Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Denise Deo Dias and Version 2 by Sirius Huang.
Magnesium is an essential mineral that plays a central role in approximately 800 biochemical reactions within the human body. Its distinctive physical and chemical attributes render it an indispensable stabilizing factor in the orchestration of diverse cellular reactions and organelle functions, thereby rendering it irreplaceable in processes directly impacting muscle health.
- magnesium
- aging
- sarcopenia
- frailty
- intrinsic capacity
1. Introduction
Skeletal muscle health is fundamental to human functionality, mobility, and overall well-being [1][66]. During the natural process of aging, an individual’s mobility transcends being a mere measure of physical capability; it evolves into a dynamic reflection of their comprehensive health status and quality of life. The ability to move autonomously and unrestrictedly significantly influences multiple facets, including vitality, cognitive function, sensory perception, and psychological well-being. Several studies have demonstrated that consistent engagement in physical activities, which relies heavily on the integrity of skeletal muscle, is associated with enhanced cognitive performance, including improved attention, executive functions, and memory. Furthermore, physical activity triggers the release of endorphins, which are natural mood-elevating agents. As aging progresses, there is a noticeable decline in muscular function, resulting in movement restrictions, increased dependency, and, subsequently, potential negative effects on emotional and mental health [1][2][3][4][5][66,67,68,69,70].
Within this context, magnesium stands out as a vital element whose distinct chemical and physical properties position it as a crucial component in the regulation of nearly all biological processes within cells. In the absence of adequate magnesium levels, the entire organism is impacted, as no other chemical element can effectively assume its multifaceted roles. Notably, skeletal muscle houses approximately 20% of the body’s total magnesium [6][71]. This essential mineral is intricately associated with various aspects of skeletal muscle function that are negatively impacted in aging subjects. It plays a central role in processes such as protein synthesis, energy production, and muscle contraction while also offering anti-inflammatory and antioxidant benefits, as illustrated in Figure 1 [7][8][9][21,47,72]. In the subsequent sections, the comprehensive role of magnesium in the skeletal muscle health of elderly individuals will be explored, highlighting its significance across these physiological processes.
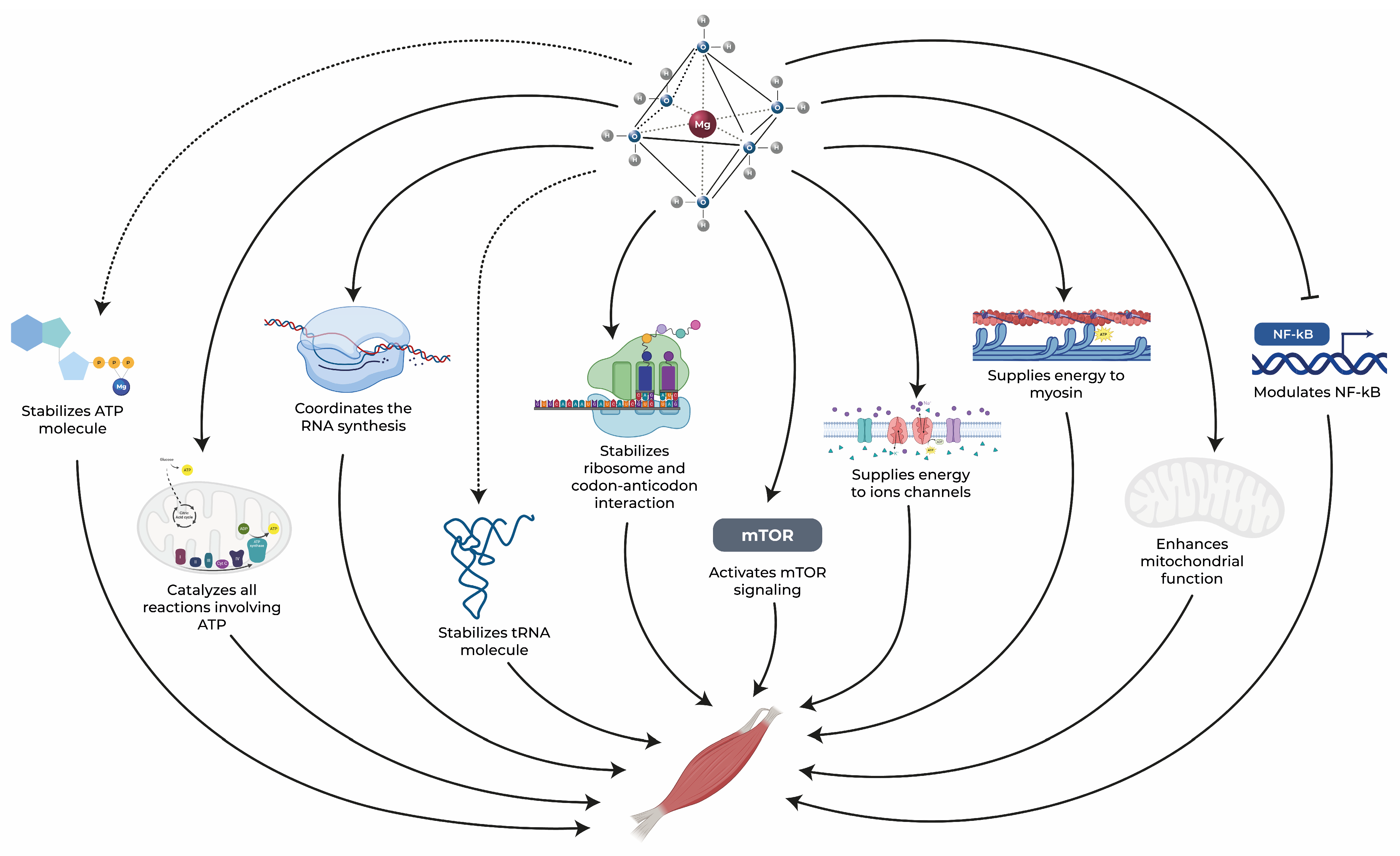
Figure 1. Magnesium exerts an essential role in maintaining muscle health through several pathways. Dotted arrows represent stabilization, solid arrows represent activation, and blunt-end arrows indicate inhibition. ATP: adenosine triphosphate, mTOR: mammalian target of rapamycin, NF-κB: nuclear factor kappa-B, RNA: ribonucleic acid, tRNA: transfer RNA [10][11][12][13][14][15][16][17][18][25,34,35,36,37,38,39,40,41].
