blisters, white and erythematous plaque, necrotizing gingivitis, salivary gland alterations, tongue depapillation, and gustatory dysfunction (Table 21) [21,78,79,80,81,82,83,84,85,86][7][8][9][10][11][12][13][14][15][16]. Importantly, multiple studies report long-term oral cavity symptoms following COVID-19 infection. These symptoms are collectively referred to as oral post-acute sequelae of COVID-19 (PASC) and include erythematous bullae on the palate, non-bleeding lesions in the palatal mucosa, tongue enlargement, and burning mouth [59,87][17][18]. In a clinical case study by the clinic “Stomatologia Rafałowicz”, out of the 1256 studied patients, 68% of patients displayed oral symptoms: discoloration, ulceration, and hemorrhagic changes on the oral mucosa (32%); mycosis on the tongue (29.69%), unilateral aphthous-like lesions on the hard palate (25.79%), and atrophic cheilitis (12.5%). These patients were diagnosed with COVID-19 2 to 6 months before oral examination [19]. Oral symptoms appear to be worsened and more prolonged in the elderly with coexisting systemic diseases and patients with severe COVID-19 [19,88][19][20].
Table 21.
SARS-CoV-2 oral manifestations. Summary of selected clinical cases that examined viral oral manifestations in humans with SARS-CoV-2.
| SARS-CoV-2 (Linear, Single-Stranded RNA, Enveloped Vírus) |
|||
|---|---|---|---|
| Oral Manifestations | Signs and Symptoms | Disease Association | Treatment |
| Necrotizing gingivitis | Necrotic interdental papillae, bleeding in gingival sulcus and halitosis, erythematous and oedematous gingivae | Oral necrotizing disease | Debridement, deep cleaning (scaling, root planning), metronidazole or ampicillin, chlorhexidine mouthwash |
| Dark pigmentation | Dark brown pigmentation in the palate and gingiva | Oral melanotic macule, oral lichen planus, oral cancer |
Ibuprofen |
| Xerostomia | Dry feeling in the mouth, frequent thirst, difficulty swallowing dry foods, diminished or altered taste | Periodontitis, caries, COVID-19 |
Artificial saliva substitutes |
| Dysgeusia | Altered taste perception (frequent metallic or bitter taste) | Periodontitis, COVID-19 |
Addressing cause of dysgeusia (e.g., vitamin supplements for vitamin deficiency, switching medications if dysgeusia is a side effect of medication) |
| Mucositis | Red pigmentation and swelling on affected mucous membranes, ulcers and sores | Gingivitis, periodontitis, COVID-19 |
Benzydamine, artificial saliva substitutes, low-level laser therapy |
| Periodontal infection | Persistent and severe inflammation of the gums due to immune response, alveolar bone resorption and loss of tooth attachment | Periodontitis (in COVID-19) | Scaling, deep cleaning, trimethoprim and sulfamethoxazole (antibiotics) |
| Erythematous bullae on palate | Erythema (redness), fluid-filled blisters, pain or tenderness on palate | Pemphigus vulgaris, HSV infection, COVID-19 |
Corticosteroids, acyclovir or valacyclovir, saline rinse |
| Tongue depapillation | Flat or glossy tongue surface, xerostomia, halitosis | Candida infection, COVID-19 | Nystatin oral suspension or fluconazole |
Drivers of COVID-19-related symptoms in the oral cavity include stress, poor oral hygiene, multi-organ disorders, and notably, immune system dysfunction [19,21,22][7][19][21]. An improper immune response may allow for opportunistic infections. This scenario may explain why oral lesions in COVID-19 subjects appear concomitant with the hypergrowth of opportunistic oral pathogens, such as herpesviruses [59][17]. Recurrent herpetic lesions and higher bacterial growth in COVID-19 subjects suggest the dysregulation of immune responses that augment opportunistic co-infections (bacterial and viral). Painful oral lesions and ulcers were reported to affect keratinized and non-keratinized tissue in a manner similar to those caused by HHV in suspected and confirmed COVID-19-infected patients [84][14]. Intriguingly, the resolution and healing of oral lesions correspond to the overall resolution of COVID-19 symptoms, suggesting that COVID-19 and the oral manifestations were linked. SARS-CoV-2 and its interaction with ACE2 on epithelial keratinocytes could influence their function, resulting in the development of lesions and ulcers [79][9]. Ultimately, SARS-CoV-2 elicits an inflammatory immune response that varies in intensity and duration among infected individuals. Thus, the virus can manifest in various oral diseases or in other parts of the body, with differing clinical manifestations between infected individuals. The following section will discuss the link between SARS-CoV-2 and oral diseases such as periodontitis, peri-implantitis, and endodontic disease.
