You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Bik-Kwoon Tye.
The origin recognition complex (ORC) selects sites for replication initiation by recruiting a pair of hexameric minichromosome maintenance (MCM) complexes to replication origins where the pre-replication complex (Pre-RC) is assembled, and the bidirectional replisomes are formed.
- origin selection
- replication initiation
- eukaryotic DNA replication
1. Introduction
Eukaryotic DNA replication is highly conserved with one guiding principle: the entire genome must be replicated precisely and accurately once in every cell division. It is not surprising that a fundamental mechanism shared by all eukaryotes is highly conserved, but how conserved is it? The replication of DNA must be coordinated with the gene expression program of a cell to synchronize with the temporal expression of genes and to limit conflicts of DNA replication and transcription forks, which are a major source of DNA damage. Yeast is an excellent model for studying DNA replication because of its simplicity, but its life cycle is very different from those of vertebrates that have elaborate developmental programs.
2. The Origin Recognition Complex Is Conserved in Structure but Diverged in DNA Binding Properties
The origin recognition complex is a six-subunit complex that was initially purified in yeast based on its affinity to yeast origin DNA in an ATP-dependent manner [33][1]. Yeast replication origins can be cloned on plasmids as autonomously replicating sequences (ARSs) [34,35][2][3]. These ARSs share a 17 bp consensus sequence known as the ARS consensus sequence (ACS) [36,37][4][5]. The six ORC subunits, Orc1, Orc2, Orc3, Orc4, Orc5 and Orc6, are found in all eukaryotes. They are highly conserved in protein sequence