2. Energy Metabolism
The human body operates through intricate physiological processes that require significant energy reserves. Key tissues, especially the brain and muscles, stand out as primary energy consumers within this biological system [19][20][73,74]. In resting conditions, an average adult brain and skeletal muscle each consume approximately 250 mL O2/min, accounting for approximately 20% of the total O2 consumption for each tissue [20][74]. Their pivotal roles in maintaining overall bodily function dictate that a consistent and sufficient energy supply is indispensable for their optimal performance. A diminished energy provision can critically impede these essential biological processes, potentially compromising the health and efficiency of these tissues [19][20][73,74]. Given this context, it becomes imperative to elucidate the mechanisms underlying energy metabolism and to understand the contribution of magnesium in this intricate system.
As with many other systems and tissues, skeletal muscle undergoes numerous structural and functional transformations with aging, including mass loss. These changes serve as key contributors to morbidity and frailty. Age-related muscle deterioration, diminished strength, and impaired cellular energy metabolism culminate in reduced physical capability [21][75].
Each muscle fiber (myocyte) contains hundreds of thousands of mitochondria, which are known as the cellular “powerhouses” [22][76]. A significant amount of the energy needed for human physiological functions is generated by these organelles via electron movement through the respiratory chain [21][75]. Diminished mitochondrial function in muscles might play a role in age-related muscle dysfunction and decreased aerobic capacity [21][75].
Energy metabolism within cells is a complex process, with magnesium serving a crucial role, which is particularly essential for mitochondrial health and function [23][77]. Insufficient magnesium levels can result in decreased mitochondrial efficiency and increased production of reactive oxygen species (ROS). ROS can subsequently result in structural and functional damage to vital molecules, including DNA and proteins [23][77], all of which are associated with aging.
Supporting this notion, a study revealed that muscle tissue from magnesium-deficient animals exhibited mitochondrial damage, which presented as swelling and ultrastructural changes [24][78]. Conversely, the oxidative mitochondrial decay associated with aging might predispose individuals to hypomagnesemia. Wilson et al. (2004) indicated that a mutation in a mitochondrial gene led to decreased magnesium levels in the bloodstream. This reduction became more pronounced as the subjects aged, possibly because reabsorption of magnesium at the distal convoluted tubule of the kidney nephron demands a significant amount of ATP, which diminishes with impaired mitochondrial function [25][79].