2. SARS-CoV-2 and Periodontal Disease
Recent studies have revealed an association between COVID-19 infection and periodontitis severity [89,90][22][23]. Clinical data indicate a positive correlation between periodontitis and COVID-19 complications. This relationship is evident in a case–control study by Marouf et al., which used the national electronic health records of the state of Qatar between February and July 2020. Compared to COVID-19 patients with early-stage or no periodontitis, the study found that COVID-19 patients (n = 528) with moderate-to-severe periodontitis had increased odds for ICU admission (OR = 3.54, 95% CI 1.39–9.05), need for assisted ventilation (OR = 4.57, 95% CI 1.19–17.4), and death (OR = 8.81, 95% CI 1.00–77.7). Marouf et al. also found significantly higher levels of D-dimer, WBC, and C-reactive protein (CRP) in the serum of COVID-19 patients with periodontitis than those without periodontitis. This result is significant because elevated D-dimer, WBC, and CRP blood levels are considered biomarkers of worsened COVID-19 prognosis, suggesting that periodontal disease could exacerbate COVID-19 clinical outcomes [83][13].
2.1. Mechanistic Link between SARS-CoV-2 and Periodontal Disease
Several hypothetical mechanisms explain the link between COVID-19 and periodontitis: (1) the elevated T helper (Th)-17 response in severe periodontitis amplifies a ‘cytokine storm’, which recent studies associated with worse prognosis and increased mortality rate due to COVID-19, (2) periodontal pockets serve as an ideal reservoir for SARS-CoV-2, (3) periodontopathic bacteria increase ACE2 expression and promote inflammatory cytokine production in the lower respiratory tract via food or salivary aspiration, (4) periodontopathic bacteria enhance the infectivity of SARS-CoV-2 by producing proteases that cleave the S protein, and (5) increased Gal-3 expression in severe periodontitis enhances Gal-3-mediated immune response and viral attachment [17,83,91,92,93,94][13][24][25][26][27][28]. Given the established link between periodontitis, a chronic inflammatory disease localized in the oral cavity, and other systemic inflammatory diseases in the existing literature, understanding the relationship between COVID-19 and periodontal disease manifestation and aggravation is critical to effectively treating oral inflammation [95,96,97][29][30][31].
Non-resolving inflammation triggered by a dysbiotic subgingival biofilm is characteristic of chronic periodontitis. This localized chronic inflammation can subsequently translate to systemic inflammation and the elevated production of inflammatory markers in circulation. In particular, chronic periodontitis often involves an IL-17/Th17-mediated pro-inflammatory response—a significant contributor to cytokine storms. Cytokine storms are characterized by an excessive release of inflammatory molecules into the bloodstream and result from the hyperactivation of immune cells, such as lymphocytes and macrophages, which primarily produce cytokines. Similar overexpression of several cytokines (IL-1β, IL-7, IL-10, IL-17, IL-2, IL-9, IL-8, IL-9, IFN-γ, TGF-β, and some metalloproteinases) is seen in chronic periodontitis and COVID-19 [83,93,94,98][13][27][28][32]. Mounting evidence suggests an association between cytokine storms and COVID-19 severity, particularly with the development of acute respiratory distress syndrome (ARDS), a life-threatening condition characterized by fluid build-up in the alveoli seen in some COVID-19 patients, resulting in hypoxemia and multi-organ failure (Figure 21) (Ragab et al., 2020). Due to a similar cytokine storm profile between chronic periodontitis and COVID-19, Sahni et al. suggest that the robust IL-17/Th17 response in chronic periodontitis could aggravate the cytokine storm seen in COVID-19 [83,93][13][27].