and structure from yeast to humans. However, despite their conservation in structure, ORC has diverged binding sites ranging from an absolute requirement for a specific sequence in the budding yeast to a total agnosticism to base sequences in humans. The binding of the human ORC on chromatin appears to be dictated by the chromatin landscape, clustering in intergenic regions [38][6] where nucleosomes are depleted, such as CpG islands, transcriptional start sites and GC-rich DNA [39][7]. An immediate question is why do metazoans and fungi use different strategies for identifying replication initiation sites? Humans developing from a single fertilized egg to an adult go through many stages of development that require the programmed expression of different sets of genes. As a result, the chromatin landscape changes throughout development. Just like transcription factors, replication initiators need to find their target sites in response to these changes [40,41][8][9]. On the other hand, yeasts are single-celled organisms that have hardly any developmental changes. Their life mission is to divide as rapidly and efficiently as possible. Their chromatin landscape is largely constant. Like metazoan replication origins, the budding yeast ARSs are located at intergenic regions. They are nested in nucleosome free regions of about 125 bp in polar T-rich and A-rich DNA segments. The essential element, ACS, is a 11 bp sequence (WTTTATRTTTW) [42][10] asymmetrically positioned near the upstream nucleosome while two important elements, B1 and B2, are located downstream from the ACS [37][5]. Upon ORC binding to the ACS, the downstream nucleosome is repositioned [43][11] presumably to make room for the loading of the MCM double hexamer at the B2 element. Of the more than 400 ARSs identified, 249 have these canonical features [44][12].3. The cryoEM Structure of Yeast ORC Bound to Origin DNA