Notably, magnesium in mitochondria constitutes one-third of its total cellular level, highlighting its pivotal role in cellular energy metabolism [9][26][29,72]. This essential mineral is critical in supporting core mitochondrial functions, including the electron transport chain, oxygen detoxification, and the production of ATP, which acts as the primary energy currency in cells, driving numerous physiological functions. In fact, magnesium is essential for all phosphorylation processes and reactions that entail the transfer and utilization of ATP [11][23][34,77]. Magnesium exists not only in complex with ATP but also as a component of nucleic acids and membranes [9][26][29,72].
Magnesium is needed for the activity of all rate-limiting glycolytic enzymes [8][47]. Moreover, the importance of magnesium in energy metabolism and muscle function is underscored by its involvement in the Mg-ATP complex, an indispensable entity for the sliding filament mechanism of myofibrillar contraction and relaxation in striated muscles [9][72]. Most of the ATP within cells is found as Mg-ATP complexes, representing its biologically active form [27][80]. Magnesium is crucial for catalyzing all reactions involving ATP, helping to maintain the polyphosphate chain of ATP in a conformation that promotes enzyme binding [28][20]. This importance of Mg binding to ATP arises from several roles it plays, as described below, ultimately enhancing the specificity of enzyme-substrate interactions by amplifying the binding energy [27][80].
Magnesium ions (Mg2+) counteract the negative charges on the ATP polyphosphate chain, which minimizes nonspecific ionic interactions between the enzyme and the polyphosphate group [27][80]. Therefore, the reactions are specific and efficient. Additionally, Mg2+ increases the number of interaction points between the ATP-Mg complex and enzymes, thereby enhancing binding energy and ensuring that the ATP molecule is positioned appropriately for enzymatic actions [27][80]. Furthermore, these magnesium-ATP interactions ensure that the nucleotide remains in well-defined structures, allowing enzymes to bind with precision. Such interactions also prime the ATP molecule by weakening its terminal O-P bond, facilitating the efficient transfer of phosphate, a foundational process in cellular energy mechanics [28][20].
It is worth noting that numerous prevailing theories on aging, which aim to elucidate the mechanisms behind sarcopenia, have turned their focus to the mitochondria due to their dual role in governing both life and death processes within myocytes [22][76]. These organelles are not just primary generators of cellular energy through Mg-ATP; they are also crucial regulators of apoptosis, which is a programmed cell death pathway [22][76]. As previously highlighted, aging is often associated with poor magnesium status. This can in turn subsequently influence the age-related deterioration of mitochondrial function, leading to energy depletion within the cell. Such dynamics can trigger apoptosis in aging skeletal muscles, further contributing to the age-associated loss of function [7][8][22][29][21,47,76,81].
Beyond its direct role in mitochondria, energy production, and muscle contraction, magnesium is also integral for cellular signaling processes. Specifically, the Mg-ATP complex plays a crucial role in phosphorylating proteins and in the synthesis and activation of cyclic adenosine monophosphate (cAMP). This important cell-signaling molecule participates in a plethora of biochemical processes, reinforcing the diverse and central roles of magnesium within cells [9][26][30][29,72,82]. Another interesting interplay is the relationship between magnesium and calcium within muscle cells. The uptake and release of calcium from sarcotubules are intimately connected to Mg-ATP levels. This association implies that even subtle changes in intracellular magnesium concentrations can profoundly influence the contractile performance of muscle cells [9][72].
Given that both magnesium deficiency and sarcopenia are more prevalent in the aging population and that magnesium plays a central role in muscle ATP production, a hypothesis has been posited suggesting that a compromised magnesium status might be a contributing factor to sarcopenia observed in the later stages of life [9][23][72,77]. Supporting this notion, several studies conducted in aging as well as young volunteers found that the magnesium status in the organism or its supplementation strongly affects muscle performance [23][31][32][33][34][77,83,84,85,86]. This is likely attributed to the pivotal role of magnesium in energetic metabolism, transmembrane transport, and muscle contraction and relaxation [23][33][77,85].
3. Protein Synthesis
Proteostasis is a term used to describe the complex and tight network responsible for maintaining protein homeostasis within cells [35][87], and this process plays a crucial role in regulating several physiological processes within the human body. Proteins, composed of carbon, hydrogen, oxygen, and nitrogen, serve as intricate macronutrients with multifaceted roles in the body. They contribute to structural integrity, regulate vital cellular and physiological processes, act as effectors, and, at times, serve as an energy source [36][37][88,89].
