Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Wendy Huang and Version 1 by Tracey Martin.
Striatins (STRNs) are generally considered to be cytoplasmic proteins, with lower expression observed in the nucleus and at cell–cell contact regions. Together with protein phosphatase 2A (PP2A), STRNs form the core region of striatin-interacting phosphatase and kinase (STRIPAK) complexes through the coiled-coil region of STRN proteins, which is crucial for substrate recruitment. STRNs and the constituent members of the STRIPAK complex have been found to regulate several cellular functions, such as cell cycle control, cell growth, and motility.
- STRNs
- STRIPAK
- cell cycle
- cytoskeleton
- apoptosis
- autophagy
- adhesion
- migration
- cell proliferation
1. Introduction
The investigation of the striatin (STRN) family of proteins arose with the identification of striatin-3 (STRN3), also known as SG2NA and PPP2R6B, as a nuclear autoantigen involved in cell cycle regulation [1]. The research on another family member, striatin (STRN1, PPP2R6A), with its name derived from striatium, was concurrently conducted by another research group [2], and was subsequently recognised to belong to the same family as striatin–3 due to shared domain structures and protein–protein interaction domains. Zinedin (striatin–4, STRN4 PPP2R6C and Zin) was later discovered to be homologous to both STRN1 and STRN3 through phylogenetic analysis [3]. The genes encoding STRN–1, STRN–3, and STRN–4 were mapped to chromosomes 2p22-p21, 14q13-q21, and 19q13.2, respectively (Figure 1).
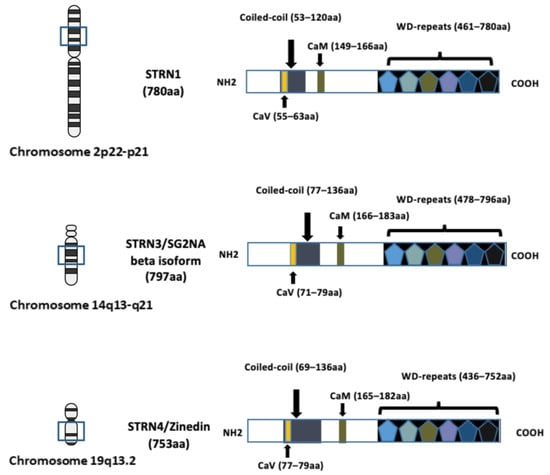
Figure 1. Schematic diagram illustrating the domain structures and chromosome locations of striatin–1 (STRN1), striatin–3 (STRN3) and striatin–4 (STRN4). The structure of each STRN includes four well-recognised domains: the caveolin-binding domain (CaV), the coiled-coil region, the calmodulin-binding domain (CaM) and the WD-repeats domain. The length of the amino acid (aa) sequence is indicated in brackets and the location of corresponding chromosome location is indicated. The arrows in the figure are to indicate location of each domain. Figure created using BioRender.com (agreement number EV266PPC09).
Through affinity purification and mass spectrometry, the STRNs, along with their regulatory protein, protein phosphatase 2A (PP2A), were identified as core constituents of the STRIPAK (striatin-interacting phosphatase and kinase) complex [4]. This complex constitutes an intricate protein network crucial in maintaining cell homeostasis and regulating essential signalling cascades in normal physiological functions. Dysregulation of STRIPAK members has been linked to disease progression, notably in cancer development, a field where their roles have been increasingly elucidated over the past decade.
The STRNs have been found to possess evolutionarily conserved functions and have been studied across different eukaryotic species, through the examination of their homologs. Serving as a cornerstone of the supramolecular STRIPAK complex, STRNs govern the regulation of various phosphatases and kinases, and have thereby been suggested to be extensively involved in a wide range of biological and cellular processes.
