2. Nanomaterials and CO2 Absorption
With quick kinetics, absorption techniques produce remarkable CO
2 removal (90%) from gases. The most-used absorbents to achieve this removal include amines, ammonia–water solutions, and alkali compounds. Both pre- and post-combustion processes can integrate these technologies into their general procedure. Besides physical absorption, chemical-driven absorption processes have three components: absorber, solvent, and stripper. Different pieces of equipment were used to capture CO
2, i.e., spray columns, packed beds, rotating packed beds, bubble columns, and tray tower absorber layouts.
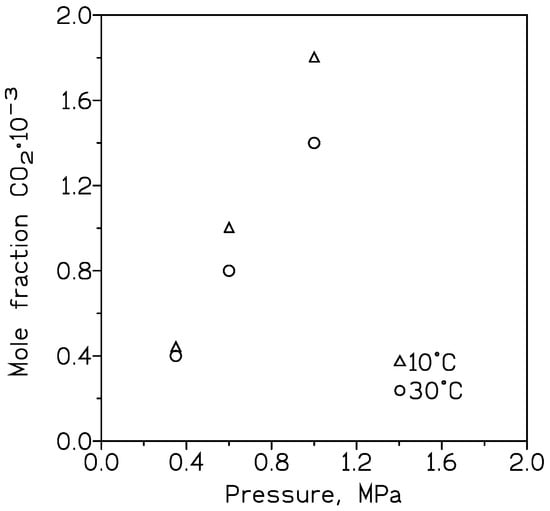
The absorption of CO
2 on Al
2O
3/MeOH
[24] was investigated, and the addition of a porous nickel metal foam increased CO
2 capture compared to pure MeOH; this increase was attributable to the forced bubble-breaking mechanism and the hydrodynamics in relation to the process. Moreover, there was a direct relation with the CO
2 absorption and the pressure and temperature used in the CO
2 capture process (
Figure 1). For a constant pressure, increasing the temperature decreased the gas capture, whereas for a constant temperature, increasing the pressure produced a greater removal of CO
2 from the gas stream. Also, an increase (0.01–0.1 wt%) in the Al
2O
3 content is accompanied by an increase in the capture of CO
2 with the alcohol.
Figure 1. Approximate CO2 removal in MeOH at various temperatures and pressures.
CO
2 capture by means of the addition of tetra-n-butyl ammonium bromide (TBAB) semiclathrate to graphite, forming a TBAB+graphite nanofluid, was investigated
[25]. The TBAB+graphite nanofluid system increased CO
2 removal with respect to the use of the TBAB or TBAB+sodium dodecyl sulfate (SDS) solutions, with 0.2 wt% graphite nanoparticles being the best concentration for hydrate growth in the TBAB+graphite nanofluid.
A metal oxide solid acid catalyst for the catalytic regeneration of a CO
2-rich 5 M monoethanolamine (MEA) solution at 90 °C was used
[26]. After the adsorption process and the formation of MEACOO
−, carbamate decomposition occurred:
This was followed by protonated amine deprotonation and the formation of MEA, H
3O
+, and H
2CO
3. The utilization of SO
42−/ZrTiO
x allowed for a 99% increase in the CO
2 desorption rate and a 43% enhancement after continuous cycles.
The performance of single-walled, carbon-deficient silicon carbide nanotubes (Si
12C
2−X; X = 1; 2) in CO
2 capture was investigated
[27]. The investigation concluded that Si
12C
11:Vc1 and Si
12C
10:Vc2 performed well for CO
2 removal and storage, surpassing the CO
2 capture efficiency of pristine SWSiCNT. The absorbent properties were due to the C vacancy effect of photoabsorption.
5,6-Dimethylbenzimidazole replaced 2-methylimidazole on the surface of ZIF-8 crystals via the shell–ligand exchange reaction (SLER)
[28], improving the thermal stability of ZIF-8. At 40 °C, CO
2 uptake using ZIF-8-SLER-PLs increased 30% with respect to that of ZIF-8-PLs. CO
2 was loaded onto the modified absorbent via a physical absorption process.