Proteostasis involves several processes that can be summarized as four main arms: protein synthesis, folding, degradation, and quality control mechanisms [38][90]. It is noteworthy that any perturbation in this delicate balance can have significant implications for the health of both individual cells and the organism as a whole [35][39][40][41][87,91,92,93]. The disruption of protein homeostasis is an important component among the seven fundamental determinants of the aging process and is linked to the pathogenesis of conditions such as neurodegenerative disorders, cardiovascular disease, and sarcopenia [40][42][43][44][45][92,94,95,96,97].
Skeletal muscle tissue is predominantly composed of proteins, as it contains 50–75% of all proteins in the body [46][98]. Consequently, maintaining protein homeostasis is crucial for the preservation of skeletal muscle mass. However, the aging process introduces a notable phenomenon termed anabolic resistance, in which the capability for protein synthesis is adversely affected. This anabolic blunting persists even in the presence of conventional anabolic stimuli, such as feeding and exercise, linking this impairment to prevalent conditions like sarcopenia and frailty among the elderly [41][47][48][49][50][93,99,100,101,102]. Using stable isotope methodologies, Wall et al. (2015) observed that postprandial muscle protein synthesis rates were 16% lower in older individuals (75 ± 1 years) than in their younger counterparts (22 ± 1 years) [51][103]. A reduction in the translation rate is one of the age-related changes observed in protein synthesis [41][93]. Furthermore, the aging process is linked to a decrease in the effectiveness of protein recycling systems, resulting in the buildup of damaged proteins and other molecules, which could hinder cell functionality and contribute to age-associated dysfunction [41][93].
Protein synthesis is one of the most intricate and energetically demanding anabolic processes in the cell [48][52][100,104]. This biologically vital process accounts for the consumption of a substantial portion of ATP, with over 70% of ATP reserves allocated to support various biosynthetic pathways [38][90]. The ribosome is a cellular organelle responsible for protein synthesis [53][105] that relies on amino acids as the fundamental building blocks for synthesizing proteins within all tissues of an organism [13][36]. In addition to ribosomes and amino acids, protein synthesis requires a diverse range of cellular components, including messenger RNA (mRNA) [13][36] and essential minerals [54][106].
The regulation of protein synthesis encompasses multiple stages of transcription and translation, and this process is crucial for the production of ribosomal RNAs and proteins that are involved in muscle contraction and metabolism [55][107]. Within the transcriptional phase, two magnesium cations (Mg2+) are localized at the active site of RNA polymerase. These cations coordinate the synthesis of RNA, playing a pivotal role in the condensation of nucleoside triphosphates (NTPs) [10][56][25,108]. One of the Mg2+ ions contributes to the formation of a new phosphodiester bond, while the other actively participates in stabilizing the pentacovalent transition state of the enzyme [56][108]. In the synthesis of ribosomal RNA (rRNA), which carries a significant negative charge, Mg2+ chelation serves to reduce electrostatic repulsion [12][38][35,90].
Mg2+ also assumes a critical role in the folding of transfer RNA (tRNA) [57][109]. In fact, Mg is indispensable for all RNA folding processes and energetic states due to its ability to form a rigid and tightly octahedral structure with oxygen atoms incorporating phosphate groups [57][109]. On the translational level, Mg2+ emerges as an indispensable component in stabilizing the secondary structure of ribosomes [54][57][58][106,109,110]. This structural stability is fundamental in all steps of translation, particularly in the initiation process of peptide bond formation [38][59][90,111].
Furthermore, Mg2+ plays an essential role in mediating the bonds between rRNA and ribosomal proteins (rProteins) [54][57][106,109] by activating water molecules that facilitate the recognition of rProteins [60][112]. Additionally, Mg2+ aids in stabilizing the codon-anticodon interaction at the A site and affects the binding of ribosome recycling factor (RRF) to ribosomes [54][106]. Notably, ribosome degradation becomes particularly important when the magnesium concentration is limited, as the recycling process releases Mg2+ ions for essential cellular activities [38][90].
Finally, the mammalian target of rapamycin (mTOR) plays a pivotal role in stimulating protein synthesis while concurrently inhibiting proteolysis [14][37]. Magnesium is intricately involved in this process, not only in its role in the energy system but also through its ability to activate mTOR signaling [61][113]. This pathway is integral for initiation, elongation, and ribosome biogenesis [62][114]. Given the central role of mTOR in anabolic and catabolic muscle pathways and the influence of magnesium on its activation, both mTOR and magnesium present promising targets for interventions aimed at countering age-related muscle loss [14][37].
