1. Introduction
Functional dyspepsia (FD) is a chronic non-organic gastrointestinal disease that is one of the most common diseases of the digestive system and one of the most common diseases of the gut–brain interaction worldwide
[1][2][1,2]. Approximately 16% of individuals within the general population are affected by epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS), which are two primary forms of FD
[3][4][3,4]. In countries outside of Asia, the incidence is approximately 10–40%, and the incidence in Asian countries is approximately 5–30%. Of the people who experience FD, approximately 40% choose to seek medical treatment due to discomfort
[5]. In approximately 80% of patients suffering from dyspepsia there is no structural explanation for their symptoms, which is then termed FD. The Rome IV criteria serve as the established diagnostic standard for FD. It is defined as a symptom of dyspepsia originating in the stomach and duodenum in the absence of evidence of organic, systemic, or metabolic disease that explains the symptom with its defining symptoms comprising postprandial satiety, epigastric pain, early satiety, or epigastric burning that persists for a minimum duration of six months
[6][7][6,7]. As far as the pathophysiology of the disease is concerned, the Rome IV criteria as well as a recent multinational consensus of European experts support the role for impaired gastric accommodation, gastric distention hypersensitivity, disturbances in gastric emptying, and altered central nervous system signal processing
[8]. The presence of lower gastrointestinal symptoms like diarrhea and constipation enhance the capacity of physicians to differentiate between individuals with functional digestive disorders and those experiencing non-FD
[9][10][9,10]. FD is a chronic condition without a known cure, and as a result, it profoundly influences both the physical and mental health and the overall quality of life of patients
[11].
The etiology and pathogenesis of FD remain unclear, but it is typically believed that it may be related to factors that include (1) gastric motility disturbance, (2) visceral hypersensitivity, (3) decreased gastric fundus receptivity diastolic function, (4)
Helicobacter pylori infection, (5) gut–brain axis disturbance, (6) mental and social factors, and (7) increased eosinophilic cells, epithelial barrier disruption, and mucosal inflammation in the duodenum accompanied by elevated mast cell levels
[12][13][14][15][16][12,13,14,15,16]. Additionally, an imbalance in intestinal flora is one of the important pathogenesis processes of FD. The imbalance in intestinal flora will lead to the disturbance of the intestinal environment, ultimately resulting in the reduction in the total amount of probiotics and a series of acute and chronic diseases
[17][18][17,18]. Thus, the correlation between the gut microbiome and the development of human diseases is of paramount importance
[19]. There are several lines of evidence suggesting that both locoregional duodenal and systemic changes may also be present in FD. Duodenal eosinophilia, epithelial barrier defect, and subtle mucosal inflammation, along with higher levels of mast cells, have been reported in FD, whereas the role of local and systemic inflammatory changes and increased small-bowel-homing T cells were not highlighted until very recently
[20][21][20,21]. Intestinal flora may improve the clinical symptoms of FD by improving intestinal barrier function and visceral hypersensitivity, and regulating gastrointestinal motility. Changing the type and composition of intestinal flora may provide a safe and effective treatment to relieve FD symptoms
[22].
Probiotics are active microorganisms that are beneficial to the host. Furthermore, strains that can meet the basic conditions of probiotics can become probiotics. Therefore, probiotics cover numerous types of microorganisms, and the main probiotics in this study include
Bifidobacterium,
Lactobacillus,
Saccharomycetes,
Bacillus, and others
[23][24][23,24]. Concurrently, the health effects of probiotics have also become a research hotspot (
Table 1). Presently, probiotics have been demonstrated to maintain the normal structure of intestinal flora, resist pathogen infection, improve constipation and diarrhea, relieve lactose intolerance, reduce serum cholesterol levels, and promote immune system development
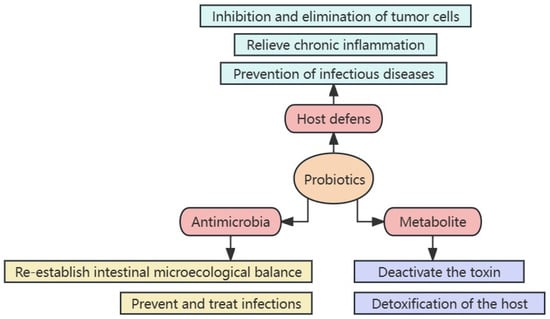
[25]. The mechanism of action of probiotics primarily includes three aspects: (1) enhancing the host defense capacity, (2) directly fighting microorganisms, and (3) metabolites playing an important function
[26] (
Figure 1). Probiotics can effectively relieve functional gastrointestinal disorders such as irritable bowel syndrome
[27]. Several intestinal flora colonize the gastrointestinal tract, an organ involved in the pathogenesis of FD due to mucosal damage and inflammation
[15]. Therefore, improving FD symptoms by regulating the gut microbiota may be beneficial.
