You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Li Lin and Version 2 by Camila Xu.
Schizophrenia is a serious and debilitating neurodevelopmental disorder that typically occurs in early adulthood. DNA methylation, a critical epigenetic modification, contributes to alter gene expression without affecting the underlying genomic sequences; 5-methycytosine (5mC) and 5-hydroxylcytosine (5hmC) are two major forms of DNA methylation in mammals.
- schizophrenia
- epigenetic
- DNA methylation
- 5mC
- 5hmC
1. Introduction
Schizophrenia is a serious and debilitating neurodevelopmental disorder that typically occurs in early adulthood. Patients experience hallucinations, delusions, disorganization, and deterioration in cognitive and social functioning, such as amotivation and social withdrawal [1]. The average lifetime prevalence of schizophrenia is just under 1% [2]. Individuals from all ages with schizophrenia have high mortality and have their life expectancy reduced by approximately 20 years compared with the general population [3]. Alterations in cortical circuits have been reported to be involved in the pathogenesis of schizophrenia. The loss of dendritic spines in pyramidal cells results in reduced excitatory activity, while, at the same time, reduced excitatory input to GABAergic interneurons results in reduced inhibition in pyramidal cells. These changes are thought to lead to an abnormal functional network [4].
The heritability of schizophrenia is as high as 80% [5]. Genetic factors, including copy number variants [6] and de novo mutations [7], have been identified to be closely associated with the development of schizophrenia. Interestingly, studies of monozygotic twins have revealed the heterogeneity of schizophrenia, suggesting that non-genetic factors may be involved in the pathogenesis of schizophrenia. Environmental factors can induce enduring changes in gene expression through epigenetic mechanisms in neurodevelopment processes, aging, and neurodegenerative diseases [8]. DNA methylation, a critical epigenetic modification, contributes to alter gene expression without affecting the underlying genomic sequences; 5-methycytosine (5mC) and 5-hydroxylcytosine (5hmC) are two major forms of DNA methylation in mammals. DNA methyltransferases (DNMTs) transfer a methyl group to the 5-position of cytosine to form 5mC; the ten-eleven translocation (TET) dioxygenases catalyze the 5mC to 5hmC, 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC) on DNA.
Altering the expression or activity of DNA methylation-related enzymes produce exacerbated phenotypes or therapeutic effects in different animal models. Cathrin Bayer et al. found that the depletion of DNMT1 increased the survival of mutant Huntington-transfected cells and accelerated perinuclear Huntington aggregation, suggesting that DNMT1-dependent degradation pathways mediate mutant Huntington-induced cytotoxicity [9]. Growing evidence suggests that epigenetic responses to environmental stimuli and plays an important role in schizophrenia [10]. By mapping thousands of 5mC sites in the prefrontal cortex of schizophrenia patients and healthy controls, differentially methylated regions (DMRs) at genes related to development and neurodifferentiation have been identified [11]. Disruptions of genome-wide 5mC have been observed in peripheral blood mononuclear cells (PBMCs) of schizophrenia patients, particularly in those experiencing their first episode of disease [12]. Despite inconsistent results from genome-wide association studies (GWAS), 108 replicable genomic loci have been identified in 2014 [6].
2. Altered 5mC Pattern in Schizophrenia
It is known that 5mC is the primary product of DNA methylation, which can influence various processes such as gene activity, individual development, and cancer progression [13][14][13,14]. DNMT1, DNMT3A, and DNMT3B possess catalytic activity and covalently add a methyl group from S-adenosylmethionine (SAM) onto the fifth carbon of the cytosine (C) pyrimidine ring, resulting in the formation of 5mC. While DNMT2 and DNMT3L contain conserved sequences of DNMT enzymes, they lack catalytic activity [15][16][15,16]. During late embryonic development and after maturation, genomic methylation patterns are established, and DNMT1 is responsible for maintaining the genomic methylation status. The DNMT1 is approximately one hundred times more active than DNMT3A and DNMT3B [17]. Abnormalities of 5mC have been widely reported in neurodegenerative diseases, such as Parkinson’s disease (PD) and Huntington’s disease (HD) [18][19][20][18,19,20]. Psychiatric disorders are characterized by impairment in cognition, emotion regulation, or behavior, and typically include bipolar disorder (BD) and schizophrenia. The pathogenesis of BD involves mitochondrial dysfunction, DNA oxidative damage, and DNA methylation changes. BD patients and their relatives exhibit genome-wide decreases in 5mC levels. After lithium treatment, 5mC levels remained unchanged in BD patients, whereas the levels were increased in their relatives, providing evidence of the significance of 5mC modification in BD pathology [21][22][23][21,22,23]. The overexpression of DNMT1 was considered to be one of the etiological factors of schizophrenia. Transcriptional dysregulation has been observed in embryonic stem cells overexpressing DNMT1. Approximately 50% of these genes have been implicated in schizophrenia, showing dysregulation independent of DNA methylation [24][25][24,25]. A novel GWAS in patients with schizophrenia identified schizophrenia-associated differential 5mC at 242 sites, of which mitotic arrest deficient 1-like 1 (MAD1L1) was robustly differentially methylated [26]. Alongside GWAS, a growing number of studies aim to identify the 5mC of candidate genes in patients with schizophrenia [27]. Specific 5mC differences have also been found in the gray and white matter of individuals with schizophrenia. These DMRs were identified within or near the Kruppel-like factor 9 (KLF9), sideroflexin 1 (SFXN1), Sprouty-related EVH1 domain-containing 2 (SPRED2), and AlS2 C-terminal-like (ALS2CL) genes [28]. Other key genes with dynamic 5mC were also involved in the pathogenesis of schizophrenia (Table 1).Table 1.
