1. The Purity of Blue Light may Affect Plants’ Elongation Responses to This Light Wavelength
Blue LEDs normal emit more pure BL than the non-LED BL sources employed in prior studies, which can affect phytochrome activity and thus affecting plant elongation response. The non-LED BL sources emit impure BL due to the presence of low levels of other light wavelengths, such as a high ratio of R/FR light, that activate phytochromes, thereby making the BL exhibit more suppressive effect on elongation growth than R light [1][43]. For instance, the blue-colored fluorescent lamp, which was previously one of the commonly used BL sources, was reported to have a R/FR ratio of 1.87 [2][11]. The white fluorescent lamp filtered through blue acetates, another previously utilized BL source, did not contain >700 nm light due to the filters employed [3][96]. In contrast to BL from non-LED lighting, blue LED light exhibits a much lower phytochrome photostationary state (PPS, an indicator of phytochrome activity), estimated as 0.5, compared to 0.9 for red LED light, as per the method established by Sager et al. [4][97]. Although the threshold value of PPS required to elicit an active phytochrome response remains a matter of debate, it is generally agreed that a PPS < 0.6 may provoke an inactive response [5][98]. The lower phytochrome activity in plants could potentially account for the elongated plants observed under blue LED lighting. Thus, thwe researchers postulate that the effects of BL on plant elongation may, in some cases, be linked to phytochrome activity, which may differ under pure and impure BL sources.
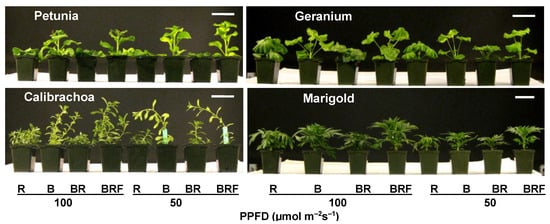
To examine the hypothesis proposed above, thwe researchers crcreated some impure BLs with different PPS by adding different ratios of R and FR to blue LEDs, in addition to the pure BL from blue LED. The results indicated that the pure BL promoted plant elongation compared to R light (Figure 1) [1][43]. However, the high-PPS impure BL had the opposite effect and inhibited elongation growth to a similar or greater extent than pure R light. The low-PPS impure BL restored the promotional effect observed with pure BL. The R/FR reversibility and the PPS changes suggest that the promotional effect observed with pure BL is linked to low phytochrome activity [1][43]. Under certain conditions, pure BL may need to co-act with R light to inhibit elongation growth by increasing phytochrome activity. Also, the promotional effect of impure BL increased gradually with the decreasing PPS values from 0.69 to 0.60. However, pure BL did not show a greater promotional effect on plant elongation than the lowest-PPS impure BL, despite a lower PPS value (0.49 vs. 0.60). It appears that the plant elongation promoted by BL gradually became saturated once the PPS values decreased below 0.60 [6][7][8][33,41,42]. It is possible that the deactivated phytochrome contributes to the maximum elongation promotion by BL [6][33].
Figure 1. Plant elongation responses to pure and impure blue light in four ornamental plant species
[1][43]. R = narrow-band red LED as a control treatment; B = pure blue light from a narrow-band blue LED; BR = impure blue light created by mixing B with a small amount (10% total PPFD) of R; BRF = impure blue light created by mixing BR with a small amount of far-red light, with red/far-red ≈ 1. The PPFD of the LED lighting was either 50 or 100 µmol m
−2 s
−1 for all treatments. The reference bar length in these pictures is 8.5 cm.
Adding low-level R light to pure BL has a similar or greater inhibitory effect than that of R light on plant elongation, so it is also interesting to know how plant elongation responds to adding low-level other wavelengths, including ultraviolet-B (UVB), ultraviolet-A (UVA), green (G) or FR light, to pure BL, since BL from some non-LED lighting sources has also been found to contain small amounts of these light wavelengths. Ouresearchers's studies indicated that adding these low-level wavelengths to pure BL only slightly changed plant elongation compared with blue LED, but still promoted plant elongation compared with red LED[9][10][32,34]. The calculated PPS values of these impure BL were similarly low (<0.6) to those of B, but they were much less than those of high-PPS impure BL by adding red light to pure BL, indicating that phytochrome activity plays an important role in plant elongation mediated by BL [9][10][32,34]. It appears that among the wavelengths possibly contained in impure BL, low-level R light has the greatest contribution to the inhibitory effect of impure BL on plant elongation.
2. Factors Affecting Plants’ Elongation Response to Pure Blue Light Relative to Red Light
Inconsistent results on plants’ elongation response to BL relative to R light have also been obtained from LED lighting studies. This may be related to differences in lighting features, plant factors, and cultivation conditions among different trials.
2.1. Lighting Features
Light intensity can interact with light quality to affect plant elongation.
Our
esearchers' lab conducted a study on arugula and mustard seedlings under blue or red LED lighting, at PPFD levels ranging from 20 to 650 µmol m
−2 s
−1 [11][35]. It was found that the hypocotyl elongation of arugula was promoted by BL at all tested PPFD levels compared to R light, while for mustard the promotional effect was limited to higher PPFD levels, i.e., 250–650 µmol m
−2 s
−1. Additionally, for arugula, the promotion of hypocotyl elongation by BL decreased as the PPFD level increased
[11][35]. The interaction effect of light intensity on BL-mediated plant elongation has also been found in other plant species
[1][9][32,43].