To understand the interactions of the yeast ORC with origin DNA, it is important to study its structure at atomic resolution. Yeast ORC (yORC) bound to origin DNA was reconstituted and subjected to single-particle cryo-electron microscopy analysis [2][13]. The structure of the yORC bound to 72 bp of ARS305 DNA containing the ACS and B1 element was determined to 3.0-Å resolution showing five of the subunits, Orc1-5, encircling the ACS (Figure 1a) while Orc6 is situated away from the ACS (Figure 1b). A prominent feature of this ORC-ARS DNA complex is the bending of the origin DNA at successive points at the ACS and B1 element (Figure 1c). When this structure is superposed with the structure of the ORC-Cdc6-Cdt1-Mcm2-7 (OCCM), an intermediate captured in the presence of ATPγS during helicase loading [45][14], the bent DNA aligns with the Mcm2-Mcm5 gate [2][13]. The implication of the orientation of the bent DNA with respect to the Mcm2-Mcm5 gate is that DNA bending facilitates a precise insertion of origin DNA into the MCM ring during the formation of the OCCM intermediate. Indeed, bending of the origin DNA is also observed in Drosophila ORC [46][15]. The salient features of yORC complexed with ARS305 that help explain the properties and function of ORC are described below.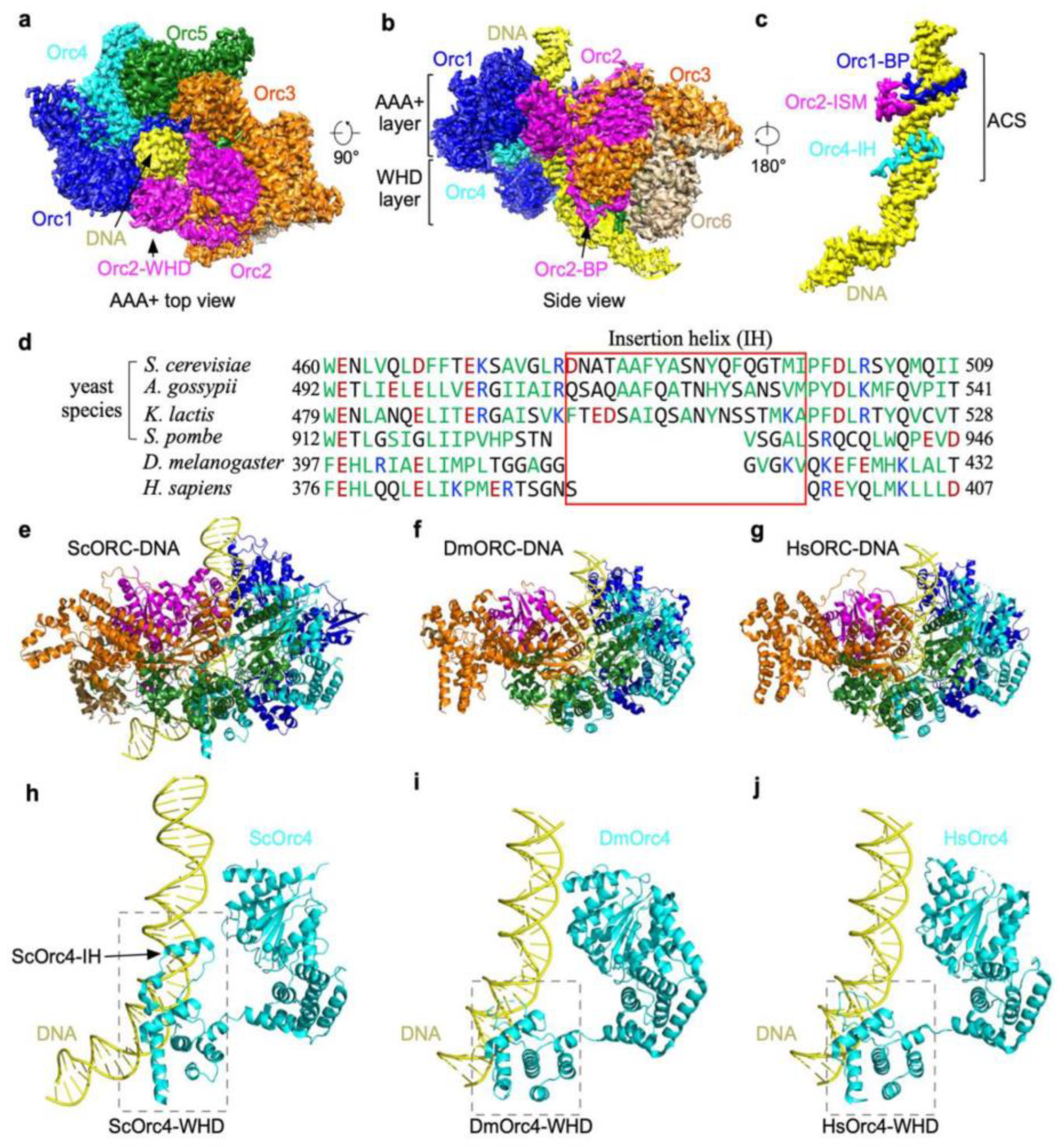
Figure 1. Base-specific recognition of ACS by yORC. (a). Top view. (b). Side view of ORC bound to ARS305. (c). Orc1 basic patch (BP) and Orc2 initiator-specific motif (ISM) interact with the minor groove while Orc4 insertion helix (IH) interacts with the major groove. (d). Multiple protein sequence alignment of Orc4 