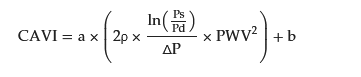A warning sign for impending cardiovascular events is not fully established. In the process of plaque rupture, the formation of vulnerable plaque is important, and oxidized cholesterols play an important role in its progression. Furthermore, the significance of vasa vasorum penetrating the medial smooth muscle layer and being rich in atheromatous lesions should be noted. The cardio-ankle vascular index (CAVI) is a new arterial stiffness index of the arterial tree from the origin of the aorta to the ankle. The CAVI reflects functional stiffness, in addition to structural stiffness. The rapid rise in the CAVI means medial smooth muscle cell contraction and strangling vasa vasorum. A rapid rise in the CAVI in people after a big earthquake, following a high frequency of cardiovascular events has been reported.
1. Introduction
Arteriosclerotic disease is the major cause of mortality and morbidity among cardiovascular diseases not only in developed countries but also in developing countries
[1]. Regarding the formation of atherosclerotic lesions in the arterial wall, many hypotheses have been proposed. Cholesterol was first thought to be a cause of atheroma formation
[2]. However, cholesterol is an important lipid component in the cell membrane. Then, denatured low-density lipoproteins (LDLs) containing cholesterol were proposed as a cause of form cell formation in atheroma
[3][4][5][3,4,5]. Later, Ross et al. proposed an injury response hypothesis
[6] to explain intimal thickening composed of the synthetic type of smooth muscle cell (SMC) proliferation, leading to stenosis of the arterial cavity. However, stenosis was not necessarily observed in the occluded coronary artery by thrombus
[7]. Then, plaque rupture theory was proposed
[8]. In that theory, vulnerable plaque is formed subsequent to a chronic inflammatory reaction in the intima. In chronic inflammatory reactions, macrophages digest the surrounding tissues, thinning a covering cap. When a thin cap ruptures, a formed thrombus occludes the lumen of the artery, leading to myocardial infarction. However, the real causes of provoked inflammation in atheromatous lesions were not fully clarified, although many cytokines relating to inflammatory reactions were investigated. Oxysterol products are toxic to cells
[9]. Oxysterol products might be a ringleader to make vulnerable plaque.
2. Reconsideration concerning the Formation of Vulnerable Plaque
2.1. The Formation of the Lipid Pool in the Intima of the Arterial Wall
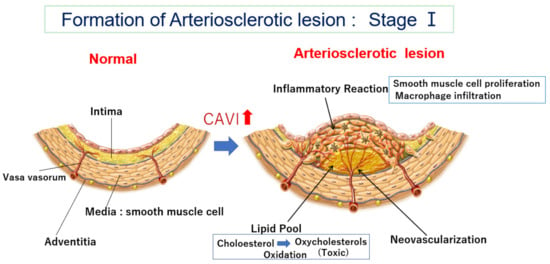
One first question is the location of the lipid pool in the arterial wall. Human pathological studies showed that cholesterol deposition in the artery is not just observed under the intimal endothelial cell layer
[10][11][21,22]. The lipid pool is situated deep in the intima, adjacent to the internal lamina. Cholesterol-rich lipoproteins such as LDL, IDL, and/or small dense LDL in the blood have been generally thought to enter through the intimal endothelial layer from the lumen of the artery
[12][13][23,24]. If the main entrance of cholesterol-rich lipoproteins was the surface of the endothelial layer of the lumen of the artery, the lipid pool would be formed just under the endothelial layer. However, why does the cholesterol pool develop in the deep area adjacent to the internal lamina in the intima and not just beneath the endothelial layer? Recently, several studies have reported that vasa vasorum exist in the arterial wall and become abundant in advanced atherosclerotic lesions
[14][15][25,26], but the significance of vasa vasorum has not been fully discussed. Nourishment of arteries had been believed mainly by diffusion from the lumen of the vessel. However, recently, it has also been reported that cholesterol-rich lipoproteins and nutrients are transported by vasa vasorum from the adventitia and enter the arterial wall
[16][27] (
Figure 1).
Figure 1. Formation of an arteriosclerotic lesion: Stage Ⅰ. Cholesterol-rich lipoproteins such as LDL, IDL, and small dense LDL are carried by vasa vasorum and enter the deep intimal area. There, cholesterols are oxidized, and several oxidative products are produced. Oxysterols are toxic and damage the surrounding tissues. For example, 7-ketocholeterol induces apoptosis of smooth muscle cells and expands the lipid pool. Then, oxysterols induced an inflammatory reaction, and macrophages infiltrated. Smooth muscle cells migrate from the media to the intima and proliferate to make intimal thickening. During this process, vasa vasorum develop from adventitia into an intimal lesion through the medial smooth muscle layer (neovascularization). The CAVI increases as arteriosclerosis develops. CAVI: cardio-ankle vascular index; IDL: intermediate-density lipoprotein; LDL: low-density lipoprotein.