It was demonstrated
[29] that the use of nano-SiO
2, with varying particle size, in glycerol solution had little effect on CO
2 removal; this capture increased with the increase in solid mass in the 0.05–0.15 wt% range, which was attributed to a better gas–liquid mass transfer area. The use of SiO
2 increased CO
2 desorption as consequence of the presence of more nucleation sites and the heating rate of the base fluid, generating bubbles.
MFCs (MgFe
2O
4@ZIF-62) containing various magnetic nanoparticle dosages (1–6 wt% of magnetic nanoparticles to ZIF-62 mass) were used in conjunction with a compatible non-penetrating solvent to form a magnetic porous liquid
[30]. The use of this absorbent material allowed continuous CO
2 capture and release for up to three cycles.
Due to certain operational and economic considerations, there is a relatively urgent necessity to find alternatives to the use of amines for CO
2 capture
[31]; amino acid salts can be one green alternative to the use of these amines. Thus, potassium L-cysteine for CO
2 capture from natural gas was investigated. Its physicochemical properties were measured at different temperatures (25–60 °C) and salt concentrations (5–30 wt%). Experimental results, at 40 °C and 20 bar, showed an important increment of CO
2 loading, from 7 to 15 mmol CO
2/g amino acid, with the increase of 10–30 wt% in the solvent concentration. CO
2 loading was attributable to the following reaction:
while carbonate formation occurred via the following reaction:
By the use of 9.01 wt% tetra butyl ammonium bromide (TBAB) mixed with water-soluble hydroxylated multiwalled carbon nanotube (MWCNTol) material, the formation of CO
2 hydrate was investigated
[32]. It was concluded that MWCNTols had negligible influence on the CO
2 hydrate generation. The use of nanoparticles such as graphene nanoribbons and MWCNTols reduced the induction time, whereas addition of various nanoparticle dosages to the TBAB solution increased the final gas consumption, with a maximum increase of 10.44% in the 9.01 wt% TBAB + 0.08 wt% GN system.
Absorption and conversion of CO
2 by an amino-acid-based nanotechnology was described
[33]. CO
2 was captured as bicarbonate nanomaterials, whereas the amino acid structure governed the formation of bicarbonate nanomaterials. Amino acids presented higher CO
2 absorption capacity and faster kinetics compared to the use of 30 wt% monoethanolamine.
Gold nanoparticles were decorated with 1,5,7-triazabicyclo [4.4.0] dec-5-ene and dispersed into methanol in order to capture CO
2 [34][35]. The photocatalyst consisted of two parts: (i) an organic shell responsible for CO
2 capture, and (ii) a plasmon-active metal nanoparticle core for activation of captured CO
2 and its involvement in the cycloaddition reaction. Results showed the efficiency of the procedure even at the temperature of −40 °C.
The mixture formed by NaP zeolite nanocrystals and 1-dodecyl-3-methylimidazolium chloride ([C
12mim][Cl]) ionic liquid was used for CO
2 removal in an isothermal high-pressure cell equipped with magnetic stirring
[35][36]. Under various experimental conditions, it was found that 0.02 wt% of zeolite nanoparticles, 0.4 wt% of [C
12mim][Cl] ionic liquid, and 0.05 wt% of sodium dodecyl benzene sulfonate in nanofluids resulted in the highest CO
2 removal compared to other conditions. This CO
2 removal increased by increasing ionic liquid and surfactant concentration up to a limiting value near the critical micelle concentration.
Methyl-diethanolamine-based Fe
3O
4 improved CO
2 absorption compared to methyl-diethanolamine-based CuO, ZnO, and SiO
2, whereas CuO nanoparticles presented higher efficiency for CO
2 removal from gas-loaded absorbent
[36][37].