The photoperiod can also interact with light quality to affect plant elongation. To determine whether periodic lighting can influence the effects of BL on plants’ elongation relative to R light, the seedlings of arugula, cabbage, mustard, and kale were subjected to B or R LED lighting at a photoperiod of 24 or 16 h d
−1 [12][30]. Regardless of the photoperiod, B consistently promoted elongation growth compared to R for arugula, cabbage, and kale. The promotional effects of BL on elongation were often more pronounced under 24 h vs. 16 h lighting. In a further study, with a photoperiod of 12 h d
−1, B vs. R LED light also promoted plant elongation for arugula
[13][31]. These findings suggest that the promotion of elongation growth by BL is not solely dependent on the 24 h lighting cycle, despite varying promotional magnitudes under different photoperiods.
BL with different peak wavelengths may have different effects on plant elongation. The PPS is very dynamic across the entire BL waveband, ranging from 0.41 to 0.60
[4][97]. A study using B LEDs with peak wavelengths ranging from 432 nm to 466 nm indicated that green perilla (
Perilla frutescens) plant elongation increased as the peak wavelength decreased below 446 nm
[14][100]. Also, when B LEDs with different peak wavelengths were used as supplemental lighting for producing two baby greens (Chinese kale and pak choi), the plants were taller under B-430 than under B-400 for both species, and also taller than under B-465 for Chinese kale
[15][101]. However, in one of
our
esearchers's studies, BL-mediated plant elongation did not differ across different peak wavelengths of BL. In this study, the growth and morphology traits of mustard and arugula seedlings were investigated under BL with three different peak wavelengths (B1: 404 nm, B2: 440 nm, or B3: 455 nm), UVA light (385 nm), FR light (730 nm), R light (665 nm), and darkness
[16][36]. It was found that B1, B2, and B3 had similar effects on hypocotyl elongation for both species, and the three BLs, compared to R, promoted plant elongation for arugula, regardless of the peak wavelength. Among the tested lights, BL had the greatest promotional effect on plant elongation for both species, despite having a smaller promotional effect than darkness
[16][36].
2.2. Plant Factors
It has been found that BL-mediated elongation can vary between plant species and even cultivars
[17][18][4,24]. In
our
esearchers' initial experiments, only four ornamental species (petunias, calibrachoa, geraniums, and marigolds) were evaluated
[1][7][8][19][40,41,42,43]. However, subsequent experiments expanded the scope to microgreens such as arugula, mustard, cabbage, and kale
[6][9][10][11][12][13][30,31,32,33,34,35], and other microgreens/baby greens such as sunflower, cilantro, celtuce, basil, and pak choi, as well as the model plant
Arabidopsis [20][21][22][46,47,48]. Most of the tested species exhibited increased plant elongation under BL relative to R light when exposed to continuous (24 h d
−1) lighting at a PPFD of 100 µmol m
−2 s
−1, except for mustard, cilantro, and celtuce. For these three species, B LEDs still promoted elongation compared with RB-LED lighting but exhibited similar or inhibitory effects on plant elongation compared to R LEDs. In another experiment, for mustard, the promotional effect of B LEDs relative to R LEDs was observed under higher PPFD (>250 µmol m
−2 s
−1) rather than lower PPFD (<250 µmol m
−2 s
−1)
[11][35]. Mustard has red pigmentation (anthocyanin) in its cotyledons, which could filter a part of R light and reduce its transmission to phytochromes. It is possible that as the light intensity increased, the transmitted R light level became high enough to induce an active phytochrome response, inhibiting elongation growth relative to BL. However,
our
esearchers're recent trial comparing red- and green-leaf cultivars from the same species under BL and R light did not confirm this explanation. Even in red-leaf cultivars, B still promoted plant elongation compared to R, suggesting that pigment filtering may not entirely account for the species differences in BL response.
2.3. Cultivation Conditions
Temperature variation can affect phytochrome activity and, thus, affect plant elongation under BL relative to R light. In
our
esearchers' previous studies on blue LEDs, only a constant temperature of around 23 °C was used. Through collaboration with the Texas A&M AgriLife Research Center,
the researchers we investigated how temperature variations affected light-mediated plant elongation
[13][31]. Arugula and mustard seedlings were grown indoors at 18 °C or 28 °C to compare plant elongation responses between R and B LED light. Regardless of temperature, B vs. R LED light promoted plant elongation in arugula, and the promotional effect was greater at 18 °C than at 28 °C, showing interactions between light and temperature. However, for mustard, there was no interaction between light and temperature with respect to plant elongation; plants were shorter under B vs. R light and were taller at 28 vs. 18 °C. In contrast to
our
esearchers' previous studies, plant elongation decreased for mustard, and plant biomass decreased for both species under B vs. R light
[13][31]. Possibly, a much shorter photoperiod (12 h d
−1 vs. 16 or 24 h d
−1) was used for this study, despite a similar PPFD (110 µmol m
−2 s
−1).
Air humidity can modulate plants’ responses to BL, including plant elongation. Researchers from Norway found that when B LEDs were added to HPS lighting, tomato and cucumber plants under high relative humidity (RH; 90%) were taller compared with those under moderate RH (60%)
[23][109]. They speculated that BL might have been used more efficiently for the development and function of chlorophyll and stomata under higher air humidity. In contrast, B LEDs inhibited shoot elongation for
in vitro cuttings of
Rehmannia glutinosa under no-ventilation conditions (at a higher air humidity), but they had a similar effect under ventilation conditions (at a lower air humidity), compared with R LEDs
[24][92].