2. Cell Cycle
STRN3 is a well-recognised protein known to have elevated expression during the S and G2 phases of the cell cycle. Upregulation of STRN3 is associated with the extension of the G2 phase duration, whereas downregulation of STRN3 induces cell cycle arrest at G0/G1 [66][5]. By manipulating the expression of individual STRIPAK members, a recent study has provided further insight into the role of STRIP1 in regulating the G1 phase of the cell cycle [39][6]. This regulation occurs through GCKIII-mediated enhancement of the expression of cyclin-dependent kinase (CDK) inhibitors p21 and p27, following STRIP1 depletion. Furthermore, STRNs have been implicated in modulating microtubule dynamics, which plays a fundamental role in key cell cycle events such as chromosome segregation, spindle assembly, and cytokinesis [70][7].3. Cytoskeleton Remodelling
The cell cytoskeleton consists of microtubules, actin, and intermediate filaments, forming a dynamic and extremely complex network that controls not only cell integrity, architecture, and motility but also signals to cells in response to external stimuli. The implication of the STRIPAK complex in cytoskeleton remodelling is often associated with the ezrin, radixin and moesin (ERM) family proteins, a group of proteins linking the cytoskeleton and cell membrane that are key modulators in cell morphology/migration and tissue morphogenesis. Inhibition of PP2A in the STRIPAK complex has been shown to affect the subcellular localisation of SLIK and downregulate ERM in Drosophila, resulting in morphological defects of cells during mitosis [14][8]. During endothelial cell migration, lamellipodia formation and membrane retraction have been demonstrated to be, in part, due to MAP4K4-regulated ERM phosphorylation, in response to growth factor stimulation [71,72][9][10]. In addition to STRIPAK association with the ERM proteins, STRIP1 and STRIP2 have been identified as regulators of the cytoskeleton [73][11]. However, their depletion induces distinct phenotypes of cells, indicating differences in their signalling partners during cell spreading. Although several lines of evidence have shown the involvement of the STRIPAK complex in cytoskeletal organisation, the study of individual STRIPAK members in this area is lacking. Until recently, STRN1 has been found to co-localise with microtubules in HEK293T cells, and its downregulation resulted in microtubule depolymerisation [70][7]. However, the exact signalling events involved in this process need further elucidation.4. Apoptosis and Autophagy
Apoptosis is recognised as programmed cell death. Although its dysregulation can induce tumour progression and angiogenesis in cancer, it is also a leading cause of resistance to chemotherapies. All three members of the STRNs family have been implicated in this manner, with decreased expression associated with increased cell susceptibility to apoptosis, under cell stress or cancer therapies [42,59][12][13]. However, in contrast to traditional results, a recent study offers a new perspective that STRNs can regulate cell apoptosis in T-cells when in complex with the scaffolding subunit of PP2A (PP2AA). The regulation is possibly via NF-κB signalling [15][14]. During cancer development, ER stress can enhance cell aggressiveness by promoting NF-κB mediated anti-apoptosis; and this activity has further been found to be facilitated by the STRN3–PP2A complex [69][15]. Beyond the core region, other STRIPAK components also play roles in apoptosis, via influencing various signalling cascades. For example, MAPKs were found to inhibit Hippo signalling via MST1/2 [42][12], whereas CCM3 regulated MST4-dependent ERM phosphorylation as a protective mechanism for cells to survive oxidative stress-induced apoptosis [74][16]. Autophagy demonstrates controversial roles in tumour formation, suppressing tumour initiation by eliminating damaged components while potentially fostering cancer development through increasing cell susceptibility to stress-induced death. A recent study has revealed interaction between STRN1 and ULK1 (Unc-51 Like Autophagy Activating Kinase 1) in a positive-feedback manner via PP2A, promoting cell autophagy [75][17]. The implication of STRIPAK in autophagy within muscle tissues has additionally been demonstrate in cancer research [76,77][18][19]. Although MAP4K2, a GCKI family member, is not considered a STRIPAK member, the depletion of STRN4 induces hyperphosphorylation and activation of MAP4K2, suggesting a crucial regulatory role of STRIPAK upstream to MAP4K2. This regulation has been proposed to be involved in autophagy during cellular stress [77][19].5. Cell Adhesion and Migration