2.2. How Does the Lipid Pool Developed in the Intima Make Progress to Vulnerable Plaque—The Role of Oxidized Cholesterol for Provoking Inflammatory Reaction
Deposited cholesterol might not be toxic because cholesterol is a natural organic compound and a part of the cell membrane and is also an ingredient of hormones and bile, as mentioned above. Then, how does the cholesterol pool form and invade the surrounding area? One possible explanation is based on oxidized cholesterols. In atherosclerotic lesions, there are many oxidized cholesterols, such as 7α-ketocholesterol and 7α-hydroxycholesterol
[17][28]. Those oxysterols are generated by various oxidative stresses, such as diabetes mellitus, smoking, and aging during deposition in the lipid pool
[18][19][29,30]. Furthermore, oxysterols are enzymatically produced
[20][21][31,32]. The toxicity of oxysterols has been investigated in several studies. 7-ketocholesterol mediated inflammation in atherosclerosis
[22][23][24][33,34,35]. Thus, oxysterols might be the main cause to provoke an inflammatory reaction in atheromatous lesions.
Chronic kidney disease (CKD) is one of the arteriosclerotic diseases, and it is known that oxidative stress influences its progression and complications. However, the role of antioxidants has not been clarified. It is known that probucol, a cholesterol-lowering agent that also lowers HDL cholesterol, has antioxidant activity
[25][40].
2.3. Neovascularization of Vasa Vasorum in the Arterial Wall
Recent observations have confirmed the presence of vasa vasorum and neocapillaries in atherosclerotic plaques, as mentioned before
[14][25]. This angiogenic process can be initiated by hypoxia in the intima
[15][26]. Hypoxia is induced by the impairment of oxygen diffusion from lumina by intimal thickening; this hypoxic condition induces the expression and release of angiogenic factors (e.g., VEGF)
[26][43]. Thus, the vasa vasorum density is higher in atherosclerotic-prone areas, and neovascularization facilitates the supply of nutrients and oxygen to intimal lesions
[16][27].
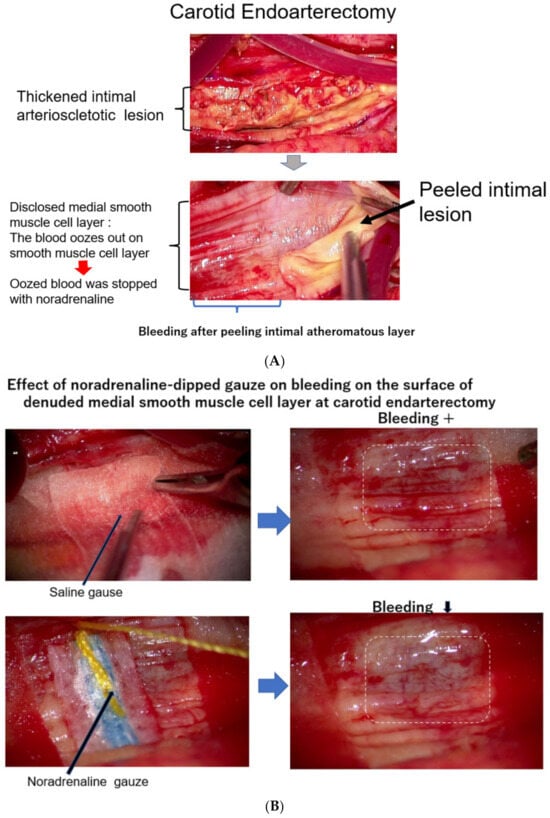
As it is not clear how much blood is carried by vasa vasorum to the intimal atheromatous lesion, scholars observed the surgical endarterectomy of the carotid artery in a patient suffering from carotid artery stenosis. Just after peeling off the proliferative intimal layer of the stenotic carotid artery, the surface of the denuded medial SMC layer was immediately covered with blood, which exuded from the medial smooth muscle layer. This observation means that the blood into the intimal lesion is obviously transported by vasa vasorum penetrating the medial SMC layers from the adventitia (
Figure 23A). During this operation, when the surface of the denuded medial smooth muscle layer was covered with a sheet of gauze dipped in saline, the bleeding on the surface of the smooth muscle layer did not stop; however, when this surface was covered with noradrenaline-dipped gauze, the blood exudation stopped (
Figure 23B)
[27][20].