Plants’ elongation response to BL relative to R light seems to be affected little by other cultivation factors, such as planting density and growth medium. In most of
theour resstudies on microgreens’ elongation response to B vs. R light, an evenly low planting density (with only one seedling per plug cell) was adopted to reduce the compound effect of plant–plant shading and provide the convenience to investigate the biometrics. However, in a follow-up experiment conducted on arugula and sunflowers at commercial (i.e., higher) planting densities, a similar promotional effect on plant elongation was observed under B vs. R LED light. In most of
the reseachers'our studies, a peat-lite mix was used for plant cultivation. However, for
Arabidopsis [20][21][22][46,47,48] and some microgreens, such as arugula and mustard growing in rockwool cubes as an alternative medium, the plants also exhibited a similar elongation response to B vs. R LED light.
3. Mechanisms Underlying Blue-LED-Promoted Plant Elongation
3.1. Shade-Avoidance Response
thWe
researchers hhave identified the promotion of stem elongation by blue LEDs as a shade-avoidance response (SAR), albeit with varying sensitivity across plant species
[1][6][33,43]. In addition to elongated stems, other typical SARs have also been observed in other plant traits under blue LEDs. In mature plants, blue LEDs reduced the side-branching, chlorophyll content, leaf mass per unit area and/or increased individual leaf area, petiole length, biomass allocation to supporting structures, and/or advanced flowering time in petunias, calibrachoa, geraniums, and marigolds, compared with red LEDs
[1][43]. Similar SARs were also observed in lettuce grown under narrow-band blue LEDs, which reduced the root dry mass, leaf chlorophyll content index, and leaf thickness compared with RB-LEDs
[25][110]. In de-etiolated seedlings such as arugula, mustard, kale, and cabbage, blue LEDs resulted in longer petioles, smaller cotyledons, lighter plant color, or greater biomass allocation to hypocotyls
[6][33]. Blue LEDs also caused leaf hyponasty in sunflower microgreens, which differed from the leaf epinasty under red LEDs , and the red-light-induced leaf epinasty in geraniums could be alleviated by blue LEDs
[26][111]. Leaf hyponasty was also promoted in lettuce plants under blue vs. red LEDs, despite shorter stems
[27][51]. Furthermore, the proteome changes in
Arabidopsis thaliana’s response to blue LEDs relative to red LEDs also appear to be involved in the pathway of SARs
[28][112].
The BL-mediated SARs in morphological traits were partially supported by the changes in anatomical structure. In arugula, the hypocotyl epidermis demonstrated greater cell elongation under blue LEDs compared to red LEDs
[10][34]. Similar results have been reported in
Arabidopsis under low-level BL
[29][113]. However, smaller cotyledon sizes in arugula, kale, and cabbage seedlings under blue vs. red LEDs resulted from decreased cell numbers rather than decreased cell size in the cotyledon epidermis; on the other hand, the leaf cell size increased to compensate for the reduced cell numbers
[10][34]. Associated with decreased leaf thickness, some anatomical changes such as reduced palisades, and spongy tissue thickness were observed in lettuce leaves under blue LEDs compared with RB-LEDs
[25][110]. The increased leaf hyponasty of sunflower seedlings under blue vs. red LEDs was due to the increased length of epidermis cells in the abaxial (or lower) sides of leaves, which also contributed to blue LED’s inhibition of leaf epinasty in geraniums under red light
[26][111].
The SARs were primarily observed under BL with low PPS, but not under BL with high PPS
[1][6][9][32,33,43]. Moreover, the BL-promoted SARs were more pronounced under lower light intensity
[1][9][32,43]. In natural vegetative shade, plants experience both decreased ratios of R/FR and reduced intensity of BL, which can trigger plants’ elongation to compete for light as one of the SARs through reduced activity of PHY and CRY
[30][31][114,115]. It is possible that the BL with low PPS is like an integrated shade signal that can be perceived by multiple photoreceptors such as PHY and CRY in plants. However, it remains unclear which factor—low BL level or low phytochrome activity—plays a more significant role in the BL-promoted SARs under specific conditions, necessitating further research.
3.2. Hormone Changes
GAs have been found to play an important role in BL-promoted plant elongation. In the case of petunias with elongated plants under blue vs. red LEDs, compared with other hormones, the contents of active gibberellic acid (GA) varied more markedly between blue and red light
[32][116]. In stem tissues under blue LEDs, much higher levels of GAs (especially GA
4 and GA
1) were detected compared with those under red LEDs
[32][33][17,116]. For the dwarf plants developed under red light, after the application of GA
3, the plants’ height increased
[32][33][17,116]. Under blue LEDs, the production of GA
20-oxidase, one of the key enzymes in the synthesis of active GAs, might have increased in the shoot tips
[34][117]. This has been supported by higher expression of
PhGA20ox-1S and
PhGA20ox-2L, two homologous genes for encoding GA
20-oxidase in
Arabidopsis, under blue LED treatment than under red LED treatment after 6 h of light treatment
[32][35][22,116]. The increased enzyme production and gene expression were closely associated with higher contents of GAs under blue vs. red LEDs
[34][117]. Another study in tomato seedlings also suggested that GA biosynthesis may be involved in the stem elongation of seedlings grown under low-BL conditions
[36][118].