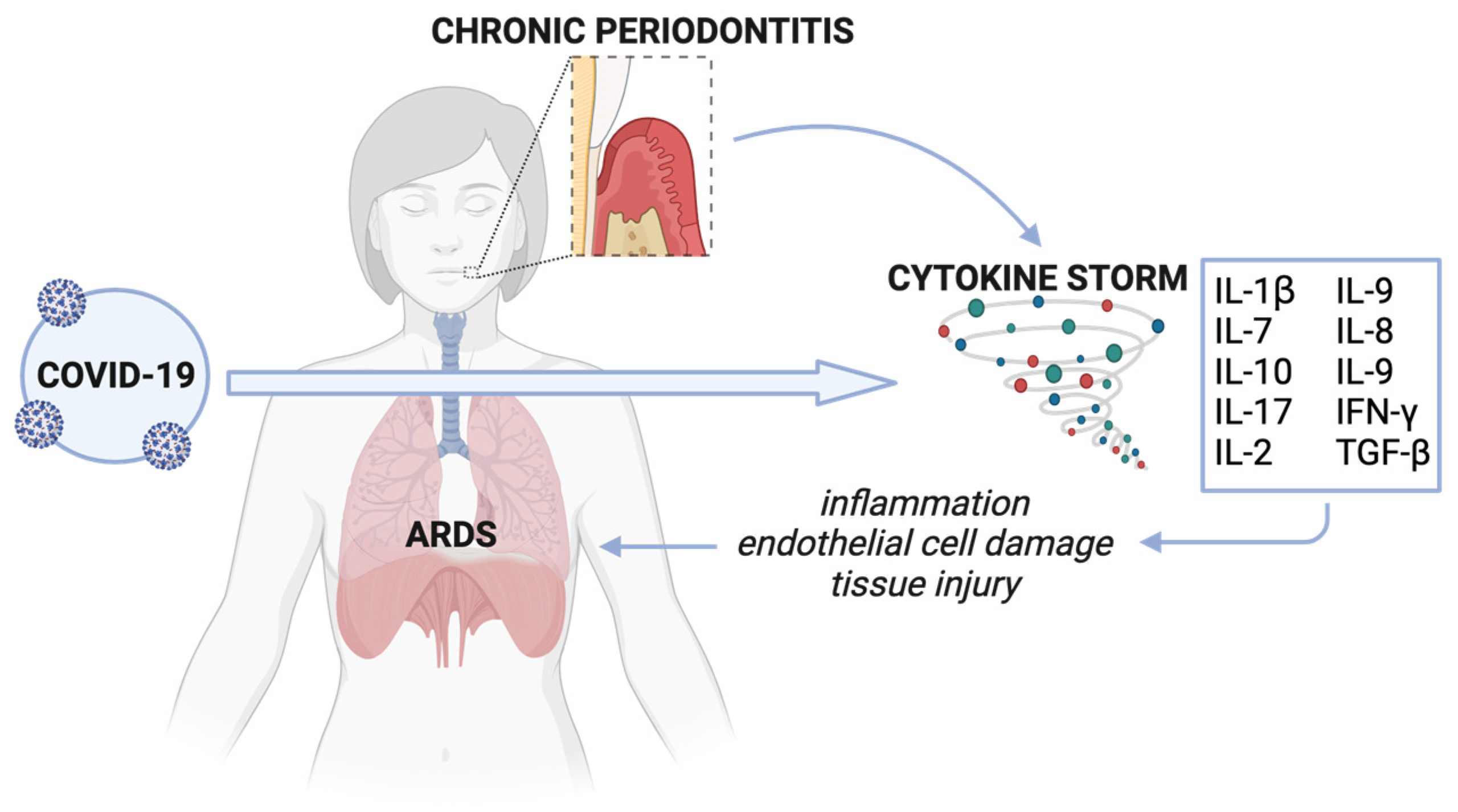
Figure 21. Cytokine storm associated with chronic periodontitis and COVID-19. Cytokines resulting from untreated chronic periodontitis disseminate intravascularly and contribute to COVID-19-associated cytokine storm. This aggravated cytokine storm worsens systemic inflammation, causing widespread tissue damage, including in the capillary and endothelial epithelium, ultimately resulting in ARDS. Created with Biorender.com (accessed on 26 December 2023).
2.2. Peiodontital Tissue as a Target for SARS-CoV-2 Infection
As periodontitis progresses, tissue damage, detachment of junctional epithelium, and chronic inflammation contribute to the formation and widening of periodontal pockets, which refer to abnormally deepened gingival sulcus (>3 mm). Periodontal pockets harbor dysbiotic subgingival plaque biofilms and viruses, such as bacteriophages and herpesviruses. In the case of SARS-CoV-2, a study by Badran et al. hypothesized that periodontal pockets could serve as plausible reservoirs for the virus. Moreover, these pockets could facilitate SARS-CoV-2 replication and migration to systemic tissues via the capillary periodontal complex [91][25]. Possible SARS-CoV-2 entry points into the periodontium involve the S protein–ACE2 and S protein–CD147 interactions. Multiple studies have shown the binding of S protein to ACE2, which Santos et al. confirmed it as expressed in human periodontal ligament fibroblasts [99][33]. Less studied, however, is CD147 and its possible role as a novel, non-canonical viral receptor for SARS-CoV-2. Wang et al. showed that administering a CD147-blocking antibody on Vero E6 and BEAS-2B cells inhibits SARS-CoV-2 amplification [100][34]. Previous studies have shown that CD147 is expressed in periodontal pockets, particularly in the buccal and subgingival regions [91,101][25][35]. Additionally, post-mortem data from Matuck et al. confirm the presence of SARS-CoV-2 in the periodontal tissue of COVID-19-positive patients [102][36]. Together, these findings suggest that periodontal tissue is a target for SARS-CoV-2 infection, particularly in cases of chronic and aggressive periodontitis.