insertion helices from indicated species. (e–g). The atomic models of the ORC-DNA structures from ScORC-DNA (PDB: 5ZR1) (e). DmORC-DNA (PDB: 7JK5) (f). and HsORC-DNA. (g). Note that the DNA from the DmORC-DNA was modeled into the HsORC structure (PDB: 7JPS). (h). ScOrc4-IH interacts with the major groove of ACS. (i). IH absent in DmOrc4-WHD. (j). IH absent in HsOrc4-WHD.
4. Yeast Orc4 Insertion Helix Encodes Sequence-Specific Binding
The yORC binds origin DNA in two modes: base-specific and base non-specific. Orc1-5 each interacts with the DNA backbone along the ACS to the B1 region at multiple points, creating a tight grip. This non-specific DNA binding property explains the affinity of ORC even for non-ARS DNA. In addition to the base non-specific binding, three of the ORC subunits interact specifically with the bases of the ACS. A basic patch (BP) from Orc1 and the Orc2 initiator-specific motif (ISM) interact with the minor groove of the ACS while an insertion alpha helix (IH) in Orc4 interacts with the major groove (Figure 1c). The hydrophobic residues of the IH are embedded in a hydrophobic environment created by the methyl groups of the invariant Ts of the ACS, forming strong interactions. An alignment of the beta-hairpin region containing the Orc4 IH showed that the IH sequence has diverged among yeast species that have different ACSs but are totally absent in metazoans (Figure 1d). For the fission yeast S. pombe, ORC binds AT-rich sequences and the IH is missing, instead SpOrc4 has acquired four A-T hooks [47,48][16][17]. As the Orc1-BP and Orc2-ISM interact with the minor groove, where A-T or T-A base pairs are indistinguishable [49[18][19],50], of the three DNA binding motifs, the IH of Orc4 is the most likely determinant for the sequence-specific