Auxin has been considered as a fundamental regulator of SARs induced by low R/ FR ratios
[37][119]; however, it appears to play a minor role in BL-mediated plant elongation as an SAR. Low-R/FR-induced phytochrome inactivation stimulates auxin biosynthesis and induces hypocotyl elongation, petiole elongation, and leaf hyponasty in
Arabidopsis [29][113]. Also, regulated transport of these enhanced auxin levels is essential to achieve elevated auxin concentrations in the hypocotyl to induce its elongation in
Arabidopsis seedlings under low R/FR ratios
[29][113]. For petunia plants, unlike GAs, the auxin content under blue LEDs was only slightly higher than that under red light treatment
[32][116]. However, it is unclear whether other plant species have a similar response.
Brassinosteroids (BRs) have been shown to contribute to the SARs mediated by low-intensity BL. In
Arabidopsis, the pathways for polar auxin transport, auxin biosynthesis, and gibberellin signaling that are involved in SARs under low R/FR ratios were not required for the SARs under low-intensity BL; in contrast, the BR response appeared to be required for the full expression of the SAS phenotype under low BL
[38][120]. However, another study in
Arabidopsis indicated that both auxin and BR play important roles in the regulation of enhanced hypocotyl elongation of seedlings in response to BL depletion, and only when both hormones are blocked simultaneously will the response be fully inhibited
[29][113]. It is difficult to explain the contrasting results from the same species. Also, it is unknown how BR is involved in BL-promoted plant elongation as an SAR in horticultural crop species.
3.3. Involved Photoreceptors
The researchers found that at least three photoreceptor systems are involved in the BL-promoted plant elongation as an SAR. Although phytochromes are primarily the receptors of R and FR light, the photoreceptors can also sense other wavelengths, including BL
[39][40][1,121]. The blue LED has a low PPS below 0.6, which normally cannot induce an active phytochrome response
[5][98]. Also, the R/FR reversibility, which is considered to be a hallmark of phytochrome action, indicates that the blue-LED-promoted elongation as an SAR is related to low-activity phytochromes
[1][43]. Through further studies on
Arabidopsis photoreceptor-deficient mutants and photoreceptor-overexpressing transgenic plants, the researchers found that not only low-activity phytochromes but also low-activity cryptochrome 1 (CRY1), high-activity cryptochrome 2 (CRY2), and phototropins (including phot 1 and phot 2) are involved in the blue-LED-mediated responses
[20][21][22][46,47,48]. Previous studies on
Arabidopsis indicated that CRY1 plays a key role in BL-mediated inhibition of hypocotyl elongation, and that CRY1-mediated inhibition of hypocotyl elongation requires active phytochromes for full expression in some cases
[41][42][104,122]. However, the detailed information about how CRY1 is deactivated under blue LEDs through crosstalk with other photoreceptors, such as phytochromes, is still unknown, especially for horticultural plant species.
One means of crosstalk between cryptochrome and phytochrome is the direct protein–protein interaction of the two photoreceptors, according to the studies of
Arabidopsis. Phytochrome A (phyA) was previously found to directly interact with CRY1, and a direct interaction was also shown between phyB and CRY2, but these interactions were not demonstrated to be light-dependent
[43][44][123,124]. Hughes et al.
[45][125] reported a direct light-dependent interaction between phyB and CRY1, where CRY1 interacts specifically with the dark/FR state (Pr) of phyB, but not with the R light-activated (Pfr). Whether these interactions can explain the crosstalk between CRYs and PHYs to mediate plant elongation under blue LEDs is unknown.
Another means of crosstalk between cryptochrome and phytochrome is indirect interaction through common signaling molecules of these photoreceptors. For example, cryptochrome and phytochrome can both bind to the SUPPRESSOR OF PHYA-105 (SPA)/CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) complex to target certain sets of transcription factors for degradation
[46][126]. They can also both bind to basic helix–loop–helix (bHLH) transcription factors, such as PHYTOCHROME INTERACTING FACTORs (PIFs) to control transcription
[46][126]. In addition, it has recently been found that the blue-light inhibitors of cryptochromes (BICs) and photoregulatory protein kinases (PPKs) may also play roles in the cryptochrome-phytochrome coaction
[47][127].
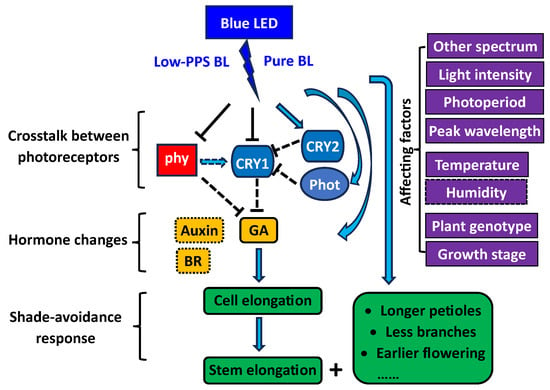
Based on the key findings of the relevant studies, especially the research conducted in the ouresearchers' lab, the researchers we propose a simple model (Figure 26) to explain the underlying mechanisms involved in blue-LED-promoted plant elongation.
Figure 26. A proposed simple model for explaining the mechanisms involved in blue-LED-promoted plant elongation. BL = blue light; PPS = phytochrome photostationary state; phy = phytochrome; cry = cryptochrome; phot = phototropin; GA = gibberellic acid; BR = brassinosteroid. The proposed model is based on the key findings from
the our
esearchers' previous studies
[1][6][7][8][9][10][11][12][13][16][19][20][21][22][30,31,32,33,34,35,36,40,41,42,43,46,47,48], except for the GA signal from Fukuda’s group
[33][17].
