Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Laquanda Knowlin.
Pediatric surgery is the diagnostic, operative, and postoperative surgical care of children with congenital and acquired anomalies and diseases. The early history of the specialty followed the classic “see one, do one, teach one” philosophy of training but has since evolved to modern methods including simulation-based training (SBT). Current trainees in pediatric surgery face numerous challenges, such as the decreasing incidence of congenital disease and reduced work hours.
- pediatric surgery
- simulation training
- education
- neonatal
1. Introduction
Pediatric surgery involves the diagnostic, operative, and postoperative surgical care of children with congenital and acquired anomalies and diseases [1]. There is no universal definition of a pediatric surgeon, as some are recognized through specific board certification while others have developed a niche based on clinical experience alone [2]. The journey to become a surgeon that operates on this special population is tedious but rich in history.
2. The Future Direction of the Simulation-Based Training of Pediatric Surgeons
As pediatric surgical simulation training continues to evolve, the future needs of the field should include the creation of more models tailored to pediatric surgery, the expansion of video gaming technology to clinical areas of limited training exposure, and more implementation of simulation-based curricula. Previous reviews have noted that very few pediatric surgical simulators are readily available for purchase, but some literature examples enable departments and hospitals to develop their own [35][3]. Now that 3D printers have become more commercialized, some hospitals have 3D printing programs or institutes for clinical and research purposes. It is possible to print anatomic structures—like bones to internal organs—that can be handled during surgical procedures. Engineers and trained technicians can therefore develop products to meet the haptic and tactile needs of surgeons (Figure 1). A hybrid of 3D printing and cadaver tissue has even been used [30][4].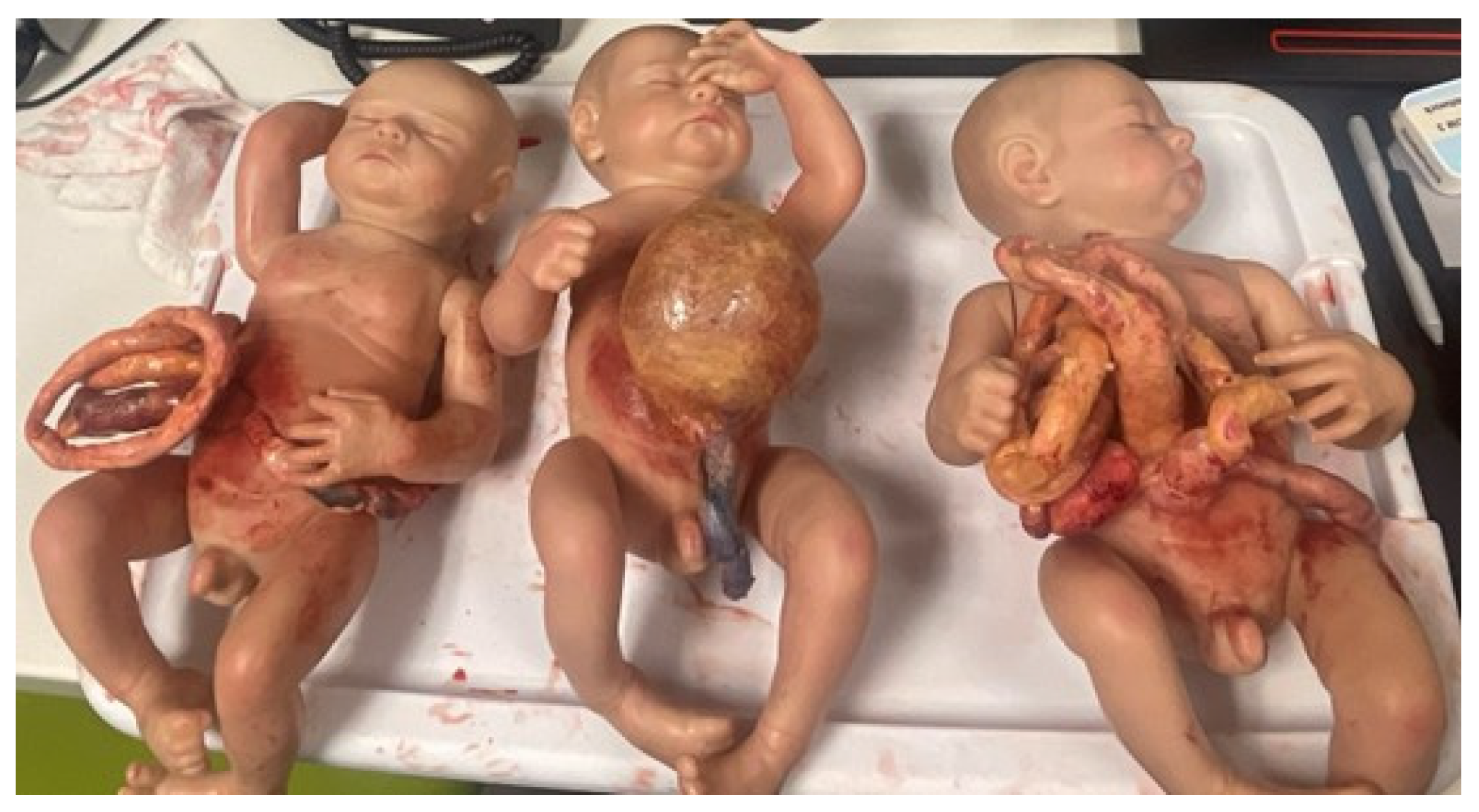