With sufficient evidence demonstrating the contribution of STRIAPK members in cytoskeleton regulation, it is not surprising that this complex exerts significant roles in cell adhesion and motility. Consequently, the complex’s members may offer novel insights into understanding cancer development and progression. Recent findings indicate that STRN1 colocalizes with APC, a regulatory protein of tumour-suppressive β-catenin, at cell contact areas, helping to maintain correct cell–cell adhesion [54][20]. STRN1 was not found to colocalize with E-cadherin, an adherens junction marker. However, its depletion reduces cell adhesion through mechanisms involving E-cadherin. Furthermore, alterations in E-cadherin affect the subcellular localisation of STRN1, subsequently influencing cell polarization and migration [54][20]. PP2A has been shown to negatively regulate tight junction assembly through interactions with several junctional proteins such as occludin [78][21]. This regulation may be mediated by different regulatory subunits of PP2A, as APC has been found to direct PP2A activity by interacting with several distinct regulatory subunits of PP2A [54][20]. Nevertheless, the contribution of the STRIPAK complex, in addition to STRN-APC or individual STRIPAK member-mediated cell behaviour changes, remains to be addressed.6. Cell Proliferation
STRIPAK complex components are involved in cell proliferation, via the regulation of several signalling pathways. Knocking down of STRN3, SIKE1, SLMAP, and STRIP1 was associated with increased MST2 activation, which, in turn, inhibits YAP translocation and oncogenic gene transcription in the gastric HGC27 cell line [79][22]. Additionally, the STRN3/PP2A complex was shown to deactivate MAP4K4 in Hippo signalling, promoting cell growth and proliferation in brain tumour cells [12][23]. With the involvement of STRIPAK in cell cycle control, the breast cancer cell line MDA-MB-231 exhibited cell cycle arrest and suppressed cell proliferation and growth upon depletion of STRIP1. This regulation was shown to involve tumour-suppressive CDK inhibitors p21 and p27 [39][6]. Furthermore, MOB4 is also a crucial STRIPAK component that controls cell proliferation by involvement in STRIPAK assembly and regulation of Hippo signalling. Downregulation of STRN1 has been shown to destabilise microtubules and inhibit the proliferation of HEK293 cells [70][7].7. DNA Damage and Repair
As key machinery involved in the loss of the tumour-suppressive Hippo signalling pathway, STRIPAK complex-mediated MST1/2 inactivation of Hippo has been shown to facilitate DNA double-stranded break (DSB), inducing resistance to drug therapies [80][24]. Interestingly, although STRIP1 depletion has a tumour-suppressive role in breast cancer proliferation via communication with P21 and P27, it showed a contradictory characteristic upon the administration of chemotherapies to cells [39][6]. The cells’ fate, after non-lethal doses of chemotherapy, is dependent on the STRIP1-mediated P21/P27 expression level prior to and during the drug treatment. Inhibition of PP2A was additionally investigated in a recent study as a way of sensitising cancer cells to radio- and chemotherapies [81][25].8. Immune Regulation
More and more research has recently appreciated the role of STRIPAK members in immune regulation in cancer. STRN4 and PP2A exhibited immunosuppressive features that were shown to limit stimulator of interferon gene (STING)-mediated antitumour immune responses, via modulating the Hippo signalling pathway [82][26]. Similarly, reduced expression of STRIP2 was associated with a higher level of immune response in lung adenocarcinoma [83][27]. Additionally, MAP4K4 has also been shown to regulate inflammation-related signalling cascades such as the NF-κB pathway [84][28]. It has been established that cancer cells can mimic ligands of immune checkpoints, as a way to escape immune surveillance. The immunotherapy drugs function as inhibitors, blocking this false interaction. Recently, PP2A has emerged as a key immune-checkpoint regulator, but again, its substrate specificity and involvement in downstream signalling is varied, depending on its regulatory subunits [85][29].9. Therapeutic Drug Responses