4. Application of Blue LEDs in Mediating Plant Elongation for Controlled-Environment Production
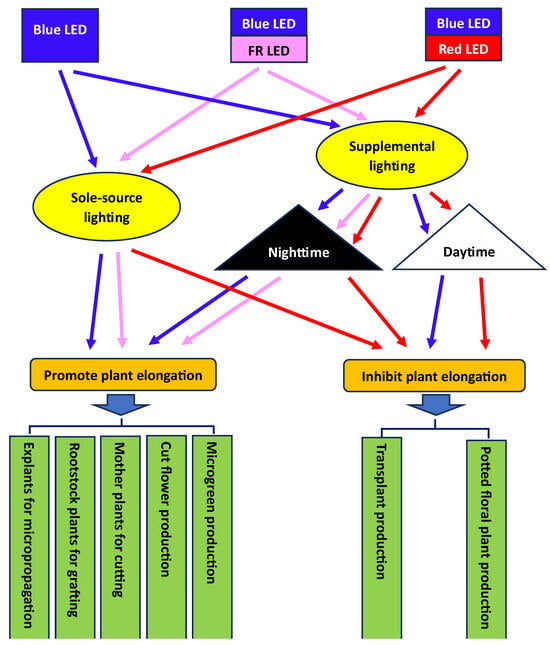
Considering that BL-mediated plant elongation can be affected by phytochrome activity, the application of blue LEDs, alone or in combination with R or FR LEDs, either as the sole source or as supplementary lighting, would potentially affect plant elongation differently during daytime or nighttime. Therefore, propose different potential ways to apply blue LEDs in controlled-environment plant production for varying purposes (Figure 37). Most of these methods have been tested in relevant studies in the ouresearchers' lab or by other groups.
Figure 37. Potential ways to apply blue LEDs in plant production in a controlled environment. FR = far-red.
4.1. Plant Propagation
4.1.1. Promoting Explant Elongation for Micropropagation
Nodal and internodal explant culture stands as a straightforward and efficient technique for micropropagation. Nonetheless, certain plants, like
Paphiopedilum and
Nepenthes, present challenges due to their short and poorly defined internodes. Consequently, obtaining precisely delineated nodal and internodal explants for micropropagation becomes a formidable task. Moreover, the dense arrangement of leaves on these plants complicates the process of surface decontamination for explants
[48][128]. For
P. delenatii, one-month-old
ex vitro single-node shoots (1.5–2.0 cm length) were grown under various light conditions, including blue or red LEDs alone, mixtures of blue and red LEDs, and darkness, for examinations of the shoot elongation. The best stem elongation was obtained under blue LEDs (100%B) after four months of culture
[49][129].
4.1.2. Promoting Hypocotyl Elongation of Rootstock Plants for Grafting
Producing seedlings with long hypocotyls is desirable in vegetable grafting. Longer hypocotyl lengths in the rootstock would both allow easier grafting and reduce the risk of scion exposure to the soil
[50][130]. A short-term (10-day) pre-grafting lighting with blue LEDs at 15 µmol m
−2 s
−1 promoted plant elongation, increased the leaf number and size, and increased the graft–take ratio in tomato seedlings compared with darkness
[51][133]. However, its beneficial effects were less than those of white fluorescent light or natural light.
4.1.3. Mediating Shoot Elongation of Mother Plants for Cuttings
Campanula mother plants have short shoots, and it is difficult to harvest cuttings; therefore, producing mother plants with long and thick side branches without flower buds is important for high-quality cuttings
[52][134]. For indoor-grown
Campanula mother plants,
the our
esearchers' lab has developed a three-stage lighting strategy, i.e., sequential lighting with red, blue, and RB-LEDs at three stages, aimed at increasing the number of side branches, promoting shoot elongation, and enhancing shoot thickness, respectively
[52][134]. The dynamic lighting increased the side branch numbers and plant height without inducing flowering, meeting the target height (≈7.5 cm) for machine harvesting. Furthermore, the dynamic lighting improved the upright growth of side branches and did not affect the cutting quality or rooting. Overall, dynamic lighting with blue and red LED light has the potential to benefit the controlled-environment production of
Campanula cuttings if the lighting strategy is further optimized.
4.2. Transplant Production
For transplant production of vegetables or ornamental plants, normally the high-quality seedlings have compact canopies, developed root systems, high chlorophyll concentrations, and the ability to withstand transplanting shock
[53][54][135,136]. Blue LEDs can be applied alone or in combination with other wavelengths as a sole or supplemental lighting source to mediate plant elongation as well as other quality indices during transplant production.
4.2.1. Sole-Source Lighting with a Combination of Blue and Red LEDs Can Produce Compact Transplants under Indoor Conditions
Sole-source lighting with RB-LEDs is commonly used for indoor transplant production. Studies on cucumber and tomato seedlings have indicated that the lack of either blue or red light negatively affects early development, but BL appears to play a more critical role than red light
[55][56][57][55,80,138]. A recent study in
the reseaour
chers' lab indicated that RB-LEDs (15%B) can potentially replace fluorescent light, but the trichromatic lights appear to be unnecessary for the indoor production of compact gerbera transplants
[58][137]. Compared with red or blue LEDs alone, RB-LEDs (50%B) caused more compact seedlings in bedding plants such as impatiens, petunias, and salvia
[59][57]. In addition to more compact transplants, RB-LEDs (50%B) also promoted the post-transplanting growth of lettuce plants, due to higher biomass and antioxidant activities in the transplants, compared with red or blue LEDs alone
[60][50].