Using 2-methylimidazole zinc salt (ZIF-8) modified by tetraethylenepentamine (TEPA), which provided pores, and 1-ethyl-3-methylimidazolium bis(trifluoro-methanesulfonyl)-imide ([EMlm][NTf
2]) ionic liquid, used as a sterically hindered diluent, an amine-functionalized type III porous liquid was formed
[37][38]. It was found that with 30TEPA@ZIF-8 nanoparticles, the best CO
2 absorption capacity was obtained. Moreover, the CO
2 absorption loading of 0.124 mmol/g presented by 5-30TEPA@ZIF-8/[EMlm][NTf
2] was 4.43 times higher than the value obtained by the use of 5-0TEPA@ZIF-8/[EMlm][NTf
2], whereas a 745% increase of the absorption rate was reached.
A mixture of 26 mol% CO
2 and 74 mol% CH
4 was used to investigate the separation of both gases
[38][39]. Silica nanoparticles, in KOH medium, modifying the surface of (3-aminopropyl) teriethoxysilane (APTES) were utilized as additives. The best results were derived with silica and KOH-bearing nanofluids; with these components, improvements of 36% (gas consumption), 29% (separation factor), and 38% (recovery factor) resulted with respect to the use of pure water.
CO
2 geological sequestration by the use of silica aerogel nanofluid was investigated
[39][40]. Using this material, non-dissolved CO
2 molecules were captured in the nanopores of the silica aerogel nanoparticles, increasing the solubility of CO
2 in the aqueous phase. Aerogel nanoparticles adsorbed at the CO
2–brine interface reduced the interfacial tension.
3. Nanomaterials and CO2 Adsorption
Adsorption processing involves the use of a solid material on which CO
2 (and other gases and solutes) is captured by means of physical or chemical processes or a combination of both. Key parameters to yield the best adsorptive properties of the materials are: porosity, pore size, operational stability, presence of reactive groups towards CO
2 adsorption, etc., whereas the equipment used is usually described as a packed or fluidized bed.
The fabrication of carbon nanofibers (CNFs) via biaxial electrospinning was investigated
[40][41]. Polymethylmethacrylate (PMMA) and polyacrylonitrile (PAN) were used as core and shell precursors, respectively. Further, Co
3O
4 nanoparticles were included in the PAN shell, increasing its roughness and surface area. The uniform distribution of Co
3O
4 resulted in a better flexibility of the hollow carbon nanofiber material (HCNF-Co), providing more vacant oxygen sites to increase CO
2 adsorption loading. HCNF-Co nanofibers exhibited CO
2 capture uptake of 3.28 mmol/g at 25 °C. Experimental results indicated that HCNF-Co had remarkable CO
2 selectivity (S = 26) over N
2.
Heterojunctions of Co
3O
4 with different morphologies and modified carbon nitride (CN) were investigated in order to optimize their properties to degrade CO
2 under UV–visible irradiation
[41][42]. A solvothermal synthesis was used to fabricate the cobalt oxide from metal–organic framework structures, yielding ultrathin 2D Co
3O
4 nanosheets (Co
3O
4-NS). These nanosheets presented improved photocatalytic properties compared to those of the bulk Co
3O
4/CN composites. CO
2 reduction was improved due to (i) the match of the planar surface of CN and the 2D structure of Co
3O
4-NS, which resulted in a larger interface, and (ii) improvement in charge carrier lifetime.
The authors of
[42][43] described the utilization of 2D nanomaterial MXenes and activated carbon (AC) to form sandwich-type materials and nanocomposites for CO
2 adsorption using a fixed-bed column. These investigations included CO
2 breakthrough measurements at a fixed 15% CO
2 concentration, with an inlet flow rate at 200 mL/min and temperatures in the 25–55 °C range. The highest CO
2 adsorption load (near 9 mg/g) was yielded with AC/MXene sandwich adsorbent at 25 °C, which was nearly a 37% improvement in CO
2 adsorption capacity over the use of pristine AC. AC/MXene sandwich-type nanomaterials can be used, with a small loss of their CO
2 adsorption uptake, under various cyclic experiments.