As shown here, magnesium plays a pivotal role in protein synthesis, influencing both transcriptional and translational processes in skeletal muscle tissue. Its involvement in RNA synthesis, ribosomal stabilization, and activation of the mTOR signaling pathway highlights its importance in maintaining muscle health. Given the age-related decline in protein synthesis, ensuring optimal magnesium levels might be key to addressing muscle degeneration and sarcopenia in elderly individuals.
4. Anti-Inflammatory and Antioxidant Activities
Inflammation is recognized as one of the seven pillars of aging [42][63][94,115]. Notably, the chronic low-grade inflammation observed in older individuals, a condition referred to as “inflammaging”, is a pivotal risk factor for frailty, morbidity, and mortality. Additionally, it is a common feature of various age-related diseases and has been associated with numerous adverse effects on muscle health [63][64][65][66][115,116,117,118]. In fact, inflammaging has been proposed as an underlying mechanism of muscle decline and sarcopenia [67][68][69][119,120,121].
The inflammatory process can inhibit muscle regeneration after injury or exercise, thereby fostering muscle disuse atrophy and rendering individuals more susceptible to recurrent muscle damage [65][70][71][117,122,123]. Additionally, the presence of chronic inflammation is closely associated with insulin resistance. Insulin resistance is a highly prevalent condition among the elderly that diminishes intracellular glucose levels and can lead to muscle loss [60][63][72][112,115,124]. Moreover, inflammation can disturb the balance between muscle protein synthesis and degradation, ultimately contributing to muscle wasting [73][74][75][125,126,127]. In a study conducted by Merritt et al. (2013), subjects with mean ages of 61 and 76 years exhibited higher expression of several genes, including the proinflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-6, as well as TNF-like weak inducer of apoptosis signaling (TWEAK), following the induction of modest muscle damage than that in individuals with a mean age of 40 years. This finding underscores the potential relevance of inflammation management as a promising strategy to mitigate age-related muscle damage [76][128].
The anti-inflammatory properties of magnesium have been extensively documented in the preclinical and clinical scientific literature [77][78][79][80][81][129,130,131,132,133]. In fact, studies have consistently shown that a low magnesium status correlates with increased low-grade systemic inflammation, as evidenced by elevated levels of proinflammatory markers such as TNF-α, IL-1β, and C-reactive protein (CRP) and a reduction in the levels of anti-inflammatory cytokines [9][82][72,134]. It also impacts immune responses by activating leukocytes and macrophages, causing endothelial dysfunction and inflammatory syndrome [82][83][134,135]. Additionally, magnesium deficiency can impact mast cells by affecting their histamine secretion, and histamine is a key component in inflammatory responses [6][71]. Conversely, magnesium repletion therapy induces an anti-inflammatory response and reduces proinflammatory marker levels in rats initially deficient in magnesium [84][85][136,137].
Recent research has focused on elucidating the pathway linking sustained chronic inflammation to oxidative stress, a process implicated in numerous chronic diseases, including sarcopenia [6][9][66][71,72,118]. The interplay of inflammation and oxidative stress can influence multiple intracellular signaling pathways, disrupting mitochondrial function and the equilibrium between protein synthesis and degradation. This prompts apoptosis and ultimately results in the loss of muscle mass [9][66][72,118]. Moreover, oxidative stress can trigger several transcription factors, including NF-κB, AP-1, and NRF2, which, when activated, modulate the expression of more than 500 genes, including inflammatory cytokines, chemokines, and molecules integral to oxidative stress defense. Thus, there is a profound interconnection between oxidative stress and chronic inflammation, and both of these processes play key roles in age-related muscle atrophy [6][66][86][71,118,138].
Previous studies on inflammation, including clinical studies and those based on animal and cellular models, have consistently shown a link between low magnesium status and the onset of oxidative stress and compromised antioxidant defense systems [9][17][87][40,72,139]. Several studies have indicated that magnesium deficiency is characterized by reduced antioxidant defenses and elevated levels of oxidative stress markers, including those related to lipid, protein, and DNA oxidative modifications, resulting in enhanced free-radical-induced tissue damage [9][87][88][89][90][91][92][93][94][72,139,140,141,142,143,144,145,146].