binding property of Saccharomyces cerevisiae ORC (ScORC). If so, removal of the IH of Orc4 should eliminate the sequence-specific binding property of the ScORC and convert it to one with properties more akin to metazoans such as Drosophila and humans (Figure 1e–j).5. Humanizing the Yeast ORC by Removing the 19 Amino Acid Insertion Helix of Orc4
To test this hypothesis, a mutant yeast strain (orc4-IHD was constructed such that the 19 amino acid insertion helix was removed from Orc4 [3][20]. Unexpectedly, this strain was viable albeit showing a prolonged G2 phase and an activated checkpoint in the late S phase as indicated by the phosphorylation of Rad53. Collisions between replication and transcription forks are a likely source of fork stalling and checkpoint activation in an uncoordinated replication initiation program. Footprints of ORC based on ChIP-seq showed that the mutant ORC (referred to as ORC-IHD) now binds many more sites in clusters throughout the yeast genome with little overlap with the canonical ARSs, indicating that ORC-binding shows an apparent decrease in sequence specificity (Figure 2a–c). As a result, ORC-IHD favors binding to transcriptional start sites (TSS) with 83% of binding sites located within 500 bp upstream of a TSS. Furthermore, this binding positively correlates with the transcriptional strength of the promoter (Figure 2d). In fact, these characteristics are precisely the binding profile observed for human ORC [39,51,52][7][21][22]. TSSs are known to have a polar bias in T-rich and A-rich sequences and well-positioned downstream nucleosomes, as found at ARSs in S. cerevisiae, suggesting that the mutant ORC-IHD has retained many of the site-selection properties but lost its base sequence specificity.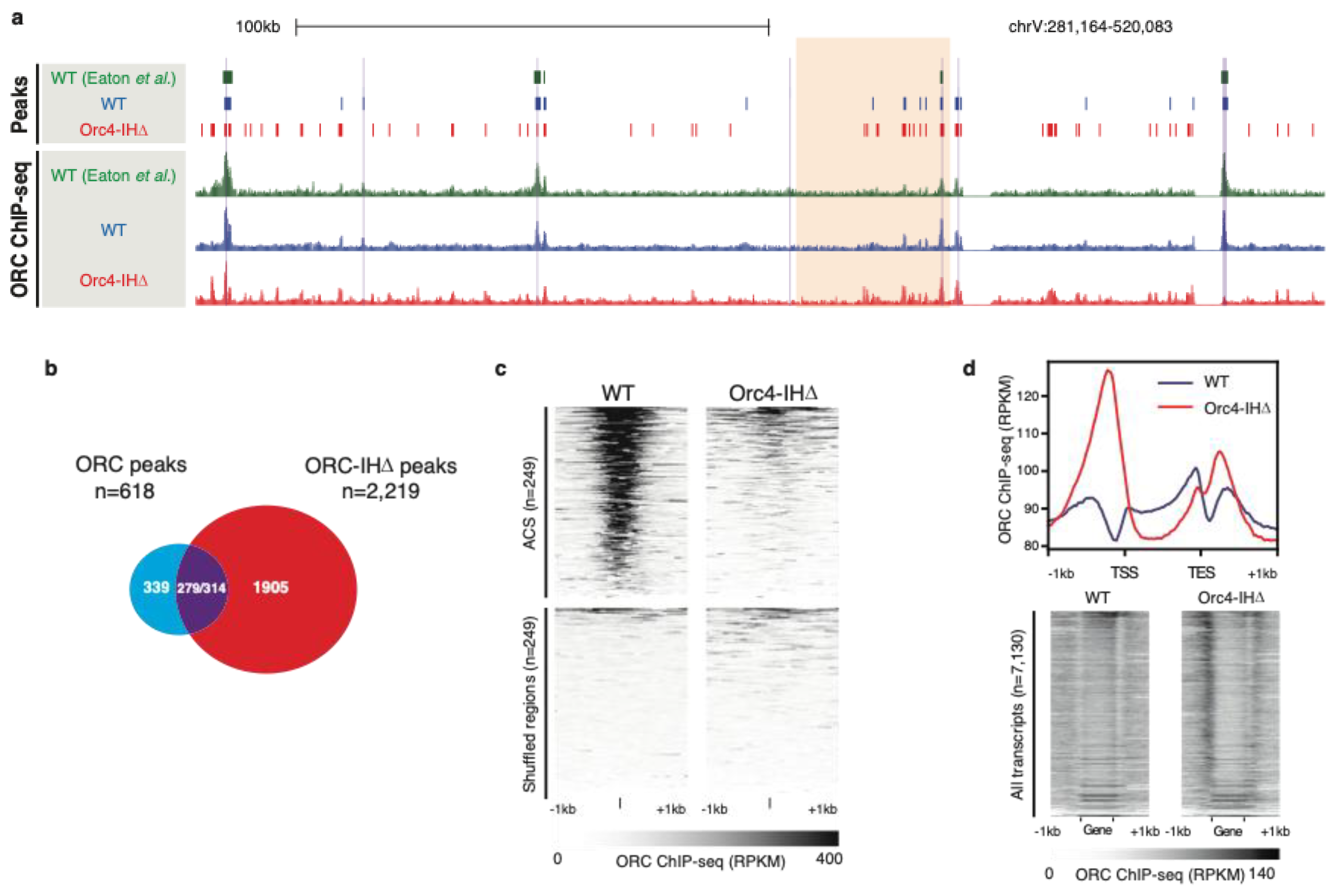