Figure 1. Mechanism of probiotic actions.
Table 1. The health effects of common probiotics.
| Probiotic Species |
Bacterial Strain |
Mechanism of Action |
Health Benefits |
References |
| Lactobacillus |
Lactobacillus acidophilus |
Adjuvant antigen-specific immune response; |
Immunity enhancement; lower inflammatory factors; restoration of nervous system function |
[28][29][28,29] |
| |
Lactobacillus casei |
Through antagonism, colonization competition, increasing antibody production, enhancing the systemic immune effect, and the antibacterial action of metabolites, it can resist the invasion of pathogens |
Inhibition of pathogenic bacteria in the gut; protecting the internal environment; maintaining intestinal microecology |
[30][31][30,31] |
| |
Lactobacillus paracasei |
Regulating the production of anti-inflammatory cytokines by Th1/Th2 cells and reducing the release of toxic nitrogen-containing metabolites |
Relieving inflammation; relieving chronic metabolic diseases |
[32][33][34][32,33,34] |
| |
Lactobacillus rhamnosus |
Competitive colonization, inhibition of H. pylori growth, and adhesion to mucosal cells |
Maintaining intestinal barrier integrity; inhibiting inflammation and oxidative stress; regulating gut–brain communication |
[35] |
| |
Lactobacillus reuteri |
Increasing the number of Bifidobacteria in the gut, and transforming the intestinal dominant flora suitable for breaking down proteins into the flora suitable for sugar metabolism, thereby reducing the production of toxic and spoilage metabolites |
Secreting antimicrobial compounds; regulating the host immune system; preventing diarrhea and colitis; reducing the prevalence of acute abdominal pain in infants |
[36][37][38][36,37,38] |
| Bifidobacterium |
Bifidobacterium longum |
Increasing the intestinal flora and inhibiting pathogenic bacteria |
Maintaining intestinal health in early life; promoting the establishment of intestinal microbiota |
[39][40][39,40] |
| |
Bifidobacterium animals |
Increasing antibody production |
Resisting foreign pathogenic microorganisms |
[41] |
| |
Bifidobacterium infantis |
Preventing excessive intestinal immune responses |
Regulating intestinal flora; anti-hepatic fibrosis; anti-infection |
[42][43][42,43] |
| Others |
Bacillus coagulans |
Enhancing the specific defense of non-specific antigens |
Pathogen suppression; improved immune ability and growth performance of the body; improved intestinal digestion and absorption of nutrients; improved utilization of mineral elements in the body |
[44][45][46][44,45,46] |
| |
Streptococcus thermophilus |
Producing vitamins and cofactors; activating immune function; affecting bile salt concentration |
Improved body immunity; improved lactose intolerance |
[47][48][47,48] |
2. Mechanism of Probiotics to Improve Functional Dyspepsia Symptoms
Probiotics improve FD symptoms by regulating immune function and regulating the probiotics of metabolites such as short-chain fatty acids, bile acids, and neurotransmitters.
2.1. Regulation of Immune Function
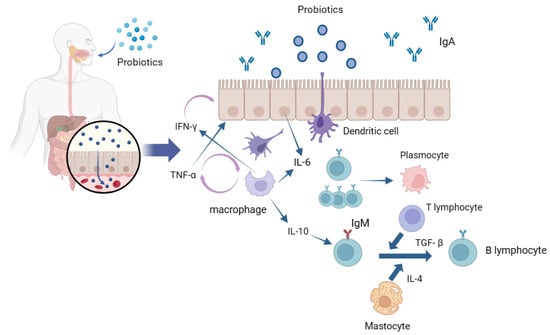
Probiotics exert a significant impact on shaping the gut microbiota composition, as they can hinder the establishment of pathogenic bacteria in the intestinal environment. They assist the host in developing a robust protective mucosal layer in the intestines, fortify the host’s immune system, and generate beneficial metabolites that contribute to maintaining overall health
[49][50][120,121]. Local intestinal immune response is induced by the interaction of probiotics with intestinal epithelial cells and mucosal lamina propria immune cells (
Figure 2). The gastrointestinal tract microbiota modulates the movement and role of neutrophils and influences the division of populations of T cells into various forms of T helper cells (Th), namely Th1, Th2, and Th17, or into regulatory T cells
