Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Mohamed F. Abdallah.
Maize is frequently contaminated with multiple mycotoxins, especially those produced by Aspergillus flavus and Fusarium verticillioides. Mycotoxin contamination is a critical factor that destabilizes global food safety.
- maize
- Aspergillus flavus
- Fusarium verticillioides
1. Introduction
Maize (Zea mays L.) is one of the strategic cereal crops which can be processed into a variety of food, feedstuff, and other industrial products [1]. The threat posed to maize production by fungal plant diseases is one of the critical factors that can destabilize global food security and safety. Preharvest losses due to fungal plant diseases are estimated to account for nearly 10–20% of cultivated maize, which can feed about 8.5% of the world’s population [2]. Among these diseases, Aspergillus Ear Rot and Fusarium Ear Rot, caused by Aspergillus and Fusarium species, respectively, are the most important [3]. Both diseases decrease the yield and quality of the maize crop and the safety of maize kernels due to the production of mycotoxins, secondary fungal metabolites toxic to animals and humans [4].
Aspergillus Ear Rot disease is mainly caused by a fungal pervasive maize invader called A. flavus [5]. The A. flavus species has been reported in several countries in Africa, America, Asia, and Europe [6,7,8][6][7][8]. Toxigenic A. flavus species produce several mycotoxins/secondary metabolites; however, due to their toxicity and widespread contamination, the most studied toxins are aflatoxins (AFs) [9]. So far, there are four members of AFs called B1, B, G1, and G2. Aflatoxin B1 (AFB1) is the most potent member of AFs, and several fatal outbreaks have been associated with the consumption of AFB1-contaminated maize in Brazil (60 deaths) and Kenya (317 cases of intoxications and 125 deaths) [10,11][10][11]. The toxicity of AFB1 has aroused widespread public concern due to its hepatotoxic, immunotoxic, mutagenic, carcinogenic, and teratogenic properties [12]. The International Agency for Research on Cancer (IARC) classified AFB1 as a group 1 carcinogen due to the sufficient evidence of causing liver cancer in humans [13].
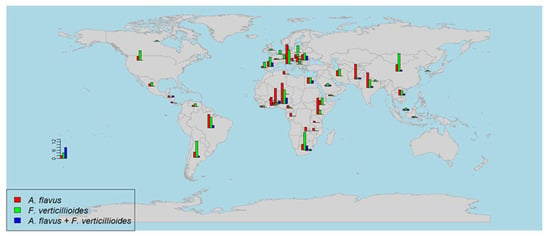
2. Global Distribution of A. flavus and F. verticillioides in Maize
A general overview of the number of studies per country reporting the (co-)occurrence of A. flavus and F. verticillioides in different continents between 1980 and 2020 is shown in Figure 1. Furthermore, their sampling years, sample numbers. Most of these studies were on A. flavus occurrence, followed by F. verticillioides, while fewer studies on the co-infection were published. The research of A. flavus was highest in Africa, with 74 scientific papers, followed by Asia (39 studies), Europe (35 studies), and the Americas (14 studies). However, Europe has a high awareness of studying the contamination of F. verticillioides that was reported in 51 papers, followed by Africa (40 studies), Asia (37 studies), and the Americas (27 studies). These data show that people are paying more attention to the contamination of both fungi [4], which reflect that the mycotoxin pollution problem of these two is increasing during these years. Figure 1 shows that more research of A. flavus and F. verticillioides is related to hot and rainy climates in African countries, which favored the growth of the two fungi. Apart from Africa, it was noticeable that southern European countries (Italy, Portugal, Spain, and Romania), some Asian countries (China, India, Iran, and Pakistan), and other countries in Latin and northern Americas (Brazil, Argentina, and United States) had a considerable number of publications. Gradually, those areas face an increased risk of A. flavus and F. verticillioides co-contamination [23][14].
Figure 1. A world map showing the number of studies that surveyed the (co-)occurrence of A. flavus and F. verticillioides. The number of studies is represented as a bar chart for A. flavus (red color), F. verticillioides (green color), and both fungi (blue color).