2.3. SARS-CoV-2 and Periodontopathic Bacteria
When food and saliva are unintentionally aspirated, periodontopathic bacteria can migrate into the lower respiratory tract [103,104,105][37][38][39]. Previous studies have identified periodontopathic bacteria in the bronchoalveolar lavage fluid of COVID-19 patients. Takahashi and Watanabe et al. hypothesize that bacterial colonization in the lungs and bronchi can promote SARS-CoV-2 infection by releasing pathogenic factors, such as endotoxins, which increase ACE2 expression [94][28]. Additionally, these bacteria stimulate the production of pro-inflammatory cytokines in the lower respiratory tract, such as IL-6 and IL-8, thereby promoting COVID-19 aggravation. To support this stance, Takahashi and Watanabe et al. demonstrate that the culture supernatant of the periodontopathic bacteria Fusobacterium nucleatum upregulates ACE2 expression and IL-6 and IL-8 production in human alveolar epithelial cells. The authors observed a similar increase in IL-6 and IL-8 in other human epithelial cells, including BEAS-2B bronchial and Detroit 562 pharyngeal epithelial cells [94][28].
Another possible mechanism by which periodontopathic bacteria enhance SARS-CoV-2 tropism is by producing proteases that cleave the S protein. After viral entry, proteolytic cleavage of the S protein at the S1/S2 and S2′ cleavage sites are necessary to mediate viral attachment to host cells and viral–host membrane fusion. Specifically, the host proprotein convertase furin cleaves at the S1/S2 site, while the transmembrane serine protease 2 (TMPRSS2) cleaves at the S2′ site. In vitro data show that treating Calu-3 human airway epithelial cells with combined TMPRSS2 and furin inhibitors displays potent antiviral activity against SARS-CoV-2 proteolytic activation and replication [106][40]. Similarly, proteases produced by periodontopathic bacteria have been shown to cleave hemagglutinin (HA) into HA1 and HA2 during influenza virus infection. Takashi and Watanabe et al. suggest that these bacterial proteases could play the same role during SARS-CoV-2 infection, although further experimentation is needed to confirm this hypothesis [94][28].
Kara et al. suggest that increased Galectin-3 (Gal-3) levels are positively associated with periodontitis severity, and this relationship could explain the link between periodontitis and COVID-19. Gal-3 is a widely expressed pro-inflammatory protein and a key regulator component of immune cell homeostasis, which it modulates by controlling T-cell-mediated inflammation. Inhibition of Gal-3 reportedly decreases the production of the pro-inflammatory cytokines IL-1 and IL-6 while increasing the production of the anti-inflammatory cytokine IL-10 [92,107][26][41]. Gallo et al. reported higher plasma levels of Gal-3 in patients with severe COVID-19 compared to healthy controls [108][42]. In terms of structure, a component of SARS-CoV-2′s S protein (i.e., S1-N terminal domain) and Gal-3 are strikingly similar. This resemblance is significant because, in other viral diseases such as HIV and HTLV, Gal-3 reportedly serves as an attachment factor that mediates viral entry into T-cells [109][43]. Therefore, the Galectin-like S1-NTD is believed to increase the SARS-CoV-2 immune response and viral attachment to host cells [92][26]. While the previously mentioned articles described the observed relationship between periodontitis, its associated pathogens, and COVID-19 infection and severity, few articles address how COVID-19 affects periodontitis. Recently, oral examinations of patients infected with SARS-CoV-2 report the presence of uncharacterized periodontal lesions, specifically necrotizing periodontal disease (NPD). NPD is a type of periodontal disease common among HIV and immunocompromised patients. It is characterized by necrosis in the alveolar bone, gingival tissues, and periodontal ligament. Metagenomic analyses of SARS-CoV-2 patients detected an increased prevalence for oral disease-implicated bacteria, including Prevotella intermedia, Fusobacterium spp., and Veillonella spp. [85,110][15][44]. Interestingly, another study examining the relationship between the nasopharyngeal microbiome and COVID-19 discovered decreased Fusobacteria presence in patients with COVID-19 [111][45]. Of note, P. intermedia and Fusobacterium spp. are frequently associated with NPD [112][46]. Bacterial co-infections with SARS-CoV-2 may predispose individuals to NPD [113][47]. Therefore, it is crucial to not only study how oral health affects SARS-CoV-2 infection and severity but to also examine how SARS-CoV-2 can lead to altered oral disease manifestation.