Figure 1. Neonatal Abdominal wall defect models created for the Pediatric Surgery Fellow Workshop at Las Madrinas Simulation Center (Los Angeles, CA, USA).
2.1. Technology Expansion
The expansion of video gaming technology also enables training in areas of limited exposure for pediatric surgery trainees. These are low-frequency, high-stakes events. One such area is pediatric trauma education. Pediatric trauma is the leading cause of mortality in children [51][5]. It is essential to prepare providers, surgeons, and non-surgeons to take care of this special population to improve outcomes. However, specific simulators for trauma are very limited, especially for pediatric and infant patients. Moulage, special makeup, is often used to simulate critical injuries on non-trauma-designated manikins (Figure 2a,b). Trauma simulators can range from USD 14,000 to USD 200,000 depending on the size of the manikin and the level of desired fidelity [52,53,54][6][7][8]. A lower-cost simulation solution is digital. Several companies are creating simulation training through VR to train providers with minimal exposure to pediatric trauma [55][9]. Specialized topics include mass casualty situations in both the military and civilian populations. It provides a psychologically safe environment to practice making life-saving decisions in preparation for actual events. Taking it a step further, augmented reality (AR) decision-making systems with algorithms for trauma activations have been designed in New Zealand, with expanded use in Asia and the Middle East [56][10]. Collaboration among adult and pediatric surgeons can expand this