For instance, Boparai et al. (2007) discovered protein and lipid oxidation in the liver and plasma of rats subjected to a magnesium-deficient diet [90][142]. In the study by Gueux et al. (1995), lipoproteins (VLDL and LDL) from magnesium-deficient rats showed increased susceptibility to CuSO4-induced oxidative damage compared to control rats. Additionally, tissues exposed to lipid peroxidation induced by iron (Fe) exhibited higher levels of thiobarbituric acid-reactive substances, indicative of increased oxidative stress [88][140]. Accordingly, rats fed a magnesium-deficient diet showed a notable reduction in both erythrocyte and plasma magnesium levels, accompanied by a significant increase in the plasma oxidative marker malondialdehyde (MDA) and a corresponding reduction in radical-trapping antioxidant markers [89][141]. Reductions in the activities and levels of important molecules and enzymes related to antioxidant defenses, including glutathione (GSH), glutathione reductase (GR), superoxide dismutase (SOD), catalase, glutathione S-transferase (GST), and vitamin E, were also observed in rodents after magnesium deprivation, which further led to an increase in oxidative stress [95][96][97][98][147,148,149,150]. Conversely, magnesium supplementation has the potential to mitigate oxidative stress. In a rat model of diabetes, low magnesium levels and increased urinary excretion were correlated with increased plasma MDA and reduced hepatic expression of SOD and GST, all of which were rectified with magnesium supplementation [99][151].
Regarding the clinical context, studies in humans have also corroborated the interplay among low magnesium status, low-grade systemic inflammation, and oxidative stress. Song et al. (2005) found an inverse association between plasma CRP concentrations and dietary magnesium content in a cohort comprising more than 11,000 women aged 45 and older participating in the Women’s Health Study [100][152]. Moreover, several meta-analyses have concluded that magnesium reduces CRP levels [78][82][101][130,134,153]. For example, more recently, Veronese et al. (2022) conducted a comprehensive meta-analysis including 17 randomized controlled trials involving 889 participants (mean age: 46 years), and their findings revealed a significant reduction in various inflammatory markers, particularly CRP, associated with magnesium supplementation [82][134]. Accordingly, in human subjects chronically exposed to stress, an inverse association was observed between magnesium levels and oxidative stress markers, specifically MDA and plasma superoxide anions [102][154]. Additional clinical research indicates an inverse relationship between serum magnesium levels and markers of oxidative stress and inflammation [103][104][155,156]. Last, in three recent randomized controlled trials, magnesium cosupplementation with zinc, melatonin, or zinc-calcium-vitamin D was shown to reduce inflammatory and oxidative stress markers in women with polycystic ovary syndrome [105][106][107][157,158,159].
While the precise pathophysiological mechanisms underlying the anti-inflammatory and antioxidant effects of magnesium remain to be fully elucidated, many studies highlight its pivotal role in mitochondrial function. Magnesium supplementation has been demonstrated to enhance mitochondrial function by inhibiting mitochondrial ROS, modulating permeability, and regulating the opening of the mitochondrial transition pore [108][160]. Moreover, several studies have proposed that magnesium deficiency can contribute to the onset and persistence of oxidative stress and inflammation, primarily through mechanisms related to mitochondrial dysfunction [6][87][109][71,139,161]. Magnesium deficiency can contribute to disrupted mitochondrial functioning by promoting the uncoupling of oxidative phosphorylation, leading to electron loss in the electron transport chain, which in turn amplifies intracellular reactive species production and consequent oxidative stress [87][110][111][112][139,162,163,164]. Furthermore, reduced magnesium concentrations cause calcium accumulation in the cytosol [111][112][163,164]. This not only contributes to the uncoupling of oxidative phosphorylation but also stimulates other peroxidation pathways [87][113][114][139,165,166]. The overproduction of peroxynitrite induced by magnesium deficiency further intensifies mitochondrial dysfunction [115][116][167,168]. Notably, proinflammatory mediators, also induced by magnesium deficiency [9][82][72,134], can further impact mitochondrial function, thereby amplifying mitochondrial oxidative stress and perpetuating an oxidative-inflammatory cycle [18][41].
