Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Michael Werner and Version 2 by Jessie Wu.
The nasoseptal flap (NSF) has become a workhorse reconstructive option in the endonasal repair of anterior skull base defects. The flap is pedicled on the posterior septal nasal artery, which branches off the sphenopalatine artery and courses along the posterior nasal cavity and inferior sphenoid face. Due to its versatile nature and customizability, the NSF can be utilized for a range of different defects extending from the frontal recess to the low clivus in the sagittal plane.
- expanded endoscopic endonasal surgery
- anterior skull base reconstruction
- nasoseptal flap
- pedicled flaps
1. Impact of Nasoseptal Flap Closure on Postoperative Cerebrospinal SFluid Leaks
Since the original nasoseptal flap (NSF)al NSF paper, several studies have demonstrated a decline in postoperative cerebrospinal fluid (CSF)CSF leaks when the NSF is used in comparison to traditional grafting techniques. In one study of pituitary skull base surgery, the authors found that the rate of CSF leak in NSF patients was 3% in comparison to 12.5% for those closed with grafts [1][20]. Likewise, a meta-analysis of post-surgery CSF fluid leaks after skull base meningioma resection reported a decrease from 22% in 2004 to 4% in 2020, which coincides with the widespread adoption of the NSF [2][21]. When comparing graft reconstruction and the NSF, a review article published in 2012 showed that the leak rate for free grafts was 15.6% in comparison to a 6.7% leak rate for NSFs [3][22]. In cases of obesity, using a NSF significantly decreased the risk of a CSF leak (from 29.6% to 15.0%), while the difference between using a NSF and a free graft was not significant for patients in a healthy weight category [4][23]. Another study found that there was an overall success rate of 91.5% when using NSFs but when stratified by location, the differences in leak rates between grafts and NSFs were only significant in the clivus [5][24].
Several studies have addressed the possible risk factors associated with postoperative CSF leaks after NSF reconstruction (Table 1). In a review of 98 patients, CSF leak incidence was reported as 11%, which was within the expected 5–20% rate reported elsewhere [6][5]. Abnormal BMI (>25 or <18.5) was the most significant risk factor associated with both CSF leaks and meningitis [4][23]. Increasing age was also associated with CSF leaks, as was the duration of lumbar drain for meningitis [6][5]. The mechanism behind a high BMI and increased CSF leak risk is thought to be related to intracranial pressure [7][25]. Intraoperative CSF leaks and combined endonasal and open craniofacial resections were also risk factors for postoperative leaks [6][5].
Table 1.
Risk factors for postoperative CSF leak.
| After endonasal endoscopic skull base surgery [4][23] |
| Longer length of stay |
| Staged procedure |
| Preoperative hydrocephalus |
| Closure with graft (instead of vascularized flap) |
| Prior endoscopic skull base surgery [8][26] |
| Prior radiotherapy [8][26] |
| After closure with nasoseptal flap [6][5] |
| Abnormal BMI (<18.5 or >25) |
| Age > 65 |
| Intraoperative CSF leak |
| Complex closure with combined open craniofacial approach |
| Surgeon experience [9][1] |
Perhaps the most important factor in a postoperative CSF leak after using the NSF is the experience of the surgeon. In a series of 225 consecutive patients in which the NSF was used to repair anterior skull base defects by a single surgeon, the postoperative CSF leak rate was 24% in the first 25 patients and 4% in the last 200 [9][1]. This highlights the nuance that goes into pre-surgical planning and decision-making about when to implement the nasoseptal flap, as well as intraoperative considerations that mitigate the risk of CSF leaks. Pre-operatively, the most important factor is whether the NSF pedicle may be compromised due to prior surgery or tumor involvement. When the NSF is available, its arc of rotation and size make it a universal reconstructive option regardless of defect size, location, and CSF output. Nevertheless, its success depends on careful dissection without perforating or tearing the mucosa and with particular attention to preserving the vascular bundle and blood supply. This includes careful elevation of the flap inferior to superior and anterior to posterior. Scarring from prior septal surgery, pre-existing perforations, and large septal spurs are the predominant obstacles. In planning the incision, some surgeons opt to map out the arterial supply using a Doppler probe. Postoperatively, most surgeons prefer to pack the nose using a combination of absorbable nasal packing and stents that secure the flap in place. This is preferred over balloons which may exceed the intravascular pressure of the flap, resulting in flap necrosis [10][11]. NSF necrosis is a relatively rare complication that could result in CSF leaks, epidural empyema, or meningitis. Risk factors include prior surgery which may predispose an individual to poor vascular supply and the use of a fat graft as part of the multilayered closure, which may reflect the need for additional support intraoperatively for a flap found to be less robust. A lack of flap enhancement on a postoperative MRI is an indication that the flap may be necrotic [11][27]. In cases of flap failure resulting in postoperative CSF leaks, the cause was determined to be flap displacement, partial dehiscence of the flap rim, or hematoma formation compromising the flap [12][28].