further and help reduce morbidity and mortality outcomes in children around the world. The surgical field is shifting to implementing artificial intelligence (AI) which encompasses machine learning, to offer feedback on trainee surgical performance after video review [57][11]. As surgical fields such as neurosurgery, urology, and some general surgery subspecialties look to artificial intelligence, the question remains if pediatric surgery will follow suit [58,59,60][12][13][14]. Similar to 3D VR, AI has been proposed for pre-operative planning with a focus on visualization [61][15]. However, there is still work that needs to be done as far as reducing implementation and production costs, providing clear evidence that this technology is superior to the current standard, and addressing intrinsic technology limitations (i.e., cybersickness, head-mounted devices, and power for graphic processors) [56][10]. Future technologies are likely to improve haptic sensations in VR and digital simulations, building on hybrid models that combine tactile sensations with digital solutions to achieve learning objectives. Haptics are not optimized for current digital simulations, despite the importance of haptic feedback in pediatric surgical procedures. The researchers predict that haptic technology will enhance future training, which will fully enable digital and distance-based simulations for pediatric surgeons [62][16]. In addition, as the future becomes even more technology-driven, we must not forget the importance of pediatric surgery training in countries with limited resources or technological access (Figure 3).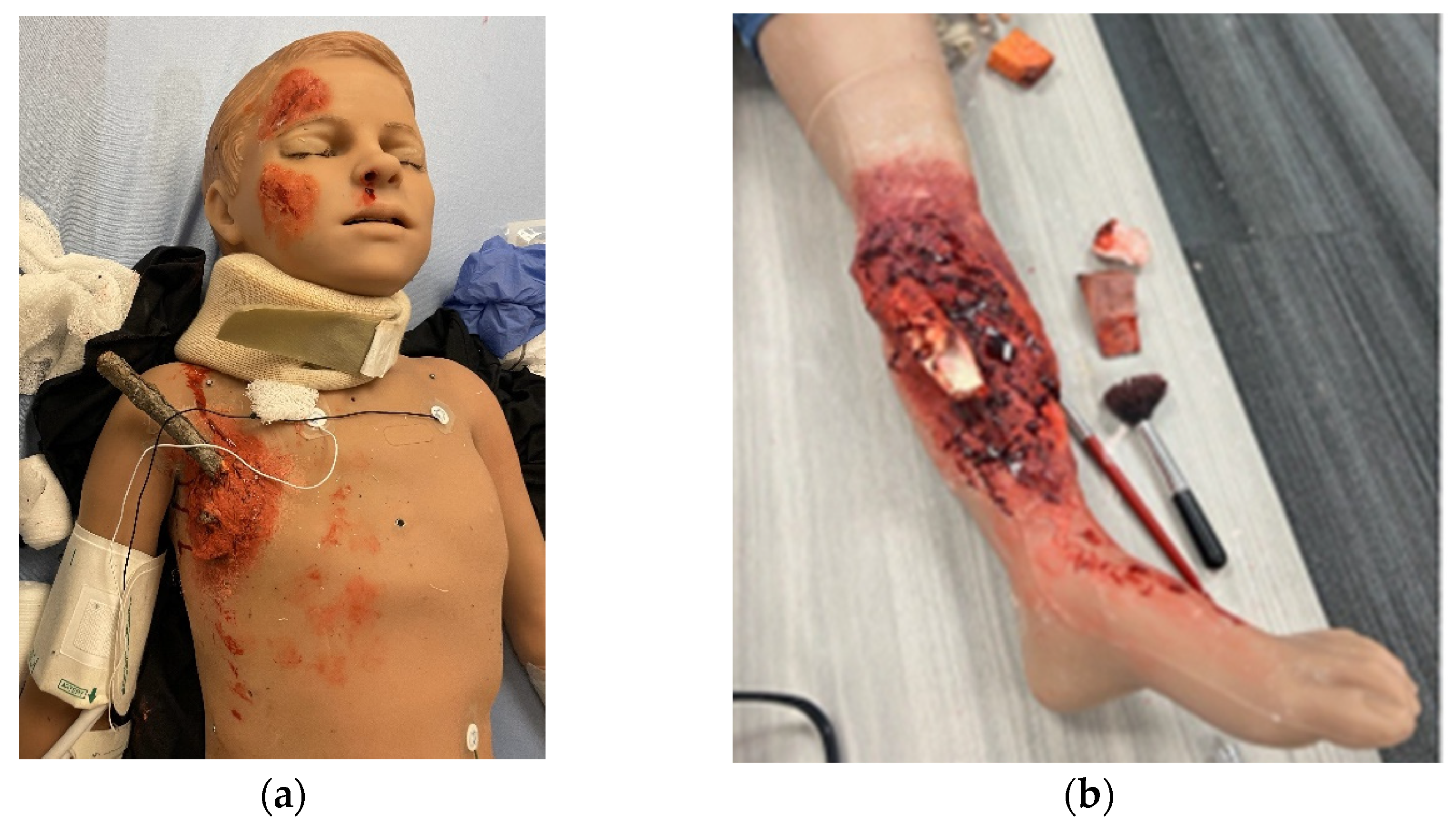
Figure 2. Trauma moulage to simulate critical injuries on a pediatric medical simulator (Pediatric HAL, Gaumard Scientific) at Las Madrinas Simulation Center (Los Angeles): (a) penetrating chest trauma and (b) open orthopedic fracture.