Association of 5mC with schizophrenia.
| DMRs | Tissues | Expression | 5mC | Phenotypes | References |
|---|---|---|---|---|---|
| KLF9 | Human cortical grey and white matter | ↓ | ↑ | rs11142387 near the KLF9 was significantly associated with psychiatric disease and poor memory function. | [28] |
| SFXN1 | Human cortical grey and white matter | ↓ | ↑ | The loss of SPRED2 leads to defective glycine and purine synthesis. | [28] |
| SPRED2 | Human cortical grey and white matter | ↓ | ↑ | The loss of SPRED2 leads to a phenotype resembling recessive Noonan syndrome. | [28] |
| ALS2CL | Human cortical grey and white matter | ↑ | ↓ | Mutations in ALS2CL may contribute to the development of schizophrenia. | [28] |
| RELN | Human peripheral blood | ↓ | ↑ | Single-allele and biallelic mutations in RELN can lead to neurodevelopmental disorders. The dysregulation of RELN expression has been observed in patients with schizophrenia and bipolar disorder. | [29][30][29,30] |
| BDNF | Human peripheral blood | ↓ | ↑ | BDNF activates the tyrosine kinase receptor B (TrkB), triggering various downstream signaling pathways. In patients with schizophrenia, there are alterations in BDNF signaling transduction. | [31] |
| SLC6A3 | Isohelix swab pack | ↓ | ↑ | SLC6A3 is associated with several neurological and psychiatric disorders, including ADHD, autism, cognitive impairments, movement disorders, and schizophrenia. | [32][33][32,33] |
| DTNBP1 | Human brain | ↓ | ↑ | The aberrant expression of DTNBP1B is associated with cognitive deficits in schizophrenia. | [34][35][36][34,35,36] |
| GAD1 | Human | ↓ | ↑ | The GAD1-knockout mouse model exhibits impairments in spatial memory and working memory. It shows reduced locomotor activity in new environments and a decreased preference for novel stimuli. | [37] |
| COMT | Human peripheral blood | ↑ | ↑ | The deletion of the COMT gene can lead to a range of complex complications, with psychiatric symptoms manifesting as schizophrenia and other mental disorders. | [38] |
↑: upregulation; ↓: downregulation.
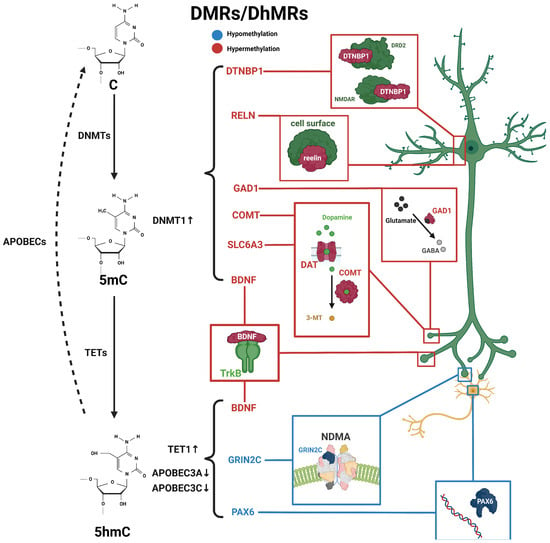
Figure 1. Schematic diagram of dynamic 5mC and 5hmC in neurons of schizophrenia patients. 5mC is catalyzed by DNMTs from cytosine and is then hydroxylated to 5hmC by TET enzymes, and further conversed into cytosine through the participation of apolipoprotein B mRNA-editing enzymes (APOBEC). Alterations in 5mC and 5hmC patterns have been identified in patients with schizophrenia. BDNF processes both 5mC and 5hmC dynamics. These distinctive patterns of 5mC and 5hmC are implicated in the pathogenesis and development of schizophrenia. Blue, hypomethylation; red, hypermethylation.