[51][122]. Mast cells (MCs) are membrane cells connected to the epithelial tissue; epithelial cells (ECs) and dendritic cells (DCs) are treated with probiotics and enter the intestinal lumen in different internalization manners. Following their interaction with epithelial cells, probiotics or their components are internalized and initially engage with antigen-presenting cells (APC), including macrophages and dendritic cells in the tunica propria of the digestive tract. The interaction between probiotics and epithelial cells triggers the release of IL-6 that, in turn, stimulates the clonal expansion of IgA-producing B lymphocytes, thereby elevating their population. These IgA-producing B lymphocytes then migrate into the protoplasmic cells of the intestinal tunica propria through these antigen-presenting cells
[52][53][123,124]. Macrophages and dendritic cells engulf probiotics or fragments, thereby inducing the production of cytokines such as TNF-α and IFN-γ to enhance epithelial excitation and mutual interference among immune-related cells
[54][125]. IL-6 and TNF-α are two important cytokines that play an important role in inflammation, anti-tumor activity, and the regulation of immune function. In the intestinal mucosal immune system, probiotic bacteria or their metabolites can be acquired and recognized by mucosal M cells as antigens. Thus, this promotes the development of the intestinal mucosal immune system, activating macrophages and B lymphocytes, forming germinal centers in the intestinal mucosal lymphoid tissue, and finally, transforming B lymphocytes into plasma cells to secrete mucosal antibody IgA to mediate mucosal immunity
[55][56][126,127]. Probiotic strains can increase the levels of anti-inflammatory cytokines such as IL-10, reduce the levels of inflammatory cytokines such as TNF-α, IL-1β, and IL-8, and exert significant effects on reducing intestinal inflammation and improving colitis
[57][128]. Additionally, different probiotics maintain gut health in different ways.
Lactobacillus and
Bifidobacterium primarily compete with pathogenic microorganisms for favorable adhesion sites, improve intestinal barrier function, regulate intestinal microflora, enhance intestinal immunity, and inhibit or kill harmful bacteria. However,
Bacillus primarily enhances intestinal immunity through its metabolites and resists the invasion of pathogenic bacteria. Additionally, the probiotic effect of probiotics is strain-specific in regard to antibodies, and different strains utilize different modes of action and exert different effects on different parts of the intestine. For example,
Bacillus subtilis MY02 is more effective at increasing SCFAs levels and
Lactobacillus paracei LC-37 is more effective at reducing abdominal pain. Based on this, it is necessary to further precisely regulate the probiotics acting on different parts to provide full play to their probiotic effect on FD and other intestinal diseases
[58][59][129,130].
Figure 2. Local intestinal immune response induced by the interaction of probiotics and intestinal epithelial cells.
2.2. Probiotics Work by Regulating the Metabolites of the Flora
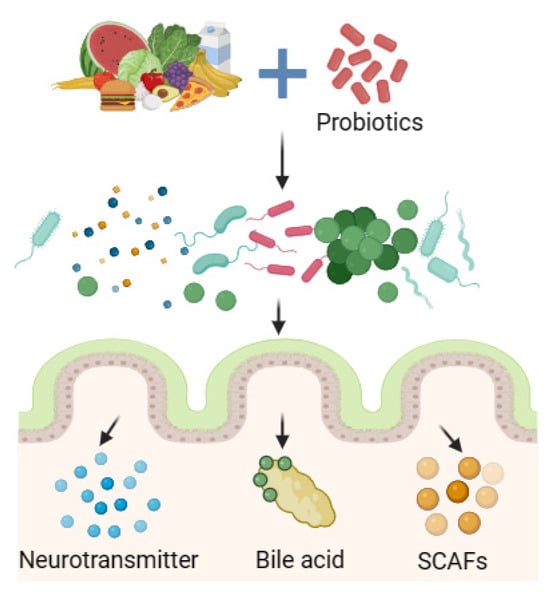
In addition to directly acting on the intestinal flora, probiotics can also play a beneficial role by indirectly regulating metabolites of the intestinal flora such as short-chain fatty acids (SCFAs), bile acids, and neurotransmitters, relieving FD symptoms to improve body health (Figure 3).
Figure 3. Probiotics regulate biota metabolites.
2.2.1. Promotion of the Production of Short-Chain Fatty Acids
As significant byproducts of gut flora, SCFAs have the ability to strengthen the immunity and function as chemical messengers for brain–gut interactions
[60][61][131,132]. Research has revealed that administering probiotics to mice led to a considerable increase in SCFAs and beneficial bacteria (including
Oscillibacter and
Prevotella) in their gut flora as compared to levels in the control group
[62][133]. The gut is the primary site where SCFAs mediate intestinal epithelial integrity or mucosal immune response. Intestinal flora disturbances that lead to reduced SCFAs are associated with colon disease. SCFAs exert an anti-inflammatory function by regulating immune cell chemotaxis, reactive oxygen species release, and cytokine release
[63][134]. Lactobacillus and
Bifidobacterium produce lactic acid and acetic acid, which are major end products of carbohydrate metabolism. These organic acids, when produced in situ, can reduce intracavitary pH and inhibit the growth of pathogenic bacteria
[64][65][135,136].