Figure 3.
Preoperative planning using 3D virtuality reality software ImmersiveView 5.0 by ImmersiveTouch (Chicago, IL, USA).
2.2. The Widespread Implementation of Simulation-Based Curricula
The idea of simulation-based training was to build on technical skills that will be transferred to improve performance in the operating room and/or to fill in the education gaps for limited exposure to certain pediatric surgical cases. In addition to evaluating technical skills, simulation has been shown to be superior to didactic training courses for non-technical skills such as leadership, communication, situational awareness, and decision-making [63][17]. This is important as up to 35% of total adverse events in children are reported during the perioperative period, and communication is thought to be a factor in 43% of errors made in surgery [64,65][18][19]. A survey of pediatric surgical trainees as part of a simulation program in France reported inadequate training in the area of non-technical skills [66][20]. More pediatric surgery programs have used simulation as part of boot camps or developed simulation-based curricula as part of trainee development [32,67,68][21][22][23]. With the implementation of minimally invasive surgery three decades ago, MIS procedures have become more integrated into the pediatric surgery training curriculum. From 2004 to 2016, there was a 30% increase in the average number of MIS cases per fellow in Canada and the United States with variation in exposure among trainees [69][24]. In contrast, 67% of pediatric surgery trainees from European countries responded to a survey of the challenges in their program performing of MIS procedures, and fewer than 5 out of 25 pediatric MIS procedures were performed by at least 50% of trainees [22][25]. Like in Northern America, there was great variability in training and exposure in Europe as years spent in a general surgery department led to a greater number of higher complex procedures performed compared to years in pediatric surgery training [22][25]. Some fellowship programs have developed and implemented MIS programs to help mitigate this weakness in training fellows. Recently, Bailez et al. wrote about the development of a minimally invasive surgery training program, onsite and telesimulated, with low-cost models in Argentina that has led to surgical efficiency and an increase in the complexity of cases performed [70][26]. In low- to middle-income countries (LMICs), there is a greater need to train more pediatric surgeons, and the method of training varies per country [71,72][27][28]. Almost half of the population in LMICs are children under the age of 15 [73][29]. Compared to high-income countries (HICs), LMICs do not have enough resources at each site, but substantial improvements have been made [74][30]. Education methods include collaborations between countries with different resources. High-income countries have sent visiting teams to teach workshops on complex procedures, acting as mentors to trainees and, through advocacy after returning home, helping bring awareness to the training needs of LMIC [75][31]. Other training methods used in the past include international pediatric surgery fellowships and fellow exchange programs in HICs to further improve the availability of diverse pediatric surgery talent [72,76,77][28][32][33]. A fellow exchange program between Montreal Children’s Hospital (Canada) and Bethany Kids of Kijabe Hospital (Kenya) resulted in more frequent exposure to neonatal, MIS, and vascular procedures for Kenyan trainees [77][33]. Reported difficulties from Kenyan surgical trainees were obtaining a training license and the financial burden of the cost of living in HICs. As of today, some pediatric surgery training centers in Africa still have limited exposure to trauma, burn, and minimally invasive surgery [78][34]. A recent survey of surgery residents in 11 Sub-Saharan African countries apart of the College of Surgeons of East, Central, and South Africa (COSECSA) strongly believed that simulation was valuable and should be part of their training program [79][35].2.3. The Metric-Based Evaluation of Surgical Trainees in Simulation Training
Prior to the introduction of anesthesia in the 19th century, the early metric in the assessment of skills for surgeons was operative time length. This type of evaluation gave no indication of the quality of performance or feedback. Metric-based assessment in surgery has evolved with the development of the Objective Structured Assessment of Technical Skills (OSATS) which is similar to the Objective Structured Clinical Examinations (OSCE) commonly used to evaluate healthcare students [80][36]. Since its first creation, the OSATS has been modified in different surgical fields such as CT surgery and gynecology [81,82][37][38]. Although the OSATS is widely accepted in surgery, the greatest issues are a lack of objectivity, it does not meet the extrapolation criteria for Kane’s validity framework, and the inability to differentiate competencies for more experienced trainees due to the ceiling effect of the rating scale [83,84][39][40]. The development of a successful metric might appear to be simple, but more attention needs to be given to breaking down procedure-specific tasks into their essential components, strictly defining what differentiates optimal from suboptimal performance, and including non-technical skills. The combination of an assessment tool with video recordings strengthens the evaluation of trainee performance and enables evaluation for discriminative validity [85][41].References
- Tomaszewski, J.; Casella, D. Pediatric Laparoscopic and Robot-Assisted Laparoscopic Surgery: Technical Considerations. J. Endourol. 2012, 26, 602–613.