The BL proportion in RB-LED lighting can be optimized based on multiple plant responses aside from plant compactness, but the optimal BL proportion seems to vary between plant species. Under sole-source lighting at a PPFD of 100 µmol m
−2 s
−1 and a photoperiod of 18 h, the optimal blue proportion in RB-LED lighting was 10% for cucumber seedlings and 30–50% for tomatoes
[61][59]. Under RB-LED lighting at 300 µmol m
−2 s
−1 for 12 h d
−1, the optimal BL percentage was 25% for sweet pepper and eggplant transplants, which showed the best performance not only in compact morphology but also in robust growth, with the highest seedling index value
[62][63][23,60]. A similar optimal BL percentage has been identified in rice seedlings grown under RB-LED lighting at 100 µmol m
−2 s
−1 for 12 h d
−1 [64][66].
The decision on the optimal proportion of BL in sole-source LED lighting needs to consider the specific goal(s) of the propagators. If plant compactness is the priority goal, as little as 6%B in RB-LED lighting at 160 µmol m
−2 s
−1 for 18 h d
−1 can elicit compact transplants in bedding plants such as impatiens, petunias, and salvia
[65][58]. For most plant species, at least 13%BL can be included in sole-source LED lighting to produce compact transplants
[66][139]. In addition to controlling the stem length, the node position of the first flower truss is also crucial for the production of high-quality tomato seedlings in Japan
[67][56]. Sole-source RB-LED lighting with less than 50%B and a BL intensity of 75 µmol m
−2 s
−1 has been recommended to suppress spindly growth and promote flowering during tomato seedling growth
[67][56]. In commercial production, the decision of optimal BL proportion in LED lighting can also be related to economics, since BL requires more energy per photon
[66][139].
4.2.2. Supplemental Lighting with Blue LEDs Only or Their Combination with Red LEDs Can Produce Compact Transplants in Greenhouse Conditions
Blue LEDs alone can be used as a supplemental lighting (SL) source for the greenhouse production of compact transplants. In cucumbers, supplemental blue LEDs at 15 µmol m
−2 s
−1 with high-pressure sodium (HPS) lamps (90 µmol m
−2 s
−1) for 18 h d
−1 not only decreased hypocotyls’ elongation, but also increased the leaf area, increased the fresh and dry weight, and enhanced their development, compared with HPS only
[68][140]. In the same species grown in a greenhouse under low-intensity sunlight (about 2.7 mol m
−2 d
−1), 10 days of SL with blue LEDs relative to white, red, or green LEDs (at 120 µmol m
−2 s
−1 for 10 h d
−1; 4.3 mol m
−2 d
−1) caused more compact plants with shorter stems and smaller leaf areas, despite similar shoot biomass
[69][141]. Furthermore, after transferring to full sunlight (10.7 mol m
−2 d
−1), plants from the blue LED treatment developed similar leaf areas and 15% higher shoot biomass, showing better acclimation ability compared to other spectral treatments
[69][141].
Blue LEDs in combination with red LEDs (RB-LEDs) can also be used as an SL source for the greenhouse production of compact transplants. For cucumbers and tomatoes, regardless of the natural light level (5–25 mol m
−2 d
−1), SL with RB-LEDs (4–16%B; PPFD = 54 µmol m
−2 s
−1; DLI = 3.6 mol m
−2 d
−1) resulted in compact transplants while improving transplant quality compared with no SL
[70][71][142,143]. In six tomato cultivars grown in a greenhouse, SL with RB-LEDs (5–20%B; 61 µmol m
−2 s
−1; 5.1 mol m
−2 d
−1) reduced the hypocotyl elongation and increased the hypocotyl diameter, epicotyl length, shoot dry weight, leaf number, and leaf expansion relative to no SL under changing solar DLIs, from 0.4 to 19.1 mol m
−2 d
−1 [72][144]. In greenhouse-grown seedlings of bedding plants (including
Antirrhinum,
Catharanthus,
Celosia,
Impatiens,
Pelargonium,
Petunia,
Tagetes,
Salvia, and
Viola), SL with RB-LEDs (15–30%B) at 100 µmol m
−2 s
−1 for 16 h daily reduced plant height by 9% to 55% and increased the stem diameter by 8% to 16%, showing a similar or higher transplant quality compared to HPS lamps
[53][135].
For SL with RB-LEDs, the optimal BL proportion varies in different situations. In the greenhouse production of transplants, within the BL percentage range of 0–30%, 15%B in RB-LEDs was found to be optimal for bedding plants when used as SL
[53][135]. However, for six cultivars of tomato transplants grown in a greenhouse under SL with RB-LEDs, the optimal BL proportion within the range of 0–20% varied between cultivars
[72][144]. Also, in greenhouse-grown cucumber transplants, the seedling morphology was not different among RB-LEDs with different B%, especially under high natural light levels, and the plants did not even show a more beneficial response to RB-LEDs compared with red LEDs under 5–24 mol m
−2 d
−1 of solar DLI
[70][71][142,143]. In this case, the impact of BL appears to be minimal, especially when background solar irradiance provides a sufficient amount of this wavelength
[66][139].