Despite the direct relationship between the alteration of many aforementioned cell functions and drug responses, the exact role of the STRIPAK complex in both cellular and patients’ responses to chemotherapies remains inconclusive and largely undetermined. This uncertainty may stem from signalling variability across different cells and tissues, as well as cancer types. Although a lower level of STRIP1 was shown to supress breast cancer cell proliferation via interference with cell cycle regulation, decreased STRIP1 exhibited tumour-promoting effects when cells were treated with a non-lethal dose of doxorubicin or cisplatin [39][6]. STRIPAK-mediated deactivating of MST1/2 in Hippo signalling also endows cells with resistance to chemo-, radiotherapies, and PARPi (poly ADP ribose polymerase inhibitor), an inhibitor to modulate DNA repair [80][24]. Furthermore, silencing of STRN4 was shown to sensitise pancreatic cancer cells to gemcitabine [86][30]. Clinically, breast cancer patients who expressed higher level of STRN3, CCM3, PPP2CA, and PPP2CB were shown to be more sensitive to chemotherapies. However, the involvement of STRN3 in breast cancer patients’ drug responses was also reported to be hormone-receptor-status dependent [87][31].10. Other Cellular Functions of the STRIPAK Complex
STRN1 has recently been identified as a regulator of ER homeostasis, as its depletion impairs the adaptive unfolded protein response (UPR) under ER stress, resulting in cell death [88][32]. SLMAP has also been found to localise in the ER, yet its role in ER homeostasis has not been clarified [89][33]. The same study has proposed the presence of SLMAP in mitochondria and the nuclear envelope, looking at the roles of STRNs, STRIP, and SLMAP in linking STRIPAK to those different locations. Indeed, several studies have established the role of STRIPAK in nuclear import of MAPKs in a dependent manner in fungi and its role in cell metabolism [40,90][34][35]. Furthermore, STRIPAK members have been demonstrated to regulate angiogenesis processes [38][36], which is a critical step involved in tumour growth and cancer progression.References
- Muro, Y.; Chan, E.K.; Landberg, G.; Tan, E.M. A cell-cycle nuclear autoantigen containing WD-40 motifs expressed mainly in S and G2 phase cells. Biochem. Biophys. Res. Commun. 1995, 207, 1029–1037.
- Castets, F.; Bartoli, M.; Barnier, J.V.; Baillat, G.; Salin, P.; Moqrich, A.; Bourgeois, J.P.; Denizot, F.; Rougon, G.; Calothy, G.; et al. A novel calmodulin-binding protein, belonging to the WD-repeat family, is localized in dendrites of a subset of CNS neurons. J. Cell Biol. 1996, 134, 1051–1062.
- Castets, F.; Rakitina, T.; Gaillard, S.; Moqrich, A.; Mattei, M.G.; Monneron, A. Zinedin, SG2NA, and striatin are calmodulin-binding, WD repeat proteins principally expressed in the brain. J. Biol. Chem. 2000, 275, 19970–19977.
- Goudreault, M.; D’Ambrosio, L.M.; Kean, M.J.; Mullin, M.J.; Larsen, B.G.; Sanchez, A.; Chaudhry, S.; Chen, G.I.; Sicheri, F.; Nesvizhskii, A.I.; et al. A PP2A phosphatase high density interaction network identifies a novel striatin-interacting phosphatase and kinase complex linked to the cerebral cavernous malformation 3 (CCM3) protein. Mol. Cell. Proteom. 2009, 8, 157–171.
- Pandey, S.; Talukdar, I.; Jain, B.P.; Tanti, G.K.; Goswami, S.K. GSK3beta and ERK regulate the expression of 78 kDa SG2NA and ectopic modulation of its level affects phases of cell cycle. Sci. Rep. 2017, 7, 7555.
- Rodriguez-Cupello, C.; Dam, M.; Serini, L.; Wang, S.; Lindgren, D.; Englund, E.; Kjellman, P.; Axelson, H.; Garcia-Mariscal, A.; Madsen, C.D. The STRIPAK Complex Regulates Response to Chemotherapy Through p21 and p27. Front. Cell Dev. Biol. 2020, 8, 146.
- Kazmierczak-Baranska, J.; Peczek, L.; Przygodzka, P.; Cieslak, M.J. Downregulation of striatin leads to hyperphosphorylation of MAP2, induces depolymerization of microtubules and inhibits proliferation of HEK293T cells. FEBS Lett. 2015, 589, 222–230.
- De Jamblinne, C.V.; Decelle, B.; Dehghani, M.; Joseph, M.; Sriskandarajah, N.; Leguay, K.; Rambaud, B.; Lemieux, S.; Roux, P.P.; Hipfner, D.R.; et al. STRIPAK regulates Slik localization to control mitotic morphogenesis and epithelial integrity. J. Cell Biol. 2020, 219, e201911035.
- Vitorino, P.; Yeung, S.; Crow, A.; Bakke, J.; Smyczek, T.; West, K.; McNamara, E.; Eastham-Anderson, J.; Gould, S.; Harris, S.F.; et al. MAP4K4 regulates integrin-FERM binding to control endothelial cell motility. Nature 2015, 519, 425–430.