3. Altered 5hmC Pattern in Schizophrenia
In as early as 1952, 5hmC was discovered in bacteriophage DNA [66] and was first detected in mammalian genomes in 1972 [67]. As of 2009, the TET family has been identified to convert 5mC to 5hmC both in vivo and in vitro [68][69][68,69]. The TET protein family includes three members, TET1, TET2, and TET3, which have a conserved double-stranded β-helix domain (DSBH) at their C-terminus, which contains α-ketoglutarate (α-KG)- and Fe(II)-binding sites. Additionally, there is a cysteine-rich domain (CD) that contains a high concentration of cysteine residues. Together, these two domains form the core catalytic domain, which mediates the oxidation reaction of 5mC to 5hmC [70]. The distribution of 5hmC in animal tissues is widespread but variable, displaying certain tissue specificity [71][72][73][71,72,73]. It has been found, through 5hmC profiling in mammals, that 5hmC is mainly enriched in the central nervous system (CNS), with lower levels in peripheral tissues, which is in sharp contrast to the global distribution of 5mC [73][74][73,74]. In the brain, 5hmC exhibits region-specific distribution, with a unique distribution pattern in the cerebellum [75][76][75,76]. This suggests that 5hmC may play an important role in tissue-specific functions. Furthermore, 5hmC levels increase during neuronal differentiation and are associated with genes critical for neuronal function during neurodevelopment [77]. In addition, 5hmC is involved in processes of cell development and differentiation, the regulation of chromatin structure, and bone marrow regeneration [78][79][80][78,79,80]. In the process of DNA methylation regulation, the epigenetic plasticity of the CNS affects the expression of neuroactivity-dependent genes, organism learning, and memory [81][82][81,82]. It is well-known that 5mC is considered a stable heritable modification and represents a static process in epigenetic regulation. In fact, the dynamic balance between 5mC and 5hmC is an important condition for organism homeostasis, and imbalances in their ratio are implicated in the pathogenesis of many neurodegenerative diseases [78][79][83][84][78,79,83,84]. Abnormalities in 5hmC have been reported to be widespread in patients and animal models of AD and PD. At the same time, it was found that dynamic changes in 5hmC were positively correlated with aging [76][80][76,80]. Genome-wide DNA methylation changes may lead to genomic instability, and DNA methylation changes at promoter regions typically affect gene transcription. In schizophrenia, genome-wide 5hmC levels were elevated in male patients and reduced in female patients compared with healthy individuals [10], suggesting a potential association between the dysregulation of 5hmC levels and the development of schizophrenia. Many candidate genes in the peripheral blood or postmortem brain from schizophrenia patients have been identified with the differential methylation status associated with schizophrenia [11][85][11,85], including nitric oxide synthase 1 (NOS1), AKT serine/threonine kinase 1 (AKT1), DTNBP1, DNMT1, protein phosphatase 3 catalytic subunit gamma (PPP3CC), glutamic acid decarboxylase 67 (GAD67), and sex-determining region Y-box containing gene 10 (SOX10) [86][87][86,87]. These genes exhibit varying degrees of changes in 5mC or 5hmC (Figure 1 and Table 2).Table 2.
Association of 5hmC with schizophrenia.
| DhMRs | Tissues | Expression | 5hmC | Phenotypes | References |
|---|---|---|---|---|---|
| GABRB2 | Human, peripheral white blood cells | ↓ | ↑ | Gabrb2-knockout mice exhibit anxiety-like and depression-like behavioral changes, as well as alterations in social behavior, learning, and memory abilities. | [85] |
| GAD67 | Human, parietal cortex | ↓ | ↑ | GAD67-knockout mice exhibit emotional and auditory abnormalities, as well as anxiety-like behavior. | [87][87[88],88] |
| APOBEC3A/C | Human, parietal cortex and prefrontal cortex | ↓ | ↑ | The deletion of APOBEC3A has been associated with an increased susceptibility to early-onset breast cancer. | [87] |
| GADD45b | ↑ | ↑ | The knockdown of Gadd45b in the amygdala of neonatal rats leads to changes in social behavior during adolescence and a decrease in the expression of several genes associated with psychiatric disorders, including MeCP2, Reelin, and BDNF. | [89] | |
| BDNF IX | ↓ | ↑ | BDNF knockout mice exhibit chronic liver disease, specifically non-alcoholic fatty liver disease (NAFLD). | [89] | |
| GRIN2C | Monkey, cerebellum | ↓ | - | The knockdown of PAX6 in differentiating human limbal epithelial cells leads to a decrease in the expression of FABP5 and DSG1 proteins. | unpublished data |
| PAX6 | ↑ | - | unpublished data |
↑: upregulation; ↓: downregulation.