At the molecular level, a key mechanism of action of magnesium involves the modulation of nuclear factor kappa-B (NF-κB), which serves as a pivotal transcription factor responsible for modulating the expression of a myriad of genes. When activated by various stimuli, including Toll-like receptors (TLRs), NF-κB translocates to the nucleus, where it upregulates the expression of genes associated with inflammatory and oxidative stress responses [117][169]. However, its excessive activation can lead to oxidative stress and chronic inflammation [117][169], a hallmark of inflammaging [67][68][119,120]. In this context, several studies have shown that magnesium can effectively reduce cytokine production via the downregulation of the TLR/NF-κB signaling pathway [6][118][71,170].
In fact, preclinical data have shown that inflammation in skeletal muscle is often marked by the activation of the NF-κB signaling pathway [119][171]. Persistent activation of this pathway has been shown to induce significant atrophy in mouse muscle [120][172]; it is also activated by muscle immobilization [121][173]. In line with this, short-term muscle fiber-specific overexpression of either the NF-κB p65 subunit or its activating enzyme, inhibitor κB kinase 2 (IKK2), results in muscle atrophy [122][174]. Conversely, targeted elimination of IKK2 and the resulting decrease in NF-κB activation have been associated with enhanced skeletal muscle strength, preserved mass, and improved regeneration [123][175]. Hence, it is hypothesized that magnesium could attenuate age-related muscle deterioration via NF-κB modulation.
In summary, the multifaceted roles of magnesium in combating inflammation and oxidative stress underscore its potential as a therapeutic agent for age-related muscle decline. The intricate interplay among magnesium status, inflammation, oxidative stress, and mitochondrial dysfunction highlights the importance of maintaining optimal magnesium levels, especially in aging populations. While the precise mechanisms through which magnesium exerts its protective effects remain a topic of ongoing research, the current evidence underscores its potential in modulating key pathways, such as the NF-κB signaling pathway, which is pivotal in muscle health.
5. Muscle Contraction and the Equilibrium of Electrolytes
Within the muscular system, muscle contraction, defined as the activation of muscle fibers leading to their subsequent shortening, is a fundamental physiological process [4][69]. Skeletal muscles, in particular, are pivotal for imparting stability and strength to the entire spectrum of bodily movements [4][69].
Muscle strength is governed by various multifaceted factors and is closely associated with the intrinsic quality of muscle and its aptitude for contractile action, particularly among elderly individuals. Notably, the pace of strength decline resulting from the aging process exceeds the rate of muscle mass reduction [124][176]. As individuals age, there is a discernible reduction in the dimensions and contractile efficacy of muscle fibers [5][70]. This culminates in a decline in muscle strength and power and an increase in frailty, ultimately impeding functionality and postural stability or even causing immobility [1][4][66,69].
These physiological transformations can increase the risk of severe injuries, limit participation in recreational activities, and ultimately impair the ability to carry out everyday tasks, thereby compromising individual independence and overall quality of life [1][4][66,69]. Nutrition can serve as a valuable ally in counteracting muscle loss, and magnesium plays a pivotal role in this context [5][70].
The cycle of muscle contraction fundamentally hinges on the supply of energy, primarily achieved through ATP hydrolysis. This process is initiated by the release of calcium ions (Ca2+) stored within the sarcoplasmic reticulum upon stimulation from the central nervous system [1][125][66,177]. Upon release, Ca2+ binds to troponin C and myosin, leading to conformational alterations in these proteins and subsequently precipitating muscle contraction [10][125][126][25,177,178].
Magnesium is an antagonist to calcium that competes for the same Ca2+-binding sites and thereby exerts regulatory control over the muscle contraction process [10][126][25,178]. In the quiescent state, magnesium is present in muscle cells in concentrations approximately 10,000 times higher than calcium, effectively occupying all available Ca2+ binding sites. It is only upon the release of Ca2+ from the sarcoplasmic reticulum that magnesium is displaced. However, under conditions of magnesium deficiency, even minimal amounts of calcium can displace magnesium. This results in hypercontractility, marked by muscle cramps and spasms [10][25], which are common events in advanced age [127][179].
Furthermore, following muscle contraction, the reuptake of calcium by the Ca2+-ATPase of the sarcoplasmic reticulum is contingent on the presence of magnesium [128][180]. This process is energy intensive, requiring one ATP molecule to transport just two Ca2+ ions [126][178], and magnesium plays an essential role in stabilizing and activating cellular ATP molecules [129][130][131][181,182,183]. In cases of inadequate ATP reserves, muscle fibers remain in a contracted state, preventing the release of actin and myosin chains and consequently leading to muscle cramps [127][179]. Another clinical consequence of magnesium deficiency is the development of cardiac arrhythmias, stemming from the disruption of cardiac muscle contraction regulation [15][38].