Figure 2. (a). Genome browser screenshots illustrate the enrichment patterns of ORC across a region on chrV. Normalized signals (Reads per kilobase per million reads (RPKM)) of WT ORC (blue), ORC-IHD (red), and previously published ORC-ChIP-seq datasets (green). (b). Venn diagram showing the overlap between ORC ChIP-seq peaks defined in WT (n = 618; blue) and Orc4-IHD cells (n = 2219; red). Of these, 279 ORC and 314 ORC-IHD peaks overlapped by at least 1 bp (purple), while 339 and 1905 peaks were identified as unique in WT and Orc4-IHD, respectively. (c). Heatmaps show the ORC enrichment patterns in WT and orc4-IHD at previously defined ACS (±1 kb of ACS; top). Size- and number-matched shuffled genomic loci were included as controls (bottom). (d). Line plot and heatmaps show the ORC enrichment patterns in WT and orc4-IHD at annotated transcripts and flanking sequences. The line plot (top) shows the aggregated ORC ChIP-seq signal (RPKM) at all annotated transcripts and the surrounding regions (±1 kb). ORC4-IHD binding (red) at the 5′ of the transcriptional start site (TSS) is dramatically higher than that of the WT ORC (blue). Heatmaps demonstrate the differential enrichment of ORC4-IHD upstream of the TSS. The transcripts in the heatmaps are arranged by descending RNA-seq signals, indicating the association of mutant ORC binding at these loci and the expression levels.
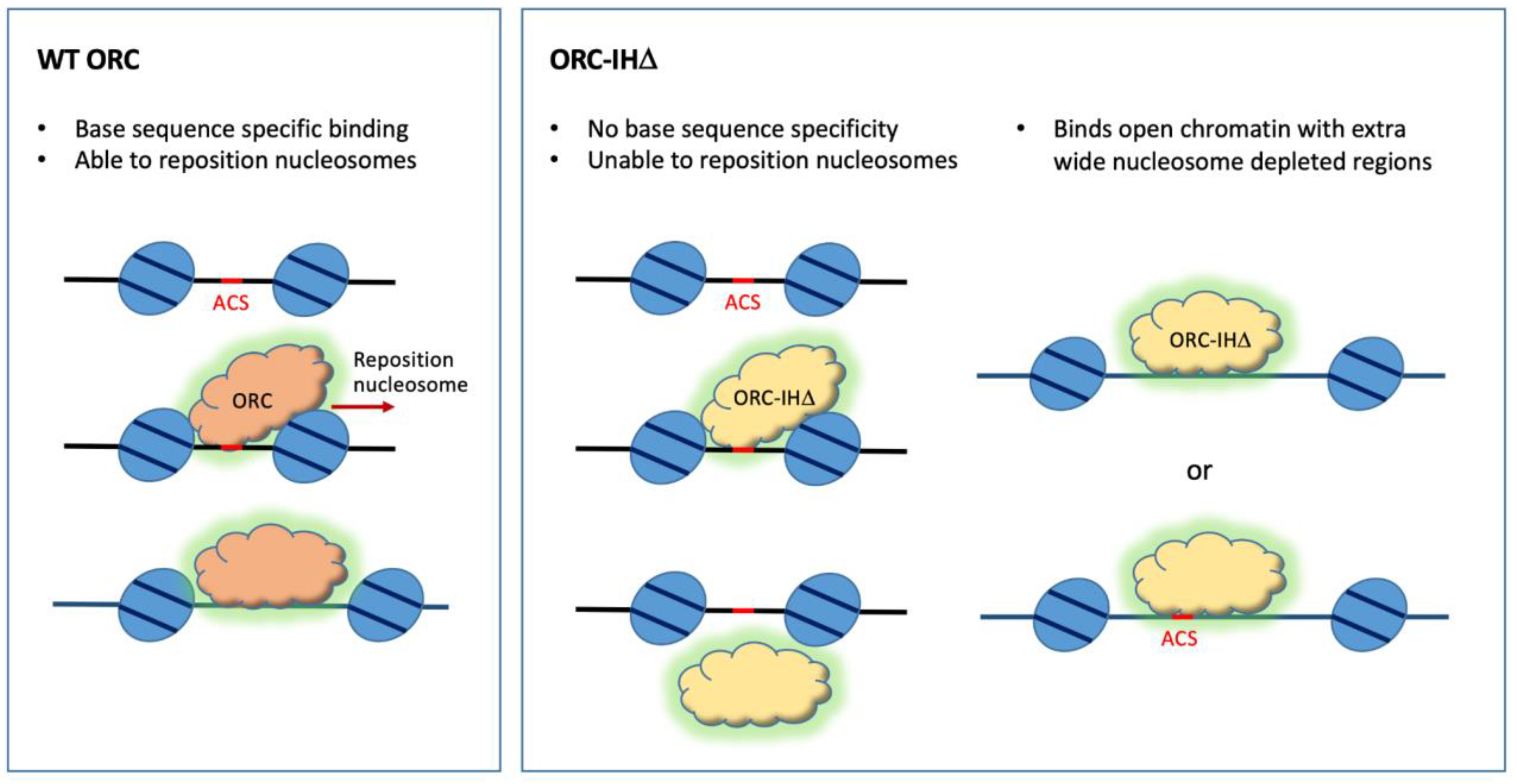
Figure 3. Model for the selection of binding sites by the WT and mutant ORC. WT ORC binds ACS and can reposition the flanking nucleosomes upon binding. ORC-IHD binds promiscuously to T-rich sequences, including ACSs, but is unable to reposition nucleosomes. Therefore, it binds wider nucleosome-depleted regions that do not require the repositioning of nucleosomes for MCM loading.