3. Worldwide Co-Occurrence of AFB1 and FB1 in Maize and Maize-Based Products
The simultaneous occurrence of several mycotoxins in a single product is a common situation, with the natural co-contamination of AFB1 and FB1 in maize and maize products as an example. The most common analytical technique (up to 66.7%) used for detecting and quantifying AFB1 and FB1 in the last decade was liquid chromatography–tandem mass spectrometry (HPLC-MS/MS). This is owing to the essential strengths of HPLC-MS/MS, including potentially high analytical specificity, a wide range of applicability to small and large molecules, the capability of multi- and mega-parametric tests, and the opportunity to develop robust assays with a high degree of flexibility within a short time frame [248][15].
In Africa, high concentrations of FB1 were 10,447 μg/kg and 18,184 μg/kg, and AFB1 concentrations that co-occur with high FB1 were 6738 μg/kg and 1081 μg/kg, respectively [249,250][16][17]. Based on this, it was found that there can be a positive relationship between AFB1 and FB1 under this co-existence condition with the collected data in Africa: the concentration of AFB1 is correspondingly high/low in the presence of high/low concentrations of FB1 according to the correlation coefficient (r > 0.8). However, Sangare-Tigori et al., Kpodo et al., and Kimanya et al. contradicted this positive relationship, which can be the selection of detection methods. In the Americas, 70% of FB1 were higher than 2000 μg/kg, and the highest was up to 53,000.0 μg/kg [251][18], almost ten times more than in Africa under the co-occurrence of AFB1 and FB1. In Asia, the highest concentrations of FB1 and AFB1 were 37,000 μg/kg and 4030 μg/kg in the analyzed samples [18,252][19][20]. Moreover, since 2010, AFB1 concentration was significantly decreased compared with before 2010. However, there was no apparent interaction between AFB1 and FB1 in the samples in the Americas (r < 0.1) and Asia (r < 0.1).
The mean of AFB1 and FB1 levels in studies from different continents is shown in Figure 32. As more than 70% of the produced maize was primarily used for animal feed in the world [286][21], the EU maximum limits for feed maize FB1(2000 µg/kg) and AFB1 (20 µg/kg) were selected as thresholds to interpret the collected data. From 1991 to 2020, 38% of AFB1 and 61% of FB1 studies exceeded the EU maximum limits separately. However, these excess issues have not happened in all continents under the co-occurrence of AFB1 and FB1. In Africa, the co-occurrence of both mycotoxins has risen to 53.8% since 2012. From 2012, 30.0% of survey studies are out of the AFB1 threshold, but all cases are below the FB1 threshold. In the Americas, 44.4% of AFB1 was higher than 20 µg/kg, and 33.3% of FB1 was higher than 2000 µg/kg. In Asia, 62.5% of studies exceeded the AFB1 limit, and only one study reported FB1 contamination exceeding the FB1 limit. There were no cases exceeding the EU maximum limits for both toxins in the last decade year.
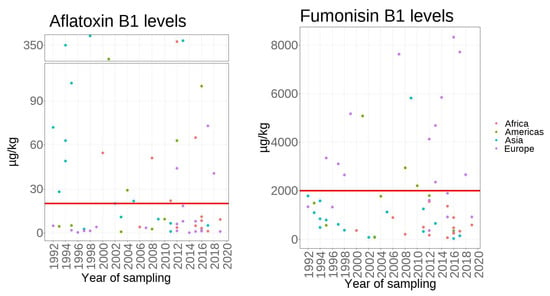

Figure 32. The reported mean values of aflatoxin B1 (AFB1) and fumonisin B1 (FB1) in studies from different continents. The red lines show the EU maximum limit for AFB1 (20 µg/kg) for cereals and FB1 (2000 µg/kg) for unprocessed maize.