4.3. Floral Plant Production
To meet the marketing requirements, not only are earlier flowering and more flowers beneficial to commercial growers, but also a compact plant morphology is helpful for production of potted floral plants, while an elongated stem is desired for production of cut flowers. Blue LEDs, alone or in combination with other LEDs, depending on the production purpose and plant genotype, can be used for mediating plant elongation as well as flowering in floral crop production.
4.3.1. Promoting Plant Compactness in Potted Floral Plant Production
The application of blue LEDs in combination with red LEDs (RB-LEDs) as sole-source lighting can produce compact potted floral plants. In roses, sole-source lighting with RB-LEDs (20%B) at 100 µmol m
−2 s
−1 for 20 h d
−1 decreased the plant height, leaf area, and shoot biomass and increased the proportion of dry mass allocated to the leaves, without affecting flowering, compared to HPS lamps
[73][145]. Indoor production with sole-source lighting with RB-LEDs (30%B) at 500 µmol m
−2 s
−1 for 16 h d
−1 also led to more compact plants in four potted floral plants (primulas, marigolds, treasure flowers, and stock plants), while causing higher numbers of flower buds and fewer days to flowering compared with greenhouse production under natural light
[74][91].
In addition to sole-source lighting, supplemental lighting (SL) with blue LEDs can also affect the compactness of potted floral plants. When narrow-band blue LEDs were used for daytime SL in the greenhouse, their effect appeared to be dependent on the natural background light level and the presence of FR light. In potted petunias, in late spring when the natural irradiance is higher (2.33 mol m
−2 h
−1), SL with blue LEDs at 50 µmol m
−2 s
−1 for 16 h d
−1 in an FR-deficient environment inhibited stem elongation similarly to red LEDs, but in early spring when the natural irradiance was low (1.35 mol m
−2 h
−1), the SL with blue LEDs did not inhibit but, rather, promoted stem elongation and plant flowering compared to red LEDs
[75][146].
Unlike blue LEDs alone, RB-LEDs can be more reliably used for daytime SL to produce compact potted floral plants, despite varying sensitivity among plant species. For potted poinsettias, strict control of plant height is essential in production, and RB-LEDs (20%B) at 100 μmol m
−2 s
−1 for 10 h d
−1 were successfully used as an SL source in greenhouses or growth chambers to produce compact plants
[76][147]. Compared with HPS lamps, the plants were 20–34% shorter and did not delay bract color formation, visible cyathia, or flowering, despite decreases in the leaf and bract area, chlorophyll content, and total dry matter accumulation
[76][147]. Similarly, for potted geranium plants, supplemental RB-LED lighting with 45%B at 90 μmol m
−2 s
−1 for 16 h d
−1 promoted canopy compactness, early flowering, and increased flower numbers compared with HPS
[77][148]. However, species-specific responses have been reported for potted roses, chrysanthemums, and campanulas grown in a greenhouse. SL with RB-LEDs (40%B) at 200 μmol m
−2 s
−1 and 16 h d
−1 reduced plant height while increasing the biomass in roses and chrysanthemums, but not in campanulas, compared with white or red LEDs
[78][149].
4.3.2. Promoting Plant Elongation in Cut Flower Production
In the winter production of chrysanthemums, a short-day (SD) plant, for cut flowers, electrical lighting is used to create long days (LDs) routinely for 2–3 weeks before the onset of short days to meet the required specific stem length, but this delays the transition to flowering. Research has shown that blue LEDs can be potentially used as a lighting source to extend the photoperiod during SD conditions for controlled-environment production of cut chrysanthemum flowers to promote stem elongation without inhibition of flowering. For example, a 4 h EOD treatment with blue LED light of 10 μmol m
−2 s
−1 after 9 h of daytime lighting with white LEDs at a PPFD of 180 µmol m
−2 s
−1 increased the plant height, leaf number, and leaf area without delaying the flowering time or reducing the flower number
[79][150]. Also, EOD illumination with BL at 70 µmol m
−2 s
−1 for 4 h daily did not inhibit the flowering of chrysanthemums growing under 12 h daytime lighting with white fluorescent light at 70 µmol m
−2 s
−1 [80][151]. Furthermore, for plants growing under RB-LEDs (20%B) at 100 µmol m
−2 s
−1 for 11 h daily, a long-day treatment with 4 h EOD or 13 h overnight exposure to blue LEDs at 100 µmol m
−2 s
−1 did not inhibit flowering but did promote stem elongation
[81][82][152,153].
It is worthwhile to note that different plant responses to prolonged-photoperiod lighting with blue LEDs have been found in chrysanthemums growing under different background light conditions as well as different reference lighting. Under an 11 h daytime condition, 4 h EOD SL with blue LEDs at 40 µmol m
−2 s
−1 inhibited chrysanthemums’ flowering in a greenhouse with daytime solar light, but not in a growth chamber with daytime lighting from RB-LEDs (40%B) at a PPFD of 100 µmol m
−2 s
−1, despite the increased stem length in both the greenhouse and chamber
[83][154]. In contrast, for chrysanthemums growing indoors under sole-source lighting with fluorescent lamps at 150 µmol m
−2 s
−1 for 12 h, a 4 h nightly interruption with blue LEDs at 1.7 µmol m
−2 s
−1 reduced the daily internode elongation rate by about 60% compared with fluorescent lamps at 150 µmol m
−2 s
−1, and the inhibitory effect of blue LEDs was maintained not only in the nighttime interruption period but also in the subsequent dark and light periods
[84][155].