- Lalchandani, P.; Dunn, J.C.Y. Global comparison of pediatric surgery workforce and training. J. Pediatr. Surg. 2015, 50, 1180–1183.
- Skertich, N.J.; Schimpke, S.W.; Lee, T.; Wiegmann, A.L.; Pillai, S.; Rossini, C.; Madonna, M.B.; Shah, A.N. Pediatric Surgery Simulation-Based Training for the General Surgery Resident. J. Surg. Res. 2021, 258, 339–344.
- Etlinger, P.; Barroso, C.; Miranda, A.; Pinto, J.M.; Lamas-Pinheiro, R.; Ferreira, H.; Leão, P.; Kovács, T.; Juhász, L.; Szabó, L.S.; et al. Characterization of technical skill progress in a standardized rabbit model for training in laparoscopic duodenal atresia repair. Surg. Endosc. 2022, 36, 2456–2465.
- Mclaughlin, C.; Zagory, J.A.; Fenlon, M.; Park, C.; Lane, C.J.; Meeker, D.; Burd, R.S.; Henri, R.; Upperman, J.S.; Jensen, A.R. Timing of Mortality in Pediatric Trauma Patients: A National Trauma Databank Analysis. J. Pediatr. Surg. 2019, 53, 344–351.
- SIMBODIES. Available online: https://simbodies.com/ (accessed on 20 September 2023).
- SIMULAB. TraumaCHild Pediatric Surgical Simulator. Available online: https://simulab.com/products/traumachild-pediatric-surgical-simulator (accessed on 20 September 2023).
- Operative Experience, Inc. Available online: https://operativeexperience.com/tcs-plus-pro/ (accessed on 20 September 2023).
- Harrington, C.M.; Kavanagh, D.O.; Quinlan, J.F.; Ryan, D.; Dicker, P.; O’Keeffe, D.; Traynor, O.; Tierney, S. Development and evaluation of a trauma decision-making simulator in Oculus virtual reality. Am. J. Surg. 2018, 215, 42–47.
- Agency for Healthcare Research and Quality. PS Net Patient Safety Network. Algorithm-Based Decision Support System Guides Trauma Staff during Initial Treatment, Leading to Fewer Medical Errors. Available online: https://psnet.ahrq.gov/innovation/algorithm-based-decision-support-system-guides-trauma-staff-during-initial-treatment (accessed on 20 September 2023).
- Satapathy, P.; Hermis, A.H.; Rustagi, S.; Pradhan, K.B.; Padhi, B.K.; Sah, R. Artificial intelligence in surgical education and training: Opportunities, challenges, and ethical considerations—Correspondence. Int. J. Surg. 2023, 109, 1543–1544.
- Turner, A.E.; Abu-Ghname, A.D.; Davis, M.J.; Ali, K.; Winocour, S. Role of Simulation and Artificial Intelligence in Plastic Surgery Training. Plast. Reconst. Surg. 2020, 146, 390e–391e.