Bifidobacterium mainly produces SCFAs through fermentation, where the oligosaccharides of Chinese yams can be used as carbon sources by
Lactobacillus plantarum,
Bifidobacterium, and other intestinal probiotics in the simulated colon environment. The content of acetic acid in the fermentation broth of
Bifidobacterium after 48 h was as high as 1.85 mg/mL, and after 8 h fermentation, was as high as 0.082 mg/mL
[66][137]. SCFAs are produced by various pathways, where the most common is through glycolysis, and certain bacterial groups such as
Bifidobacterium can also use the pentose phosphate pathway to produce the same metabolite
[67][138].
2.2.2. Promotion of Neurotransmitter Production
There is bidirectional regulation between the gastrointestinal tract, enteric nervous system, and central nervous system. Certain probiotic strains produce small molecules that exert different effects on the host and its gut microbes
[68][139]. Neurotransmitters are important signaling molecules between neurons, and between neurons and effector cells and include dopamine, gamma-aminobutyric acid (GABA), and 5-HT
[69][140]. Both dopamine and GABA are important neurotransmitters that regulate various functions in the central nervous system. Studies have demonstrated that a partially specific gut microbiota produces neurotransmitters such as dopamine and GABA. 5-HT, also known as serotonin, is an important metabolite of tryptophan, and the majority of 5-HT is produced by enterochromaffin cells that play an important role as an important neurotransmitter and signaling molecule in the two-way communication system between the brain and the gut
[70][71][141,142]. The gut microbiota can promote the production of some neurotransmitters, which are often associated with central nervous system diseases such as the brain. Therefore, targeting the regulation of microbial metabolites, such as neurotransmitters, may be a potential way to improve neurological-system-related diseases. It has also been shown that
Candida,
Streptococcus, and
Enterococcus can produce neurotransmitters such as serotonin;
Bacillus and
Saccharomyces species can produce noradrenaline; while
Lactobacillus and
Bifidobacterium species can synthesize and release GABA. These microbially synthesized neurotransmitters can act locally and also cross the intestinal mucosa to act locally, but potentially also the central nervous system via nerval signaling
[72][143]. Through the regulation of neurotransmitters, the brain–gut axis can improve the symptoms of intestinal peristalsis, upper abdominal discomfort, loss of appetite, and constipation in FD patients
[73][144].
2.2.3. Promotion of Bile Acid Production
Under the action of gut bacteria, the primary bile acids formed in the liver are modified into secondary bile acids. The circulation process between the liver and the intestine is called enterohepatic circulation, which is also an important means to regulate the composition of bile acids
[74][75][145,146]. Based on the important role of intestinal flora in the modification of bile acids, probiotics, as an intervention targeting intestinal flora, may indirectly regulate the metabolism of intestinal flora by bile acids, thereby playing a probiotic role in alleviating FD symptoms.
3. Probiotics as a Potential Treatment for Functional Dyspepsia
Interactions between the microbiota and host crosstalk are plausible underlying mechanisms, which will help to establish probiotics as a novel, tailored therapeutic approach for FD. Probiotics play a multifaceted role, and they contribute to the amelioration of FD symptoms through several mechanisms, such as eliminating pathogenic bacteria to reestablish microbial balance
[76][98], modulating epithelial barrier permeability, influencing visceral hypersensitivity, exerting both local and systemic anti-inflammatory effects, and regulating intestinal motility
[77][78][147,148]. These factors are very beneficial to intestinal health. Probiotics play a certain role in maintaining the integrity of the duodenal mucosa. FD is related to a defect in the duodenal barrier that is caused by the immune response of food and microorganisms in the local area and the whole body, thus producing FD symptoms
[79][149].
E. coli/
Shigella bacteria represent a significant origin of toxic lipopolysaccharides that may impede gastric emptying. The intake of probiotics, particularly
Bifidobacteria, can efficiently lower their concentrations and reinstate normal motor function in the small intestine
[80][150]. Patients with FD exhibit both local and systemic immune activation. Probiotics exert their influence by modulating toll-like receptors (specifically TLR2 and TLR4) and generating pro-inflammatory cytokines through the metabolites produced by the gut microbiota. The interaction between intestinal neurons and microorganisms increases neuronal survival and gastrointestinal motility. The TLR4 agonist lipopolysaccharide promotes the survival of intestinal neurons by activating TLR4 and NF-B. Factors that regulate neuronal TLR4 signaling may alter gastrointestinal motility
[81][151]. Thus, TLR4 and its downstream signaling molecules could be potential therapeutic targets for the treatment of gastrointestinal motility disorders.