4. Interactions between A. flavus and F. verticillioides and Their Toxins in Maize
The outcome of the interactions between A. flavus and F. verticillioides differs depending on the applied laboratory conditions for each experiment. This includes the substrate, culture media (in vitro) or maize (in vivo), and the related incubation conditions. Fakhrunnisa and Ghaffar have proved that A. flavus inhibited the growth of F. verticillioides (inhibition rate 16.67%) by producing a zone of inhibition in the dual agar culture plate assay [288][22]. In case the incubation conditions are changed (e.g., temperature, CO2, and humility), the interaction between A. flavus and F. verticillioides can also change, as reported by Camardo Leggieri et al. [289][23]. In their study, the growth of A. flavus was affected by the co-inoculum of F. verticillioides, and colony diameter was significantly lower than that measured in pure colonies if the incubation was between 20 °C and 25 °C. On the contrary, at 35 °C, A. flavus growth was enhanced by the presence of F. verticillioides [289][23]. Consistently, Giorni et al. reported that the co-existence of A. flavus and F. verticillioides was influenced by the temperature and water activity [290][24]. They reported that with the presence of both fungi, F. verticillioides nutritionally dominated all the strains of A. flavus at 20 °C and 0.95 aw, while A. flavus always nutritionally dominated F. verticillioides at 30 °C with either high aw (0.98 aw) or reduced aw (0.87 aw) [290][24]. The interaction between A. flavus and F. verticillioides under in vivo environment is also highly dynamic. It depends on the experimental conditions, the variable measured, and how they colonize the host. Chen et al. observed the symptoms of the lesion and mycotoxin production to evaluate the interaction of A. flavus and F. verticillioides in maize [290][24]. The dual inoculation resulted in reduced lesions of A. flavus. In contrast, the lesion size and toxin production of F. verticillioides were unaffected in the presence of A. flavus in maize at 25 °C. In contrast, their mixed inoculation resulted in more extensive lesions than a single A. flavus inoculation and higher FB production than a single F. verticillioides inoculation [290][24]. The study indicates that A. flavus can be more affected by F. verticillioides in maize. A previous study underlined the different abilities of A. flavus and F. verticillioides to grow simultaneously on maize since they usually occupy different niches regarding carbon sources [290][24]. It is stated that F. verticillioides seems to be dominant because it can use more carbon sources at the lowest temperatures (15 °C) and the highest aw levels (> 0.95 aw), while A. flavus becomes dominant at higher temperatures (>25–30 °C) and dry conditions (0.87 aw) [290,293][24][25].References
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. New York Acad. Sci. 2014, 1312, 105–112.
- Bebber, D.P.; Gurr, S.J. Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Genet. Biol. 2015, 74, 62–64.
- Masiello, M.; Somma, S.; Ghionna, V.; Francesco Logrieco, A.; Moretti, A. In vitro and in field response of different fungicides against Aspergillus flavus and Fusarium species causing ear rot disease of maize. Toxins 2019, 11, 11.
- Gurikar, C.; Shivaprasad, D.P.; Sabillón, L.; Nanje Gowda, N.A.; Siliveru, K. Impact of mycotoxins and their metabolites associated with food grains. Grain Oil Sci. Technol. 2022, 6, 1–9.
- Munkvold, G.P.; Weieneth, L.; Proctor, R.H.; Busman, M.; Blandino, M.; Susca, A.; Logrieco, A.; Moretti, A. Pathogenicity of fumonisin-producing and nonproducing strains of Aspergillus species in section nigri to maize ears and seedlings. Plant Dis. 2018, 102, 282–291.
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus Section Flavi isolated from maize in northern Italy. Int. J. Food Microbiol. 2007, 113, 330–338.
- Kortei, N.K.; Annan, T.; Kyei-Baffour, V.; Essuman, E.K.; Okyere, H.; Tettey, C.O. Exposure and risk characterizations of ochratoxins A and aflatoxins through maize (Zea mays) consumed in different agro-ecological zones of Ghana. Sci. Rep. 2021, 11, 23339.
- Massomo, S.M.S. Aspergillus flavus and aflatoxin contamination in the maize value chain and what needs to be done in Tanzania. Sci. Afr. 2020, 10, e00606.