In addition, blue LEDs can also be combined with other LEDs to mediate the plant morphology and flowering of chrysanthemums. In a greenhouse, 4 h of supplemental lighting with blue LEDs combined with FR LEDs (75%B + 25%FR) enhanced stem elongation and promoted early flowering
[85][156]. In a walk-in growth chamber, nightly interruption with 2 h of blue LEDs first and then 2 h of FR LEDs at an intensity of 10 µmol m
−2 s
−1 promoted both plant elongation and the number of flowers per plant, compared with 10 h short-day treatments
[86][157]. In a growth chamber under 13 h daytime lighting with white LEDs at a PPFD of 180 µmol m
−2 s
−1, 4 h of EOD lighting with blue LEDs at 10 µmol m
−2 s
−1 promoted flowering and increased plant height
[79][150].
Blue LEDs have also been found to show a promotional effect on the elongation of some other cut flower species. In tulips, sole-source lighting with blue LEDs for 12 h d
−1 at a PPFD of 200 µmol m
−2 s
−1 increased the cut flower stem length and cut flower fresh weight compared with red LEDs
[87][45]. Sole-source lighting with blue LEDs for 16 h d
−1 also caused taller tulip plants than red or white LEDs and resulted in an earlier sprouting and flowering and a higher biomass compared with darkness
[88][158].
4.4. Microgreen Production
Microgreens are typically harvested at 7 to 21 days from seeding, and a minimum height of 5 cm is required before the final harvest
[89][159]. In recent years, the trend in commercial microgreen production has been to switch from hand to machine harvesting to reduce labor costs. However, machine harvesting of microgreens with hypocotyls shorter than 5 cm can be challenging. LED lighting can be used to mediate the hypocotyl elongation of microgreens during controlled-environment production.
4.4.1. Application of Blue LEDs in Daytime Lighting to Promote Hypocotyl Elongation
RB-LEDs have been popularly used as daytime lighting sources for indoor-grown microgreens, and the BL proportion in RB-LEDs can be optimized to promote plant elongation while maintaining the yield and other quality traits. For daytime sole-source lighting at 300 µmol m
−2 s
−1 for 16 h d
−1 during indoor microgreen production, the BL proportion in RB-LEDs (between 5–30%B) was optimized in terms of plant elongation, yield, and other appearance qualities for cabbage at 15%B, and at 5%B for kale, arugula, and mustard
[90][160].
Although the BL proportion in RB-LEDs can be optimized, it can still cause shorter plants than blue LEDs. For example, under RB-LED lighting at 220 µmol m
−2 s
−1 for 18 h d
−1, RB-LEDs with only 10%B reduced the hypocotyl length in
Brassicaceae microgreens compared to blue LEDs only
[91][79]. However, microgreens grown under either blue or red LEDs alone cannot meet the commercial requirements in terms of both plant height and appearance quality, so a potential approach using sequential lighting, with blue LEDs first to increase plant height and then red LEDs to improve leaf size and plant color (i.e., temporal combination of blue and red LEDs), has been suggested by
the researcheour
s' lab to address this problem
[12][30]. Another approach developed by us to address the problem of short plants under RB-LED lighting is delaying the start of the lighting for several days—in other words, using early-stage dark treatment
[92][93][161,162], since darkness, relative to RB-LED lighting, can also promote hypocotyl elongation during the early development stage of plants
[94][102]. However, this approach is better for larger-seed species such as sunflowers, due to a potential yield loss in smaller-seed species such as arugula, despite the promotion of plant elongation in both species
[92][161].
4.4.2. Application of Blue LEDs in Nighttime Lighting to Promote Hypocotyl Elongation
For indoor-grown microgreens under electrical lighting, blue LEDs can be used for nighttime lighting to promote microgreen elongation without affecting yield or quality. For two microgreen species grown indoors under sole-source lighting with RB-LEDs (20%B) at a PPFD of 300 µmol m
−2 s
−1 during 16 h of daylight, nighttime lighting with blue LEDs alone at 20 µmol m
−2 s
−1 for 8 h or at 40 µmol m
−2 s
−1 for 4 h increased plant height by 34% and 18% for mustard and arugula, respectively, compared with no nighttime lighting
[95][163]. Nighttime lighting with 20 µmol m
−2 s
−1 blue LEDs and 20 µmol m
−2 s
−1 FR LEDs together for 8 h further improved the promotional effect on elongation. The 8 h lighting with blue LEDs alone also increased the fresh weight of arugula by 12% compared to darkness. Additionally, nighttime treatments with blue LEDs, alone or in combination with FR LEDs, increased the chlorophyll content index, leafy index, or dry matter content, depending on the species
[95][163].
During winter greenhouse microgreen production, overnight lighting with low-level blue LEDs alone can also promote plant elongation while improving the appearance quality and crop yield, without negatively affecting nutritional quality. For mustard and arugula microgreens, overnight lighting with 14 µmol m
−2 s
−1 from blue LEDs promoted stem elongation by 16% and 10%, respectively, and increased crop yield by 32% and 29%, respectively, compared to no overnight lighting
[96][164]. Furthermore, blue LEDs increased the cotyledon area in mustard and the leaf mass unit area in arugula, and they enhanced the cotyledon color in both species, without affecting the total chlorophyll, carotenoid, and phenolic contents. However, overnight lighting with 14 µmol m
−2 s
−1 from FR LEDs did not have a positive effect on the above plant traits compared to blue LEDs
[96][164].