- Cacciamani, G.E.; Anvar, A.; Chen, A.; Gill, I.; Hung, A.J. How the use of the artificial intelligence could improve surgical skills in urology: State of the art and future perspectives. Curr. Opin. Urol. 2021, 31, 378–384.
- Ledwos, N.; Mirchi, N.; Yilmaz, R.; Winkler-Schwartz, A.; Sawni, A.; Fazlollahi, A.M.; Bissonnette, V.; Bajunaid, K.; Sabbagh, A.J.; Del Maestro, R.F. Assessment of learning curves on a simulated neurosurgical task using metrics selected by artificial intelligence. J. Neurosurg. 2022, 137, 1160–1171.
- Park, J.J.; Tiefenbach, J.; Demetriades, A.K. The role of artificial intelligence in surgical simulation. Front. Med. Technol. 2022, 4, 1076755.
- Singapogu, R.; Burg, T.; Burg, K.J.; Smith, D.E.; Eckenrode, A.H. A perspective on the role and utility of haptic feedback in laparoscopic skills training. Crit. Rev. Biomed. Eng. 2014, 42, 293–318.
- Pena, G.; Altree, M.; Field, J.; Sainsbury, D.; Babidge, W.; Hewett, P.; Maddern, G. Nontechnical skills training for the operating room: A prospective study using simulation and didactic workshop. Surgery 2015, 158, 300–309.
- Matlow, A.G.; Baker, G.R.; Flintoft, V.; Cochrane, D.; Coffey, M.; Cohen, E.; Cronin, C.M.; Damignani, R.; Dubé, R.; Galbraith, R.; et al. Adverse events among children in Canadian hospitals: The Canadian paediatric adverse events study. Can. Med. Assoc. J. 2012, 184, E709–E718.
- Gawande, A.A.; Zinner, M.J.; Studdert, D.M.; Brennan, T.A. Analysis of errors reported by surgeons at three teaching hospitals. Surgery 2003, 133, 614–621.
- Breaud, J.; Talon, I.; Fourcade, L.; Podevin, G.; Rod, J.; Audry, G.; Dohin, B.; Lecompte, J.-F.; Bensaid, R.; Rampal, V.; et al. The national pediatric surgery simulation program in France: A tool to develop resident training in pediatric surgery. J. Pediatr. Surg. 2019, 54, 582–586.
- Bergmeister, K.D.; Aman, M.; Kramer, A.; Schenck, T.L.; Riedl, O.; Daeschler, S.C.; Aszmann, O.C.; Bergmeister, H.; Golriz, M.; Mehrabi, A.; et al. Simulating Surgical Skills in Animals: Systematic Review, Costs & Acceptance Analyses. Front. Vet. Sci. 2020, 7, 570852.
- Breaud, J.; Azzie, G. Development and assessment of a simulation-based curriculum in pediatric surgical education: Conventional wisdom and lessons learned from the national training program in France. Semin. Pediatr. Surg. 2020, 29, 150902.
- Simulation Enhanced Learning Course for Paediatric Surgical Trainees AKA Bootcamp Virtual. British Association of Paediatric Surgeons. Available online: https://www.baps.org.uk/events/simulation-enhanced-learning-course-for-paediatric-surgical-trainees-aka-bootcamp-virtual/ (accessed on 20 September 2023).
- Cairo, S.B.; Harmon, C.M.; Rothstein, D.H. Minimally invasive surgical exposure among US and Canadian pediatric surgery trainees, 2004–2016. J. Surg. Res. 2018, 231, 179–185.
- Markel, M.; Lacher, M.; Hall, N.J.; Martynov, I.; Hinojosa, A.S.; de Augustin Asensio, J.C.; Fortmann, C.; Hukkinen, M.; Mutanen, A.; Ford, K.; et al. Training in minimally invasive surgery: Experience of paediatric surgery trainees in Europe. Br. J. Surg. 2023, 110, 1397–1399.
- Bailez, M.M.; Maricic, M.A.; Falcioni, A.G.; Yang, H.C.; Martinez, P.S. Development of a simulation minimally invasive surgery (MIS) training program in a curricula of pediatric surgery: A replicable experience. J. Pediatr. Surg. 2023, 3, 100052.