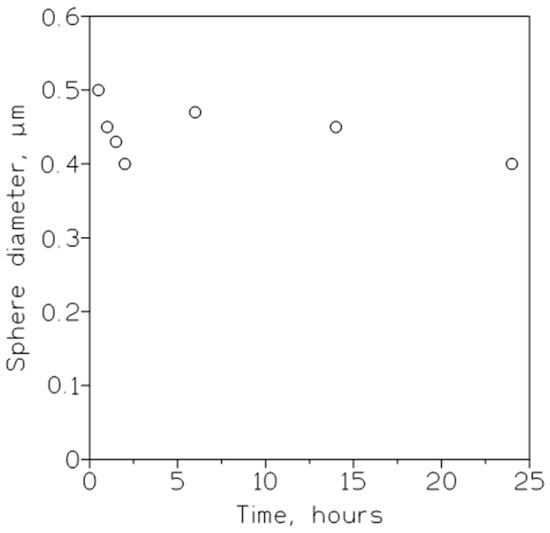
ZIF-8 hollow nanospheres, for selective CO
2 separation and storage, were developed
[43][44]. The optimum hollow ZIF-8 nanosphere material, with a uniform size distribution (
Figure 2), had a CO
2 adsorption uptake of 2.24 mmol/g at 0 °C and 1.75 bar, selective (12.15) CO
2/N
2 separation, 1.5–1.75 wt% CO
2 storage capacity, and a reasonable stability, up to four CO
2 adsorption/desorption cycles, at 25 °C.
Figure 2. Influence of synthesis time on the average diameter of soft template hollow ZIF-8 nanospheres. Surfactant/oil ratio: 75 g/L.
A heterogeneous catalyst comprising silver nanoparticles and a porous N-heterocyclic carbene polymer (Ag@POP-NL-3) was developed
[44][45]. This nanomaterial has a regular distribution of silver nanoparticles and nitrogen activation groups. The catalyst presented good properties for the selective adsorption and activation of CO
2, allowing the conversion, under mild conditions, of low CO
2 (30 vol%) concentrations, as presented in lime kiln waste gas, into cyclic carbonate. CO
2 was loaded onto the adsorbent by carboxylative cyclization of the gas with propargylic alcohols also present in the system.
The adsorption uptake of CO
2 on NaY@polyacrylate matrix was increased by 17.9% while H
2O adsorption uptake decreased by 36.6% compared to pristine NaY
[45][46]. In addition, H
2O adsorption was reduced by 54.8% after adding ZIF into composites.
The authors of
[46][47] described a maximum CO
2 loading (0.75 mmol/g) on triethylamine-doped rice husk silica nanoparticles, with an average increase in CO
2 adsorption with the increase (1 to 5 wt%) in the amine loading on the surface modifiers. Amine loadings greater than 5 wt% produced agglomeration of the particles which is detrimental with respect to CO
2 capture. CO
2 uptake corresponded to the Langmuir isotherm model.
The use of Zn-N pillar MOFs resulted in: CO
2 capture of 3.82 mmol/g (25 °C and 101 kPa), a selectivity CO
2/N
2 factor of 132, and stable structure (no change after exposure to 1000% RH environment for seven days)
[47][48].
The performance of graphene oxide (GO)-coated zinc tetraphenylporphyrin (ZnTPP/GO) nanocomposites in the photocatalytic degradation of CO
2 was investigated
[48][49]. The encapsulation of GO in ZnTPP nanocrystals promotes CO
2 adsorption, interfacial reaction, and stability and accelerates the separation of photoinduced carriers on ZnTPP (0.1 ps vs. 425.9 ps), the transportation from ZnTPP to GO (2.3 ps vs. 83.6 ps), and their final enrichment on GO.
A porous ZIF-11@ZIF-8 core–shell composite structure metal–organic framework was fabricated using the solvent-assisted linker exchange (SALE) procedure
[49][50]. Adsorptions at 25 °C and equilibrium pressures up to 4 bar showed an increase (near 100%) in CO
2 adsorption uptake of ZIF-11@ZIF-8 nanoparticles (8.21 mmol/g) compared to the pristine ZIF-11 (4.35 mmol/g). Experimental results on gas uptake fitted well with the Langmuir isotherm equation. CO
2/N
2 and CO
2/CH
4 selectivities also increased by 131% and 92%, respectively.