2. Indications, Modifications, and Postoperative Considerations
The nasoseptal flap is the default reconstructive option for most anterior skull base defects. Several modifications to the original design have been proposed for when additional tissue or reach is needed. In this section, reswearchers will highlight some of these variations. Though the flap is very versatile with a robust arc of rotation and the ability to include a large mucosal surface, it has limited reach superiorly toward the posterior nasal wall of the frontal sinus, laterally toward the orbits, and inferiorly beyond the upper two thirds of the clivus [13][29] (Figure 1). Cadaver studies have shown that total sphenoidotomy reduces the mean length of the nasoseptal flap by increasing the distance to the posterior wall of the frontal sinus [14][30]. Therefore, several modifications have been proposed to extend its reach.
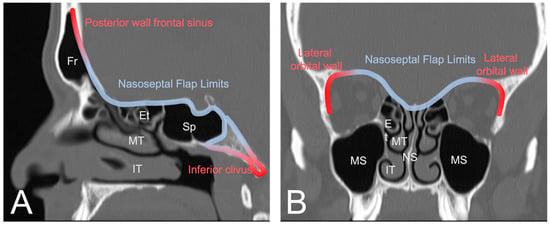
Figure 1. (A) Sagittal view of CT sinus without contrast through frontal sinus outflow tract demonstrating the general reach of the nasoseptal flap (blue). The posterior wall of the frontal sinus and the lower third of the clivus are typically too distal for the conventional nasoseptal flap (red). (B) Coronal view demonstrating lateral reach covering the ethmoidal roof and medial bony orbit (blue) with distal limits at the lateral orbital wall (red). Image from Radiopaedia.org. Fr: Frontal sinus; Et: Ethmoid air cells; Sp: Sphenoid sinus; MT: middle turbinate; IT: inferior turbinate; MS: maxillary sinus; NS: nasal septum.
To facilitate the coverage of anatomic areas such as the posterior table of the frontal sinus and inferior third of the clivus, several modifications have been proposed to extend the reach of the NSF. The vascular pedicle can be released laterally toward the sphenopalatine foramen with removal of overlying bone and extension of the inferior mucosal incision laterally across the choana above the eustachian tube to the medial pterygoid plate. Sacrifice of the vidian neurovascular bundle allows exposure for drill-out of the base of the pterygoid bone, posterior to the greater palatine canal [15][31]. Exposure of the pterygoid fossa through a maxillary antrostomy and subsequent drilling of the orbital, vertical, and sphenoid processes of the palatine bone may allow for the flap to extend further in the ventral direction. This technique has the added advantage of allowing additional nasal mucosa to be harvested to greatly expand the breadth of the flap [16][17][32,33].
Additional reach can also be achieved by making additional mucosal cuts. A releasing incision along the maxillary crest from the posterior edge to the middle of the flap has been shown to increase the surface area of the flap (Figure 2A) [18][34]. To reach more laterally for the reconstruction of medial orbital wall defects, the anterior incision can be extended superiorly and outward laterally in a circumferential manner down the lateral nasal wall toward the inferior turbinate, allowing the mucosa of the entire ipsilateral septum and nasal floor to be included (Figure 24B) [19][35]. Alternatively, the NSF can be combined with the inferior turbinate flap (Figure 24C). The robust dual vascular supply from the posterior septal and lateral nasal arteries allows this large mucosal flap to be advanced over large defects [20][36].