3. SARS-CoV-2 and Peri-Implantitis
Peri-implantitis is characterized by the bacteria-induced destructive inflammatory process affecting the implant-surrounding hard and soft tissues. Similarities between peri-implantitis and periodontitis suggest an association between SARS-CoV-2 and peri-implantitis. The clinical manifestations and cellular processes shared by peri-implantitis and periodontitis include alveolar bone loss, decreased osseointegration, increased pocket depth, and bleeding on probing [114][48]. Moreover, peri-implant and periodontal infections share an analogous sequence of inflammatory events and qualitative composition of immune cells (i.e., predominant neutrophils, macrophages, T cells, and B cells). Nonetheless, fundamental differences exist between the two oral diseases. Relative to periodontitis, peri-implantitis involves a higher proportion of immune cells and associated inflammatory mediators. Compounding this larger inflammatory infiltrate is the absence of periodontal ligament and Sharpey’s fibers around implants, resulting in less vascularization and connective tissue attachment, respectively. Altogether, these differences can help to explain the more severe and rapid progression of tissue destruction in peri-implantitis compared to periodontitis [115][49].
These immunopathological events necessitate the question: does peri-implantitis affect COVID-19 infectivity and severity? Unfortunately, there remains a tremendous gap in knowledge in the literature. While most research focuses on the association between SARS-CoV-2 and periodontitis, with the increasing prevalence of peri-implant diseases, the same attention should be paid to elucidating the link between SARS-CoV-2 and peri-implantitis.
4. SARS-CoV-2 and Endodontic Disease
A higher percentage of endodontic emergencies in the post-pandemic period compared to the pre-pandemic period further suggests an association between virus infection and the exacerbation of oral diseases. A retrospective survey of ~2500 patients (2–92 years; mean age 38.9 ± 19.3 years) in China indicated a marked increase in the proportion of dental and oral infections [85,116,117][15][50][51]. Yu et al. reported that an increase in irreversible pulpitis was the most common pathology observed in a cohort of ninety-six subjects (mean age 42.24 ± 18.32 years) from Wuhan, China. In this study, SARS-CoV-2 confirmed or suspected subjects reported significantly higher pain levels, suggesting more severe disease manifestation [117][51].
Though bacteria (i.e., Streptococci and Staphylococci spp.) are the usual drivers of pulpitis, co-infection with viruses may play a critical role in the development of endodontic pathosis. Herpesviruses, specifically EBV, are associated with irreversible pulpitis [65][52]. In the case of SARS-CoV-2, research regarding the association between COVID-19 and pulpitis is sparse, as is the case for other endodontic diseases. A transcriptome-wide effect cross-analysis suggests that the dental pulp is susceptible to SARS-CoV-2 infection [18][3]. Galicia et al. showed that ACE2 and TMPRSS2 are expressed in the dental pulp, but whether endodontic diseases predispose individuals to SARS-CoV-2 infection remains unclear.
The potential underlying roles of SARS-CoV-2 in worsening clinical manifestations of oral diseases remain elusive. Perhaps SARS-CoV-2 infection (1) takes advantage of preexisting periodontal and endodontic diseases or (2) creates new avenues for extended tissue damage by enhancing the severity and aggressiveness of tissue degradation, resulting in intense pain and dentinal and cemental pathology. The underlying mechanisms of hyper-inflammation in COVID-19 subjects reflect the crosstalk between SARS-CoV-2 and oral diseases. Whether SARS-CoV-2 and oral virus/bacteria interact synergistically demands further investigation. Persistent inflammation of periodontal and endodontic tissues has been shown to affect systemic health and may facilitate crosstalk of the underlying operative mechanisms mediating an increase in the oral and systemic clinical symptoms of COVID-19 through common chronic inflammatory pathways [118,119][53][54]. To demonstrate the impact of the SARS-CoV-2 virus on oral health and its role in exacerbating oral infections, larger cohort studies comparing SARS-CoV-2-negative and positive subjects are necessary [59][17]. However, there is promising evidence that the management of good oral hygiene can potentially prevent COVID-19 aggravation [120][55].
References
- Hoffmann, D. The Role of the Oral Cavity in SARS-CoV-2- and Other Viral Infections. Clin. Oral Investig. 2023, 27, 15–22.
- Ganesan, S.M.; Peter, T.K.; Withanage, M.H.H.; Boksa, F.; Zeng, E.; Martinez, A.; Dabdoub, S.M.; Dhingra, K.; Hernandez-Kapila, Y. COVID-19 Associated Oral and Oropharyngeal Microbiome: Systematic Review and Meta-Analysis. Periodontol. 2000 2023.
- Galicia, J.C.; Guzzi, P.H.; Giorgi, F.M.; Khan, A.A. Predicting the Response of the Dental Pulp to SARS-CoV2 Infection: A Transcriptome-Wide Effect Cross-Analysis. Genes. Immun. 2020, 21, 360–363.
- Bajaj, N.; Granwehr, B.P.; Hanna, E.Y.; Chambers, M.S. Salivary Detection of SARS-CoV -2 ( COVID -19) and Implications for Oral Health-care Providers. Head Neck 2020, 42, 1543–1547.