Figure 23. (
A) The surface of the medial layer peeled off the intimal atheromatous layer. At endarterectomy of the carotid artery, the intimal atheromatous layer could be peeled away from the medial smooth muscle cell layer. The denuded surface of the medial smooth muscle layer was promptly covered with blood, indicating that the intimal arteriosclerotic lesion was supplied by blood with vasa vasorum, which penetrated through the medial smooth muscle layer from the adventitia. (
B) Bleeding at the surface of the peeled medial layer of the arteriosclerotic artery at endarterectomy. When the surface of the smooth muscle cell layers was covered with a sheet of gauze dipped with norepinephrine, the blood exuding from the medial smooth muscle layer was stopped, indicating that contraction of the medial smooth muscle stopped blood supply from the adventitia and caused ischemia of the intimal lesion. The medial smooth muscle contraction would bring a rapid increase in the CAVI.
3. The Meaning of a Rapid Increase in the CAVI
3.1. What Is the CAVI?
To know the stages of arteriosclerosis noninvasively is difficult. Arterial stiffness indicates the degree of arteriosclerosis, and several indices reflecting arterial stiffness have been proposed. PWV reflects arterial stiffness and can be easily calculated by dividing the length of the artery by the time during the pulse spreading from one end to the other end. Various kinds of pulse wave velocity (PWV), such as carotid–femoral PWV (cfPWV
[28][44] and brachial-ankle PWV (baPWV)
[29][45], have been presented in the many papers published in the last 30 years. However, PWV depends on blood pressure at the measuring time
[30][13].
More recently, the cardio-ankle vascular index (CAVI) was proposed as a new arterial stiffness index of the arterial tree from the origin of the aorta to the ankle
[31][14]. The CAVI was derived from the combination of the stiffness parameter β
[32][46] and the modified Bramwell–Hill equation
[33][47], and the formula is shown below (Equation (1)). The notable feature of the CAVI is its independence from blood pressure at measuring time.
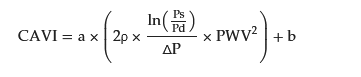
(PWV, pulse wave velocity of the arterial tree from the origin of the aorta to the ankle; Ps, systolic blood pressure; Pd, diastolic blood pressure; ρ, blood density; and
ΔP, Ps–Pd; a, b, coefficients
[9].)
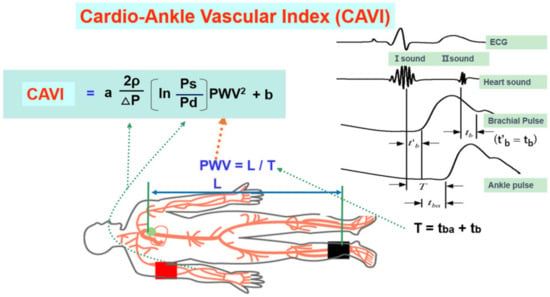
The CAVI is measured in the supine position using the VaSela system (Fukuda Denshi, Tokyo, Japan), as illustrated in
Figure 34 by Equation (1). The independen from blood pressure is based theoretically on the original stiffness parameter β, which is independent from blood pressure
[32][46]. And this has also been experimentally proven in clinical studies using an α-blocker, doxazosin, and a β-blocker
[34][16].
Figure 34. Equation of the cardio-ankle vascular index and measuring methods (adapted from Ref.
[31][14]). Ps: systolic blood pressure, Pd: diastolic pressure, In: natural logarithm, ΔP: Ps-Pd, ρ: blood viscosity, a,b: coefficiency, PWV: pulse wave velocity, L: length from the origin of the aorta to the ankle, T: time taken for the pulse wave to propagate from the aortic valve to the ankle, tba: time between the rise of brachial pulse wave and the rise of ankle pulse wave, tb: time between aortic valve closing sound and the notch of brachial pulse wave, t’b: time between aortic valve opening sound and the rise of brachial pulse wave.
3.2. The CAVI Reflects the Degree of Atherosclerosis
The cut-off value of the CAVI for arteriosclerosis was tentatively defined as nine. The CAVI increases with age, and it is reported to be high in arteriosclerotic patients with coronary artery disease, cerebral infarction, and chronic kidney disease (CKD)
[35][15].