Activated carbon (AC) was synthesized from date fruit seeds and chemically activated with KOH to improve CO
2 loading
[50][51]. From thermogravimetric analyses, 94% and 67% higher average CO
2 capture loads were measured for KOH-promoted ACs compared to the original adsorbents. The activated carbon improved its fluidization by the use of hydrophobic silica nanoparticles (NPs). The SiO
2-decorated (2.5 wt%) modified ACs had a 45% higher bed expansion ratio, which was associated with the absence of bubbles and a homogeneous fluidized regime.
Mesoporous CeO
2, ZrO
2, and Ce-Zr composite nanoparticles with a large surface area were fabricated using the hydrothermal template-assisted synthesis procedure, and CO
2 adsorption properties of these materials were investigated under equilibrium and dynamic operations
[51][52]. Better CO
2 adsorption was yielded for Ce-Zr nanomaterial due to the presence of strong O
2− base sites and many surface oxygen species. After five adsorption/desorption cycles, the composites presented a reasonable stability with a slight decrease in CO
2 adsorption uptakes in dry flow and in the presence of water vapor.
Treatment via surface N
2 plasma of zinc porphyrin (ZnTCPP) ultrathin nanosheets induced nitrogen vacancies (NVs) and resulted in a material with photocatalytic CO
2 reduction activity and selectivity
[52][53]. It was shown that the photocatalytic activity of NVs-ZnTCPP can be attributed to nitrogen-vacancy-induced spin polarization by reducing the reaction barriers and inhibiting the recombination of photoexcited carriers.
It was reported
[53][54] that CO
2 uptake (11.8 mmol/g (78% total adsorption)) after four cycles on a MOF-derived nano-CaO (average size of 100 nm) was due to high stability produced in the final material by the change in the original fiber-bundle-like MOF structure to nanosheets, and further to regular CaO spheres. CO
2 uptake onto the adsorbent corresponded to the following equation:
Lewis base and dual hydrogen bond donor (HBD) units were integrated into an organosilicon precursor, and triazine and hydrazo site co-modified periodic mesoporous organosilicas (THPMOs) were prepared via a hydrothermal self-assembly method
[54][55]. The THPMOs had BET surface areas in the 699–876 m
2/g range and low-pressure CO
2 adsorption loadings at 0 °C. If combined with tetrabutylammonium iodide (TBAI) ionic liquid, the mixture promoted the model cycloaddition of CO
2 in an effective form, with the gas fixed to epoxides.
In
[55][56], nano-TiO
2 was added to cement pastes to investigate its performance regarding the CO
2 uptake rate. Prismatic samples with dimensions of 16 × 4 × 4 cm of 0.5 water/binder cement paste with and without nano-TiO
2 particles were used. CO
2 uptakes showed that nano-TiO
2 addition improves the CO
2 uptake rate of cement pastes, changing the pore structure and allowing the removal of more CO
2 at lower gas concentrations. CO
2 loaded similarly to Equation (4), but Ca(OH)
2 reacted with the gas to form CaCO
3 and water.
MIL-101(Cr)-NH
2 has higher CO
2 adsorption capacity than MIL-101(Fe)-NH
2 [56][57], whereas the adsorption of methane and nitrogen by MIL-101(Cr)-NH
2 is lower than the adsorption of these gases by MIL-101(Fe)-NH
2, leading to a higher selectivity of CO
2 over the two gases for MIL-101(Cr)-NH
2. At elevated temperature and pressure, the chemisorption mechanism is predominant, which is attributable to the performance of amines, which adsorbed more CO
2 at these higher temperatures and pressure. Gas adsorption was explained by the use of a hybrid equation between Langmuir and Khan models:
where q
s, Q, and n represent the parameters of the model, with P being the pressure of the vapor phase at the equilibrium.