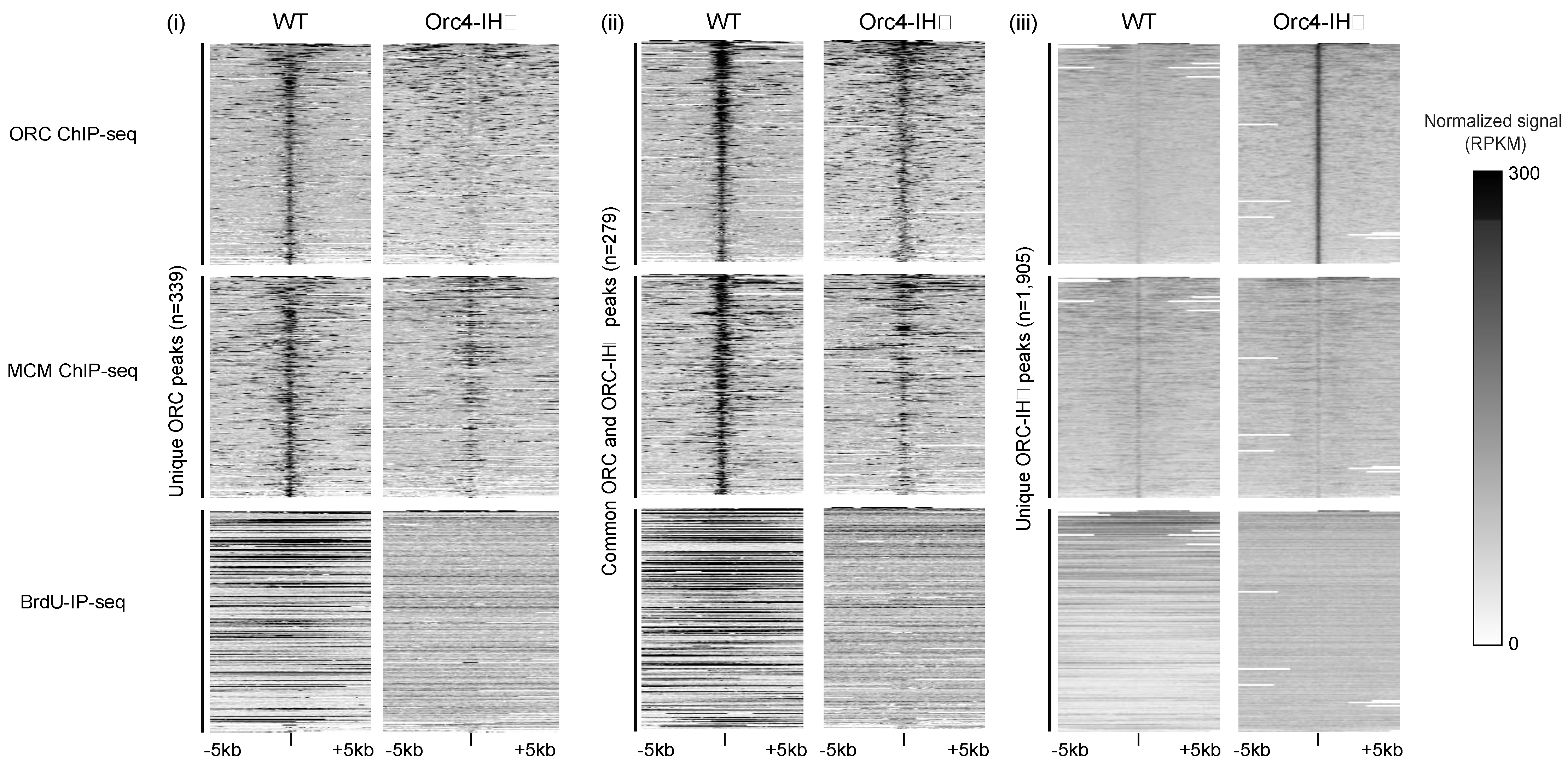
Figure 4. Heatmaps demonstrate the global changes in ORC and MCM ChIPseq and BrdUIP-seq signals. Focusing on ±5 kb surrounding ORC ChIP-seq peaks unique to WT (i), common to both WT and ORC4-IHD (ii), and unique to ORC4-IHD (iii), the normalized signals (RPKM) in WT and orc4-IHD cells are shown.
References
- Bell, S.P.; Stillman, B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature 1992, 357, 128–134.
- Struhl, K.; Stinchcomb, D.T.; Scherer, S.; Davis, R.W. High-frequency transformation of yeast: Autonomous replication of hybrid DNA molecules. Proc. Natl. Acad. Sci. USA 1979, 76, 1035–1039.
- Chan, C.S.; Tye, B.K. Autonomously replicating sequences in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1980, 77, 6329–6333.
- Chang, F.; Theis, J.F.; Miller, J.; Nieduszynski, C.A.; Newlon, C.S.; Weinreich, M. Analysis of chromosome III replicators reveals an unusual structure for the ARS318 silencer origin and a conserved WTW sequence within the origin recognition complex binding site. Mol. Cell. Biol. 2008, 28, 5071–5081.
- Hu, Y.; Stillman, B. Origins of DNA replication in eukaryotes. Mol. Cell 2023, 83, 352–372.
- Dijkwel, P.A.; Hamlin, J.L. The Chinese hamster dihydrofolate reductase origin consists of multiple potential nascent-strand start sites. Mol. Cell. Biol. 1995, 15, 3023–3031.
- Miotto, B.; Ji, Z.; Struhl, K. Selectivity of ORC binding sites and the relation to replication timing, fragile sites, and deletions in cancers. Proc. Natl. Acad. Sci. USA 2016, 113, E4810–E4819.
- Rivera-Mulia, J.C.; Gilbert, D.M. Replication timing and transcriptional control: Beyond cause and effect-part III. Curr. Opin. Cell Biol. 2016, 40, 168–178.