- Chirdan, L.B.; Ameh, E.A.; Abantanga, F.A.; Sidler, D.; Elhalaby, E.A. Challenges of training and delivery of pediatric surgical services in Africa. J. Pediatr. Surg. 2010, 43, 610–618.
- Butler, M.W. Developing pediatric surgery in low- and middle- income countries: An evaluation of contemporary education and care delivery models. Semin. Pediatr. Surg. 2016, 25, 43–50.
- PRB. 2022 World Population Date Sheet. Available online: https://www.prb.org/wp-content/uploads/2022/09/2022-World-Population-Data-Sheet-Booklet.pdf (accessed on 18 December 2023).
- Bandyopadhyay, S.; Lakhoo, K. Emerging Optimism in Paediatric Surgery in Africa. Afr. J. Paediatr. Surg. 2023, 20, 252–253.
- Hayton, R.A.; Donley, D.K.; Fekadu, A.; Woods, B.K.; Graybill, C.K.; Fitzgerald, T.N. Surgical volunteerism as a collaborative teaching activity can benefit surgical residents in low-middle income countries. Int. Surg. J. 2017, 48, 34–37.
- Reed, C.R.; Commander, S.J.; Sekabira, J.; Kisa, P.; Kakembo, N.; Wesonga, A.; Langer, M.; Villanova, G.A.; Ozgediz, D.; Fitzgerald, T.N. Comparison of Ugandan and North American Pediatric Surgery Fellows’ Operative Experience: Opportunities for Global Training Exchange. J. Surg. Educ. 2020, 77, 606–614.
- Baird, R.; Poenaru, D.; Ganey, M.; Hansen, E.; Emil, S. Partnership in fellowship: Comparative analysis of pediatric surgical training and evaluation of a fellow exchange between Canada and Kenya. J. Pediatr. Surg. 2016, 51, 1704–1710.
- Jooma, U.; Numanoglu, A.; Cox, S. Paediatric surgery training in South Africa: Trainees’ perspectives. Pediatr. Surg. Int. 2020, 36, 1489–1494.
- Traynor, M.D., Jr.; Owino, J.; Rivera, M.; Parker, R.K.; White, R.E.; Steffes, B.C.; Chikoya, L.; Matsumoto, J.M.; Moir, C.R. Surgical Simulation in East, Central, and Southern Africa: A Multinational Survey. J. Surg. Educ. 2021, 78, 1644–1654.
- Martin, J.A.; Regehr, G.; Reznick, R.; Macrae, H.; Murnaghan, J.; Hutchison, C.; Brown, M. Objective structured assessment of technical skill (OSATS) for surgical residents. Br. J. Surg. 1997, 84, 273–278.
- Hance, J.; Aggarwal, R.; Stanbridge, R.; Blauth, C.; Munz, Y.; Darzi, A.; Pepper, J. Objective assessment of technical skills in cardiac surgery. Eur. J. Cardiothorac. Surg. 2005, 28, 157–162.
- Larsen, C.R.; Grantcharov, T.; Schouenborg, L.; Ottosen, C.; Soerensen, J.L.; Ottesen, B. Objective assessment of surgical competence in gynaecological laparoscopy: Development and validation of a procedure-specific rating scale. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 908–916.
- Hiemstra, E.; Kolkman, W.; Wolterbeek, R.; Trimbos, B.; Jansen, F.W. Value of an objective assessment tool in the operating room. Can. J. Surg. 2011, 54, 116–122.
- Munz, Y.; Moorthy, K.; Bann, S.; Shah, J.; Ivanova, S.; Darzi, S.A. Ceiling effect in technical skills of surgical residents. Am. J. Surg. 2004, 188, 294–300.
- Strandbygaard, J.; Scheele, F.; Sørensen, J.L. Twelve tips for assessing surgical performance and use of technical assessment scales. Med. Teach. 2017, 39, 32–37.
More