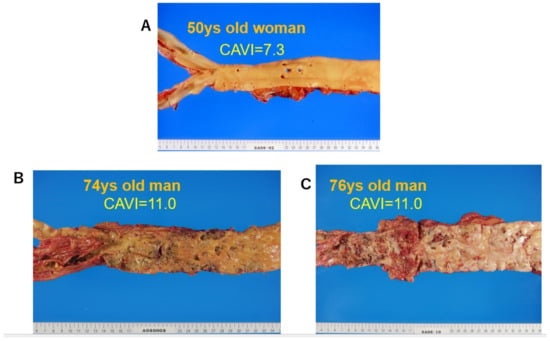
Figure 45 shows pictures of various atherosclerotic stages of the aorta. A CAVI = 7.3 indicates a nearly normal aorta in a 50-year-old woman. A CAVI = 11.0 represents far-advanced stages of atherosclerosis.
Figure 45. Various stages of atherosclerosis of the aortae and the CAVI. (
A) Aorta of a 50-year-old woman. The CAVI was 7.3, which is a nearly normal level (−0.5SD). (
B) Aorta of a 74-year-old man. The CAVI was 11.0, which is high for his age (+2SD). (
C) Aorta of a 76-year-old man. The CAVI was 11.0, which is high for his age (+2SD). A high CAVI value over +2SD from the average value for ages might indicate the presence of arteriosclerosis with vulnerable plaque. CAVI: cardio-ankle vascular index.
3.3. The CAVI Also Reflects the Functional Stiffness of the Artery
An interesting feature of the CAVI is that it reflects functional stiffness, which is derived from the contractional state of arterial smooth muscles. This conclusion can be deduced by the following studies. The administration of the adrenaline receptor α-blocker, doxazosin, decreases the CAVI, as stated above
[34][16]. Nitroglycerin administration also decreases the CAVI among control subjects and patients with arteriosclerotic diseases
[36][17]. The latter evidence indicates that the medial smooth muscle layer, even in advanced stages of atherosclerosis, retains the ability to contract or dilate in response to various stimuli. Septic conditions decrease the CAVI, accompanied by a decrease in blood pressure
[37][48].
3.4. The CAVI Just after a Huge Natural Disaster and Stress
There were several papers reporting that many more cardiovascular events occurred immediately after huge disasters, sometimes accompanying high blood pressure
[38][50]. Trichopoulos et al. reported that psychological stress-induced fatal heart attacks at the time of the earthquake
[39][51]. Dobson et al. reported that the number of heart attacks increased after the Newcastle earthquake
[40][52]. Leor et al. also reported that sudden cardiac death was triggered by an earthquake
[41][53]. Regarding the cause of those earthquake-related sequences of cardiovascular events, psychological stress was mentioned; however, the precise mechanism was not fully evaluated.
3.5. Smooth Muscle Cell Contraction Hypothesis for Plaque Rupture: The Role of Rapid Rise in the CAVI
The proposed mechanism for the occurrence of cardiovascular events after the rapid rise in the CAVI in cases showing a high CAVI is as follows. At first, an atheromatous lesion is formed by infiltration of cholesterol-rich lipoproteins such as LDL, IDL, and small dense LDL in a lipid pool. Then, deposited cholesterols become oxidative products due to oxidative stress. The resultant oxysterols act as a toxic substance in the intima, which provokes inflammatory reactions, such as the stimulation of macrophages, promoting the migration and proliferation of SMCs and inducting apoptosis of SMCs, eventually leading to the expansion of the lipid pool.
4. Treatments to Improve the CAVI
- (1)
-
Lifestyle changes
-
Weight reduction decreases the CAVI in obese diabetic patients. Continuous positive airway pressure therapy for sleep apnea syndrome decreases the CAVI (refer to 15). Waon therapy, in which patients are placed in a dry sauna at 60 degrees for 15 min, improves the CAVI
[42][55].
- (2)
-
Controlling high blood pressure
-
Antihypertensives, such as angiotensin-receptor blockers (olmesartan) and calcium channel blockers (cilnidipine and efonidipine), are reported to decrease the CAVI (refer to
[35][15]).
- (3)
-
Control of diabetic mellitus
-
The response of the CAVI to glucose-lowering treatment depends on the type of agent. Pioglitazone, a rapid-acting insulin, dipeptidyl peptidase 4 inhibitors, anagliptin, and the new sulfonylurea, glimepiride, are reported to decrease the CAVI (refer to
[35][15]).
- (4)
-
Among lipid-lowering agents
-
Among the many available statins, pitavastatin decreases the CAVI. Bezafibrate and eicosapentanoic acid are also reported to decrease the CAVI (refer to
[35][15]).
- (5)
-
Health supplements
-
For supplements, sirtuin enhancers, resveratrol, and s-equal are reported to decrease the CAVI in a double-blind study. The CAVI might be a useful index to evaluate the effects of supplements (refer to
[35][15]).