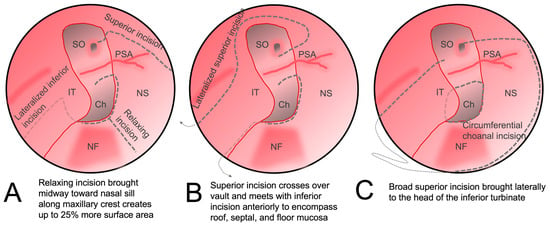
Figure 2. Alterations to the original nasoseptal flap design to increase coverage. (A) The inferior incision is lateralized to the nasal wall and third incision is made along the maxillary crest which augments the final surface area of the flap. (B) The superior incision is carried laterally superior to the sphenoid ostium and brought anteriorly along the inferior turbinate and then swung circumferentially across the nasal roof to meet with the inferior incision on the anterior septum. (C) The mucosa around the choana is released and then a superior incision is brought across the nasal floor to the head of the inferior turbinate to incorporate an inferior turbinate flap. IT: inferior turbinate; NF: nasal floor; NS: nasal septum; Ch: choana; PSA: posterior septal artery; SO: sphenoid os.
Another option is to suture a free mucosal graft from the contralateral nasal cavity to the NSF [21][37].
In high-flow CSF leak repair, the NSF is often used as the vascularized portion of a multi-layer closure. In a series of 38 patients with large skull base defects, a fascia “button graft”, which is a single inlay/onlay graft created by suturing two pieces of autologous fascia lata together in the middle [22][38], was used for primary dural repair, and then covered with a NSF. This potentially avoided the need for a lumbar drain for CSF diversion, and no postoperative CSF leaks or cases of meningitis occurred [23][39]. The role of lumbar drains after reconstruction using NSFs remained undefined until recently, with some surgeons believing that the NSF avoids the need for a lumbar drain and allows for earlier ambulation and a reduced risk of meningitis [24][40]. However, in a recent randomized control trial in patients with high-flow anterior skull base defects, lumbar drains were shown to reduce the postoperative CSF leak rate from 21.2% in the control group to 8.2% in the lumbar drain group, the majority of which were closed using a NSF [25][2]. Importantly, there was no increased risk of meningitis. This was an important study providing level 1 evidence supporting the use of postoperative lumbar drains when high-flow leaks are encountered surgically, even when the NSF is used for the defect repair.
The NSF has also been shown to be useful in pediatric endoscopic skull base surgery. The theoretical concern in this population is that cranial growth may exceed facial growth; thus, the size of the NSF may be limited relative to the size of the skull base defect [26][41]. However, the NSF was successfully used in a series of 55 patients and reduced the CSF leak rate from 12.5% to 8.9% [27][42]. Another group reported 12 pediatric cases (age range 1-17 years old) with both benign and malignant anterior skull base pathologies, for which the NSF was used for reconstruction and only one postoperative CSF leak was reported. Importantly, there was no evidence of altered craniofacial growth during the follow-up period in these patients [28][43]. Partial middle turbinectomy may help with exposure of the NSF pedicle in younger patients with small cavities. The NSF was also used to successfully treat a frontonasal meningoencephalocele defect in the anterior skull base that had already failed four prior transcranial attempts at closure [29][44].
The indication for NSFs in endoscopic endonasal surgery has also expanded beyond their traditional use in covering skull base defects. They have been successfully deployed in the marsupialization of rathke cleft cysts, in which the floor of the cavity is lined with the NSF to create a drainage pathway for cyst contents [30][45]. Likewise, a similar method has been used for obliterating recurrent cholesterol granulomas in the petrous bone [31][46].
As discussed above, lumbar drains are an important consideration when high-flow CSF leaks are encountered, even when the NSF is used to close the defect. Other postoperative considerations include the use of antibiotics with central nervous system penetration to reduce the risk of meningitis and anti-staphylococcal coverage when non-absorbable nasal packing such as Merocel sponges (Medtronic Inc., Minneapolis, MN, USA) are placed. Stool softeners, antitussives, antiemetics, and guidelines for avoiding Valsalva maneuvers, nasal blowing, and strenuous activity should also be provided to patients [24][32][40,47]. To address nasal crusting, nasal saline is often started 1–3 days after surgery, and in-office debridement under endoscopic visualization is often scheduled to start two weeks after surgery and continued on a weekly or biweekly basis until the crusting has resolved. However, there are no comparative studies or consensus guidelines for this practice after NSF reconstruction, and most postoperative management is often institution- or surgeon-dependent.