- Haque, S.M.; Ashwaq, O.; Sarief, A.; Azad John Mohamed, A.K. A Comprehensive Review about SARS-CoV-2. Future Virol. 2020, 15, 625–648.
- Gupta, S.; Mohindra, R.; Chauhan, P.K.; Singla, V.; Goyal, K.; Sahni, V.; Gaur, R.; Verma, D.K.; Ghosh, A.; Soni, R.K.; et al. SARS-CoV-2 Detection in Gingival Crevicular Fluid. J. Dent. Res. 2021, 100, 187–193.
- Tomo, S.; Miyahara, G.I.; Simonato, L.E. Oral Mucositis in a SARS-CoV-2-infected Patient: Secondary or Truly Associated Condition? Oral Dis. 2022, 28, 963–967.
- Amorim dos Santos, J.; Normando, A.G.C.; Carvalho da Silva, R.L.; De Paula, R.M.; Cembranel, A.C.; Santos-Silva, A.R.; Guerra, E.N.S. Oral Mucosal Lesions in a COVID-19 Patient: New Signs or Secondary Manifestations? Int. J. Infect. Dis. 2020, 97, 326–328.
- Brandão, T.B.; Gueiros, L.A.; Melo, T.S.; Prado-Ribeiro, A.C.; Nesrallah, A.C.F.A.; Prado, G.V.B.; Santos-Silva, A.R.; Migliorati, C.A. Oral Lesions in Patients with SARS-CoV-2 Infection: Could the Oral Cavity Be a Target Organ? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, e45–e51.
- Díaz Rodríguez, M.; Jimenez Romera, A.; Villarroel, M. Oral Manifestations Associated with COVID-19. Oral Dis. 2022, 28, 960–962.
- Glavina, A.; Biočina-Lukenda, D.; Mravak-Stipetić, M.; Markeljević, J. Oral Symptoms and Lesions in SARS-CoV-2-positive Patient. Oral Dis 2022, 28, 979–980.
- Jimenez-Cauhe, J.; Ortega-Quijano, D.; de Perosanz-Lobo, D.; Burgos-Blasco, P.; Vañó-Galván, S.; Fernandez-Guarino, M.; Fernandez-Nieto, D. Enanthem in Patients With COVID-19 and Skin Rash. JAMA Dermatol. 2020, 156, 1134.
- Marouf, N.; Cai, W.; Said, K.N.; Daas, H.; Diab, H.; Chinta, V.R.; Hssain, A.A.; Nicolau, B.; Sanz, M.; Tamimi, F. Association between Periodontitis and Severity of COVID-19 Infection: A Case–Control Study. J. Clin. Periodontol. 2021, 48, 483–491.
- Martín Carreras-Presas, C.; Amaro Sánchez, J.; López-Sánchez, A.F.; Jané-Salas, E.; Somacarrera Pérez, M.L. Oral Vesiculobullous Lesions Associated with SARS-CoV-2 Infection. Oral Dis. 2021, 27, 710–712.
- Patel, J.; Woolley, J. Necrotizing Periodontal Disease: Oral Manifestation of COVID-19. Oral Dis. 2021, 27, 768–769.
- Riad, A.; Klugar, M.; Krsek, M. COVID-19-Related Oral Manifestations: Early Disease Features? Oral Dis. 2022, 28, 940–942.
- Brandini, D.A.; Takamiya, A.S.; Thakkar, P.; Schaller, S.; Rahat, R.; Naqvi, A.R. Covid-19 and Oral Diseases: Crosstalk, Synergy or Association? Rev. Med. Virol. 2021, 31.
- Tapia-Orihuela, R.K.A. Hypertension and Coronavirus Disease 2019 Mortality. J. Hypertens. 2020, 38, 1197–1198.
- Rafałowicz, B.; Wagner, L.; Rafałowicz, J. Long COVID Oral Cavity Symptoms Based on Selected Clinical Cases. Eur. J. Dent. 2022, 16, 458–463.
- Paradowska-Stolarz, A. Oral Manifestations of COVID-19: Brief Review. Dent. Med. Probl. 2021, 58, 123–126.
- Paces, J.; Strizova, Z.; Smrz, D.; Cerny, J. COVID-19 and the Immune System. Physiol. Res. 2020, 379–388.
- Campisi, G.; Bizzoca, M.E.; Lo Muzio, L. COVID-19 and Periodontitis: Reflecting on a Possible Association. Head Face Med. 2021, 17, 16.
- Larvin, H.; Wilmott, S.; Wu, J.; Kang, J. The Impact of Periodontal Disease on Hospital Admission and Mortality During COVID-19 Pandemic. Front. Med. 2020, 7, 604980.