- Baumgartner, M.; Sillman, A.L.; Blackwood, E.M.; Srivastava, J.; Madson, N.; Schilling, J.W.; Wright, J.H.; Barber, D.L. The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. Proc. Natl. Acad. Sci. USA 2006, 103, 13391–13396.
- Bai, S.W.; Herrera-Abreu, M.T.; Rohn, J.L.; Racine, V.; Tajadura, V.; Suryavanshi, N.; Bechtel, S.; Wiemann, S.; Baum, B.; Ridley, A.J. Identification and characterization of a set of conserved and new regulators of cytoskeletal organization, cell morphology and migration. BMC Biol. 2011, 9, 54.
- Seo, G.; Han, H.; Vargas, R.E.; Yang, B.; Li, X.; Wang, W. MAP4K Interactome Reveals STRN4 as a Key STRIPAK Complex Component in Hippo Pathway Regulation. Cell Rep. 2020, 32, 107860.
- Chen, W.; White, M.A.; Cobb, M.H. Stimulus-specific requirements for MAP3 kinases in activating the JNK pathway. J. Biol. Chem. 2002, 277, 49105–49110.
- Chen, C.; Shi, Z.; Zhang, W.; Chen, M.; He, F.; Zhang, Z.; Wang, Y.; Feng, M.; Wang, W.; Zhao, Y.; et al. Striatins contain a noncanonical coiled coil that binds protein phosphatase 2A A subunit to form a 2:2 heterotetrameric core of striatin-interacting phosphatase and kinase (STRIPAK) complex. J. Biol. Chem. 2014, 289, 9651–9661.
- Bonsignore, G.; Martinotti, S.; Ranzato, E. Endoplasmic Reticulum Stress and Cancer: Could Unfolded Protein Response Be a Druggable Target for Cancer Therapy? Int. J. Mol. Sci. 2023, 24, 1566.
- Fidalgo, M.; Guerrero, A.; Fraile, M.; Iglesias, C.; Pombo, C.M.; Zalvide, J. Adaptor protein cerebral cavernous malformation 3 (CCM3) mediates phosphorylation of the cytoskeletal proteins ezrin/radixin/moesin by mammalian Ste20-4 to protect cells from oxidative stress. J. Biol. Chem. 2012, 287, 11556–11565.
- Siva Sankar, D.; Hu, Z.; Dengjel, J. The complex interplay between ULK1 and protein phosphatases in autophagy regulation. Autophagy 2022, 18, 455–456.
- Guo, Y.; Zeng, Q.; Brooks, D.; Geisbrecht, E.R. A conserved STRIPAK complex is required for autophagy in muscle tissue. Mol. Biol. Cell 2023, 34, ar91.
- Seo, G.; Yu, C.; Han, H.; Xing, L.; Kattan, R.E.; An, J.; Kizhedathu, A.; Yang, B.; Luo, A.; Buckle, A.L.; et al. The Hippo pathway noncanonically drives autophagy and cell survival in response to energy stress. Mol. Cell 2023, 83, 3155–3170.e3158.
- Lahav-Ariel, L.; Caspi, M.; Nadar-Ponniah, P.T.; Zelikson, N.; Hofmann, I.; Hanson, K.K.; Franke, W.W.; Sklan, E.H.; Avraham, K.B.; Rosin-Arbesfeld, R. Striatin is a novel modulator of cell adhesion. FASEB J. 2019, 33, 4729–4740.
- Nunbhakdi-Craig, V.; Machleidt, T.; Ogris, E.; Bellotto, D.; White, C.L., 3rd; Sontag, E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J. Cell Biol. 2002, 158, 967–978.
- Tang, Y.; Fang, G.; Guo, F.; Zhang, H.; Chen, X.; An, L.; Chen, M.; Zhou, L.; Wang, W.; Ye, T.; et al. Selective Inhibition of STRN3-Containing PP2A Phosphatase Restores Hippo Tumor-Suppressor Activity in Gastric Cancer. Cancer Cell 2020, 38, 115–128.e119.