- Peles, F.; Sipos, P.; Győri, Z.; Pfliegler, W.P.; Giacometti, F.; Serraino, A.; Pagliuca, G.; Gazzotti, T.; Pócsi, I. Adverse effects, transformation and channeling of aflatoxins into food raw materials in livestock. Front. Microbiol. 2019, 10, 2861.
- Lewis, L.; Onsongo, M.; Njapau, H.; Schurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A.; et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005, 113, 1763–1767.
- Wouters, A.T.B.; Casagrande, R.A.; Wouters, F.; Watanabe, T.T.N.; Boabaid, F.M.; Cruz, C.E.F.; Driemeier, D. An outbreak of aflatoxin poisoning in dogs associated with aflatoxin B1-contaminated maize products. J. Vet. Diagn. Investig. 2013, 25, 282–287.
- Meissonnier, G.M.; Pinton, P.; Laffitte, J.; Cossalter, A.M.; Gong, Y.Y.; Wild, C.P.; Bertin, G.; Galtier, P.; Oswald, I.P. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 2008, 231, 142–149.
- IARC. Aflatoxin: Scientific Background, Control, and Implications—Google Books; IARC (International Agency for Research on Cancer): Lyon, France, 2012.
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328.
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252.
- Kimanya, M.E.; De Meulenaer, B.; Tiisekwa, B.; Ndomondo-Sigonda, M.; Devlieghere, F.; Van Camp, J.; Kolsteren, P. Co-occurrence of fumonisins with aflatoxins in home-stored maize for human consumption in rural villages of Tanzania. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 1353–1364.
- Kimanya, M.E.; Shirima, C.P.; Magoha, H.; Shewiyo, D.H.; De Meulenaer, B.; Kolsteren, P.; Gong, Y.Y. Co-exposures of aflatoxins with deoxynivalenol and fumonisins from maize based complementary foods in Rombo, northern Tanzania. Food Control 2014, 41, 76–81.
- De Oliveira, C.A.F.; Cruz, J.V.S.; Rosim, R.E.; Bordin, K.; Kindermann, A.C.P.; Corassin, C.H. Simultaneous occurrence of aflatoxins and fumonisins in corn intended for the pet feed industry and for human consumption. J. Food Chem. Nanotechnol. 2016, 2, 1–5.
- Rosiles, M.R.; Bautista, J.; Fuentes, V.O.; Ross, F. An Outbreak of Equine Leukoencephalomalacia at Oaxaca, Mexico, associated with fumonisin B1. J. Vet. Med. A Physiol. Pathol. Clin. Med. 1998, 45, 299–302.
- Yang, X.; Gao, J.; Liu, Q.; Yang, D. Co-occurrence of mycotoxins in maize and maize-derived food in China and estimation of dietary intake. Food Addit. Contam. Part B Surveill. 2019, 12, 124–134.
- Dowswell, C.R.; Paliwal, R.L.; Cantrell, R.P. Maize in the Third World; CRC Press: Boca Raton, FL, USA, 2019.
- Fakhrunnisa, H.M.; Ghaffar, A. In vitro interaction of Fusarium spp.; with other fungi. Pak. J. Bot. 2006, 38, 1317–1322.
- Camardo Leggieri, M.; Giorni, P.; Pietri, A.; Battilani, P. Aspergillus flavus and Fusarium verticillioides interaction: Modeling the impact on mycotoxin production. Front. Microbiol. 2019, 10, 2653.
- Giorni, P.; Magan, N.; Battilani, P. Environmental factors modify carbon nutritional patterns and niche overlap between Aspergillus flavus and Fusarium verticillioides strains from maize. Int. J. Food Microbiol. 2009, 130, 213–218.
- Marin, S.; Sanchis, V.; Vinas, I.; Canela, R.; Magan, N. Effect of water activity and temperature on growth and fumonisin B1 and B2 production by Fusarium proliferatum and F. moniliforme on maize grain. Lett. Appl. Microbiol. 1995, 21, 298–301.
More