The maintenance of proper electrolyte balance is integral to muscle contraction functionality, as it serves to stabilize and uphold membrane potential [132][133][134][135][136][184,185,186,187,188]. This, in turn, regulates the flow of ions, fluids, and other molecules within the aqueous milieu of muscle [133][185] while preventing the onset of muscle fatigue [137][189]. Magnesium ions play a pivotal role in maintaining the electrolyte equilibrium of calcium, potassium, and sodium within skeletal muscle cells [126][135][178,187]. Last, magnesium facilitates the energization of ion channels, thus supporting their proper functioning [130][182]. Thus, the multifaceted role of magnesium in muscle contraction, from energy provision to electrolyte balance, underscores its indispensable nature in ensuring optimal muscle function, highlighting the need for adequate magnesium levels, especially as individuals age.
6. Magnesium and Muscle Health: Evidence from Human Studies
The examination of oral magnesium supplementation’s impact on muscle-related outcomes has been the focus of numerous interventional clinical studies. This section reviews key findings, emphasizing study design, magnesium supplementation specifics, and implications for muscle health.
Out of 26 studies reviewed, 19 were randomized placebo-controlled trials [31][34][138][139][140][141][142][143][144][145][146][147][148][149][150][151][152][153][154][83,86,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206] and three were randomized controlled trials without a placebo group [155][156][157][207,208,209]. The remaining studies had varying designs [80][158][159][160][132,210,211,212]. Dosage and duration of magnesium supplementation varied significantly across studies, ranging from 10 days to 32 weeks, and various magnesium sources were utilized, including Mg oxide, Mg sulfate, Mg citrate, Mg lactate, and chelated forms like Mg creatine chelate and Mg bisglycinate chelate.
Despite methodological limitations, such as small sample sizes, collective findings generally highlight magnesium’s positive impact on muscle health, attributed to its performance-enhancing, analgesic, and anti-inflammatory properties.
A substantial portion of the research, involving 15 studies [31][34][80][139][140][142][143][144][145][147][155][156][157][159][160][83,86,132,191,192,194,195,196,197,199,207,208,209,211,212] focused on the effects of magnesium on muscle-related physical performance in healthy individuals, with participants spanning untrained subjects to elite athletes. Of these studies, 10 [31][34][80][82][140][142][143][145][147][159][83,86,132,134,192,194,195,197,199,211] reported favorable outcomes with magnesium supplementation, enhancing muscle power, torque, exercise performance, lean body mass, handgrip strength, and reducing muscle soreness and markers of muscle damage. Although not directly linked to muscle health, improvements in metabolic response [147][199] and reductions in blood pressure [142][155][194,207] were also noted.
Studies also explored magnesium’s role in clinical populations, including patients with alcoholic liver disease [138][190], chronic alcoholism [141][193], and chronic or acute musculoskeletal low back pain [153][158][205,210]. While benefits in muscle strength were not observed in patients with alcoholic liver disease [138][190], improvements in muscle strength were noted in chronic alcoholics [141][193]. In cases of low back pain, magnesium supplementation demonstrated an analgesic effect [153][158][205,210].
The efficacy of magnesium in managing nocturnal leg cramps (NLC) yielded mixed results. Among the six studies [148][149][150][151][152][154][200,201,202,203,204,206] focusing on NLC, three specifically addressed pregnant women [148][151][152][200,203,204], a group particularly prone to NLC. Findings were divergent: three studies found no magnesium effect on NLC [149][151][154][201,203,206], whereas the others [148][150][152][200,202,204] observed decreases in frequency, intensity, and subjective discomfort. Factors influencing efficacy were discussed, including high baseline magnesium levels and dietary intake, potentially low magnesium supplementation dosages, the chemical form of magnesium administered, and the supplementation duration [139][142][143][191,194,195].
Among the studies listed, while several investigations included older participants, it is noteworthy that only one study specifically targeted the aging population [156][208] and none focused on the broader aspects of muscle health in this demographic, emphasizing the need for more targeted clinical research in this area. Such research is essential to establish evidence-based guidelines for magnesium supplementation to support muscle function and overall health and quality of life in the aging population.
In conclusion, while there is promising evidence supporting magnesium supplementation’s role in muscle health, further well-designed randomized controlled trials are necessary to conclusively establish its therapeutic potential in diverse muscle-related conditions, especially in the elderly.