- Vouzas, A.E.; Gilbert, D.M. Replication timing and transcriptional control: Beyond cause and effect-part IV. Curr. Opin. Genet. Dev. 2023, 79, 102031.
- Broach, J.R.; Li, Y.Y.; Feldman, J.; Jayaram, M.; Abraham, J.; Nasmyth, K.A.; Hicks, J.B. Localization and sequence analysis of yeast origins of DNA replication. Cold Spring Harb. Symp. Quant Biol. 1983, 47 Pt 2, 1165–1173.
- Lipford, J.R.; Bell, S.P. Nucleosomes positioned by ORC facilitate the initiation of DNA replication. Mol. Cell 2001, 7, 21–30.
- Eaton, M.L.; Galani, K.; Kang, S.; Bell, S.P.; MacAlpine, D.M. Conserved nucleosome positioning defines replication origins. Genes Dev. 2010, 24, 748–753.
- Li, N.; Lam, W.H.; Zhai, Y.; Cheng, J.; Cheng, E.; Zhao, Y.; Gao, N.; Tye, B.K. Structure of the origin recognition complex bound to DNA replication origin. Nature 2018, 559, 217–222.
- Yuan, Z.; Riera, A.; Bai, L.; Sun, J.; Nandi, S.; Spanos, C.; Chen, Z.A.; Barbon, M.; Rappsilber, J.; Stillman, B.; et al. Structural basis of Mcm2-7 replicative helicase loading by ORC-Cdc6 and Cdt1. Nat. Struct. Mol. Biol. 2017, 24, 316–324.
- Bleichert, F.; Leitner, A.; Aebersold, R.; Botchan, M.R.; Berger, J.M. Conformational control and DNA-binding mechanism of the metazoan origin recognition complex. Proc. Natl. Acad. Sci. USA 2018, 115, E5906–E5915.
- Chuang, R.Y.; Kelly, T.J. The fission yeast homologue of Orc4p binds to replication origin DNA via multiple AT-hooks. Proc. Natl. Acad. Sci. USA 1999, 96, 2656–2661.
- Kong, D.; DePamphilis, M.L. Site-specific DNA binding of the Schizosaccharomyces pombe origin recognition complex is determined by the Orc4 subunit. Mol. Cell. Biol. 2001, 21, 8095–8103.
- Kielkopf, C.L.; White, S.; Szewczyk, J.W.; Turner, J.M.; Baird, E.E.; Dervan, P.B.; Rees, D.C. A structural basis for recognition of A.T and T.A base pairs in the minor groove of B-DNA. Science 1998, 282, 111–115.
- White, S.; Szewczyk, J.W.; Turner, J.M.; Baird, E.E.; Dervan, P.B. Recognition of the four Watson-Crick base pairs in the DNA minor groove by synthetic ligands. Nature 1998, 391, 468–471.
- Lee, C.S.K.; Cheung, M.F.; Li, J.; Zhao, Y.; Lam, W.H.; Ho, V.; Rohs, R.; Zhai, Y.; Leung, D.; Tye, B.K. Humanizing the yeast origin recognition complex. Nat. Commun. 2021, 12, 33.
- Dellino, G.I.; Cittaro, D.; Piccioni, R.; Luzi, L.; Banfi, S.; Segalla, S.; Cesaroni, M.; Mendoza-Maldonado, R.; Giacca, M.; Pelicci, P.G. Genome-wide mapping of human DNA-replication origins: Levels of transcription at ORC1 sites regulate origin selection and replication timing. Genome Res. 2013, 23, 1–11.
- Petryk, N.; Kahli, M.; d’Aubenton-Carafa, Y.; Jaszczyszyn, Y.; Shen, Y.; Silvain, M.; Thermes, C.; Chen, C.L.; Hyrien, O. Replication landscape of the human genome. Nat. Commun. 2016, 7, 10208.
More