- Migliavacca, J.; Zullig, B.; Capdeville, C.; Grotzer, M.A.; Baumgartner, M. Cooperation of Striatin 3 and MAP4K4 promotes growth and tissue invasion. Commun. Biol. 2022, 5, 795.
- An, L.; Cao, Z.; Nie, P.; Zhang, H.; Tong, Z.; Chen, F.; Tang, Y.; Han, Y.; Wang, W.; Zhao, Z.; et al. Combinatorial targeting of Hippo-STRIPAK and PARP elicits synthetic lethality in gastrointestinal cancers. J. Clin. Investig. 2022, 132, e155468.
- Mazhar, S.; Taylor, S.E.; Sangodkar, J.; Narla, G. Targeting PP2A in cancer: Combination therapies. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 51–63.
- Ho, W.S.; Mondal, I.; Xu, B.; Das, O.; Sun, R.; Chiou, P.; Cai, X.; Tahmasebinia, F.; McFadden, E.; Wu, C.Y.; et al. PP2Ac/STRN4 negatively regulates STING-type I IFN signaling in tumor-associated macrophages. J. Clin. Investig. 2023, 133, e162139.
- Zhang, X.; Chen, Q.; He, Y.; Shi, Q.; Yin, C.; Xie, Y.; Yu, H.; Bao, Y.; Wang, X.; Tang, C.; et al. STRIP2 motivates non-small cell lung cancer progression by modulating the TMBIM6 stability through IGF2BP3 dependent. J. Exp. Clin. Cancer Res. 2023, 42, 19.
- Singh, S.K.; Roy, R.; Kumar, S.; Srivastava, P.; Jha, S.; Rana, B.; Rana, A. Molecular Insights of MAP4K4 Signaling in Inflammatory and Malignant Diseases. Cancers 2023, 15, 2272.
- Ruvolo, P.P. The broken “Off” switch in cancer signaling: PP2A as a regulator of tumorigenesis, drug resistance, and immune surveillance. BBA Clin. 2016, 6, 87–99.
- Wong, M.; Hyodo, T.; Asano, E.; Funasaka, K.; Miyahara, R.; Hirooka, Y.; Goto, H.; Hamaguchi, M.; Senga, T. Silencing of STRN4 suppresses the malignant characteristics of cancer cells. Cancer Sci. 2014, 105, 1526–1532.
- Li, A.X.; Zeng, J.J.; Martin, T.A.; Ye, L.; Ruge, F.; Sanders, A.J.; Khan, E.; Dou, Q.P.; Davies, E.; Jiang, W.G. Striatins and STRIPAK complex partners in clinical outcomes of patients with breast cancer and responses to drug treatment. Chin. J. Cancer Res. 2023, 35, 365–385.
- Jain, B.P.; Pandey, S.; Saleem, N.; Tanti, G.K.; Mishra, S.; Goswami, S.K. SG2NA is a regulator of endoplasmic reticulum (ER) homeostasis as its depletion leads to ER stress. Cell Stress Chaperones 2017, 22, 853–866.
- Nordzieke, S.; Zobel, T.; Franzel, B.; Wolters, D.A.; Kuck, U.; Teichert, I. A fungal sarcolemmal membrane-associated protein (SLMAP) homolog plays a fundamental role in development and localizes to the nuclear envelope, endoplasmic reticulum, and mitochondria. Eukaryot. Cell 2015, 14, 345–358.
- Kuck, U.; Radchenko, D.; Teichert, I. STRIPAK, a highly conserved signaling complex, controls multiple eukaryotic cellular and developmental processes and is linked with human diseases. Biol. Chem. 2019, 400, 1005–1022.
- Dettmann, A.; Heilig, Y.; Ludwig, S.; Schmitt, K.; Illgen, J.; Fleissner, A.; Valerius, O.; Seiler, S. HAM-2 and HAM-3 are central for the assembly of the Neurospora STRIPAK complex at the nuclear envelope and regulate nuclear accumulation of the MAP kinase MAK-1 in a MAK-2-dependent manner. Mol. Microbiol. 2013, 90, 796–812.
- Suryavanshi, N.; Furmston, J.; Ridley, A.J. The STRIPAK complex components FAM40A and FAM40B regulate endothelial cell contractility via ROCKs. BMC Cell Biol. 2018, 19, 26.
More