- Basso, L.; Chacun, D.; Sy, K.; Grosgogeat, B.; Gritsch, K. Periodontal Diseases and COVID-19: A Scoping Review. Eur. J. Dent. 2021, 15, 768–775.
- Badran, Z.; Gaudin, A.; Struillou, X.; Amador, G.; Soueidan, A. Periodontal Pockets: A Potential Reservoir for SARS-CoV-2? Med. Hypotheses 2020, 143, 109907.
- Kara, C.; Çelen, K.; Dede, F.Ö.; Gökmenoğlu, C.; Kara, N.B. Is Periodontal Disease a Risk Factor for Developing Severe Covid-19 Infection? The Potential Role of Galectin-3. Exp. Biol. Med. 2020, 245, 1425–1427.
- Sahni, V.; Gupta, S. COVID-19 & Periodontitis: The Cytokine Connection. Med. Hypotheses 2020, 144, 109908.
- Takahashi, Y.; Watanabe, N.; Kamio, N.; Kobayashi, R.; Iinuma, T.; Imai, K. Aspiration of Periodontopathic Bacteria Due to Poor Oral Hygiene Potentially Contributes to the Aggravation of COVID-19. J. Oral Sci. 2021, 63, 1–3.
- Sukumar, K.; Tadepalli, A. Nexus between COVID-19 and Periodontal Disease. J. Int. Med. Res. 2021, 49, 3000605211002695.
- Hajishengallis, G. Interconnection of Periodontal Disease and Comorbidities: Evidence, Mechanisms, and Implications. Periodontol. 2000 2022, 89, 9–18.
- Martu, M.A.; Maftei, G.A.; Sufaru, I.G.; Jelihovschi, I.; Luchian, I.; Hurjui, L.; Martu, I.; Pasarin, L. COVID-19 and Periodontal Disease—Ethiopathogenic and Clinical Implications. Rom. J. Oral. Rehabil. 2020, 12, 116–124.
- Takahashi, Y.; Hayakawa, A.; Sano, R.; Fukuda, H.; Harada, M.; Kubo, R.; Okawa, T.; Kominato, Y. Histone Deacetylase Inhibitors Suppress ACE2 and ABO Simultaneously, Suggesting a Preventive Potential against COVID-19. Sci. Rep. 2021, 11, 3379.
- Santos, C.F.; Morandini, A.C.; Dionísio, T.J.; Faria, F.A.; Lima, M.C.; Figueiredo, C.M.; Colombini-Ishikiriama, B.L.; Sipert, C.R.; Maciel, R.P.; Akashi, A.P.; et al. Functional Local Renin-Angiotensin System in Human and Rat Periodontal Tissue. PLoS ONE 2015, 10, e0134601.
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.-Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.-X.; Gong, L.; et al. CD147-Spike Protein Is a Novel Route for SARS-CoV-2 Infection to Host Cells. Sig. Transduct. Target Ther. 2020, 5, 283.
- Feldman, M.; La, V.D.; Lombardo Bedran, T.B.; Palomari Spolidorio, D.M.; Grenier, D. Porphyromonas Gingivalis-Mediated Shedding of Extracellular Matrix Metalloproteinase Inducer (EMMPRIN) by Oral Epithelial Cells: A Potential Role in Inflammatory Periodontal Disease. Microbes Infect. 2011, 13, 1261–1269.
- Fernandes Matuck, B.; Dolhnikoff, M.; Maia, G.V.A.; Isaac Sendyk, D.; Zarpellon, A.; Costa Gomes, S.; Duarte-Neto, A.N.; Rebello Pinho, J.R.; Gomes-Gouvêa, M.S.; Sousa, S.C.O.M.; et al. Periodontal Tissues Are Targets for Sars-Cov-2: A Post-Mortem Study. J. Oral Microbiol. 2021, 13, 1848135.
- Anand, P.S.; Jadhav, P.; Kamath, K.P.; Kumar, S.R.; Vijayalaxmi, S.; Anil, S. A Case-control Study on the Association between Periodontitis and Coronavirus Disease (COVID-19). J. Periodontol. 2021, 93, 584–590.
- Shen, Z.; Xiao, Y.; Kang, L.; Ma, W.; Shi, L.; Zhang, L.; Zhou, Z.; Yang, J.; Zhong, J.; Yang, D.; et al. Corrigendum to: Genomic Diversity of Severe Acute Respiratory Syndrome-Coronavirus 2 in Patients With Coronavirus Disease 2019. Clin. Infect. Dis. 2021, 73, ciab900.
- Yamasaki, K.; Kawanami, T.; Yatera, K.; Fukuda, K.; Noguchi, S.; Nagata, S.; Nishida, C.; Kido, T.; Ishimoto, H.; Taniguchi, H.; et al. Significance of Anaerobes and Oral Bacteria in Community-Acquired Pneumonia. PLoS ONE 2013, 8, e63103.
- Bestle, D.; Heindl, M.R.; Limburg, H.; Van Lam van, T.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; et al. TMPRSS2 and Furin Are Both Essential for Proteolytic Activation of SARS-CoV-2 in Human Airway Cells. Life Sci. Alliance 2020, 3, e202000786.
- Chen, S.-S.; Sun, L.-W.; Brickner, H.; Sun, P.-Q. Downregulating Galectin-3 Inhibits Proinflammatory Cytokine Production by Human Monocyte-Derived Dendritic Cells via RNA Interference. Cell Immunol. 2015, 294, 44–53.
- Gallo, V.; ISERC-Team; Reis, A.; Miranda, A.; Martins, C.; Serre-Miranda, C.; Nobrega, C.; Silva, C.S.; Sarmento, H.; Cotter, J.; et al. Increased Gal-3BP Plasma Levels in Hospitalized Patients Infected with SARS-CoV-2. Clin. Exp. Med. 2022, 23, 151–155.
- Wang, W.-H.; Lin, C.-Y.; Chang, M.R.; Urbina, A.N.; Assavalapsakul, W.; Thitithanyanont, A.; Chen, Y.-H.; Liu, F.-T.; Wang, S.-F. The Role of Galectins in Virus Infection - A Systemic Literature Review. J. Microbiol. Immunol. Infect. 2020, 53, 925–935.
- Chakraborty, S. Metagenome of SARS-CoV2 Patients in Shenzhen with Travel to Wuhan Shows a Wide Range of Species—Lautropia, Cutibacterium, Haemophilus Being Most Abundant—And Campylobacter Explaining Diarrhea. 2020. Available online: https://osf.io/preprints/osf/jegwq (accessed on 29 November 2023).
- Nardelli, C.; Gentile, I.; Setaro, M.; Di Domenico, C.; Pinchera, B.; Buonomo, A.R.; Zappulo, E.; Scotto, R.; Scaglione, G.L.; Castaldo, G.; et al. Nasopharyngeal Microbiome Signature in COVID-19 Positive Patients: Can We Definitively Get a Role to Fusobacterium Periodonticum? Front. Cell Infect. Microbiol. 2021, 11, 625581.
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute Periodontal Lesions (Periodontal Abscesses and Necrotizing Periodontal Diseases) and Endo-Periodontal Lesions: Dd56II Joint EFP-AAP Workshop. J. Periodontol. 2018, 89, S85–S102.
- Patel, J.; Sampson, V. The Role of Oral Bacteria in COVID-19. Lancet Microbe 2020, 1, e105.
- Carcuac, O.; Berglundh, T. Composition of Human Peri-Implantitis and Periodontitis Lesions. J. Dent. Res. 2014, 93, 1083–1088.
- Belibasakis, G.N. Microbiological and Immuno-Pathological Aspects of Peri-Implant Diseases. Arch. Oral Biol. 2014, 59, 66–72.
- Guo, H.; Zhou, Y.; Liu, X.; Tan, J. The Impact of the COVID-19 Epidemic on the Utilization of Emergency Dental Services. J. Dent. Sci. 2020, 15, 564–567.
- Yu, J.; Zhang, T.; Zhao, D.; Haapasalo, M.; Shen, Y. Characteristics of Endodontic Emergencies during Coronavirus Disease 2019 Outbreak in Wuhan. J. Endod. 2020, 46, 730–735.
- Li, H.; Chen, V.; Chen, Y.; Baumgartner, J.C.; Machida, C.A. Herpesviruses in Endodontic Pathoses: Association of Epstein-Barr Virus with Irreversible Pulpitis and Apical Periodontitis. J. Endod. 2009, 35, 23–29.
- Cosme-Silva, L.; Dal-Fabbro, R.; Cintra, L.T.A.; dos Santos, V.R.; Duque, C.; Ervolino, E.; Mogami Bomfim, S.; Gomes-Filho, J.E. Systemic Administration of Probiotics Reduces the Severity of Apical Periodontitis. Int. Endod. J. 2019, 52, 1738–1749.
- Fischer, R.G.; Lira Junior, R.; Retamal-Valdes, B.; Figueiredo, L.C.d.; Malheiros, Z.; Stewart, B.; Feres, M. Periodontal Disease and Its Impact on General Health in Latin America. Section V: Treatment of Periodontitis. Braz. Oral Res. 2020, 34, e026.
- Sampson, V.; Kamona, N.; Sampson, A. Could There Be a Link between Oral Hygiene and the Severity of SARS-CoV-2 Infections? Br. Dent. J. 2020, 228, 971–975.
More
