1. Introduction
Drawing inspiration from nature’s super-liquid-repellent phenomena, such as lotus leaves and moth eyes, superhydrophobic surfaces possess exceptional water-repellent properties. When water droplets are deposited on these surfaces, they exhibit almost perfect spherical shapes due to the high receding contact angle, typically above 150°, and a sliding or rolling-off angle below 10°
[1,2,3][1][2][3]. The superhydrophobicity of these surfaces is primarily attributed to the combination of micro/nano/hierarchical physical structures and low-surface energy materials. This synergistic integration enables water droplets to establish a
Cassie–Baxter contact state, wherein an air layer forms between the droplet and the solid substrate. This unique wetting status ensures that the droplets maintain their spherical morphology while preventing the liquid from wetting the rough protrusions
[4,5][4][5]. Thus, retaining the
Cassie–Baxter contact with an air layer is crucial for mechanical durability and operational stability when fabricating superhydrophobic surfaces.
Due to their low liquid adhesion and the extremely small contact area between water droplets and the solid substrate, superhydrophobic surfaces hold significant potential in diverse fields, including antifouling
[6[6][7][8][9],
7,8,9], oil–water separation
[10[10][11][12][13][14][15],
11,12,13,14,15], self-cleaning
[16[16][17][18],
17,18], and anti-corrosion applications
[19[19][20],
20], among others
[21,22,23,24,25,26,27][21][22][23][24][25][26][27]. Researchers have already explored various strategies to achieve superhydrophobic surfaces
[28,29,30,31,32,33,34,35][28][29][30][31][32][33][34][35]. However, significant limitations for practical applications still exist due to cost-intensive manufacturing processes and the durability drawbacks associated with rigid and fragile inorganic micro/nano/hierarchical structures
[36,37,38,39][36][37][38][39]. Consequently, the development of superhydrophobic surfaces with enhanced durability is a significant challenge.
In contrast to rigid and brittle inorganic materials, soft materials, such as polymers, rubbers, and elastomers, can maintain their properties after experiencing mechanical deformations such as bending, pressing, and stretching. This distinctive feature offers an opportunity to create soft superhydrophobic surfaces with greatly improved durability. Based on this, stretchable superhydrophobic surfaces have emerged in recent years
[40,41,42,43,44,45,46][40][41][42][43][44][45][46]. The primary objective is to extend the lifetime of super hydrophobic surfaces in practical applications and expand their use in wearable electronics, strain sensors, and other flexible devices
[47,48,49,50,51][47][48][49][50][51]. The fundamental principle behind developing stretchable surfaces involves incorporating soft materials into fabrication. The incorporation imparts flexibility to the superhydrophobic surfaces, enabling them to withstand mechanical deformations and be applied on curved or irregular surfaces, thereby widening their range of applications. The incorporation of soft materials also facilitates the recovery of intrinsic hydrophobicity after mechanical deformation, ensuring that the micro/nanostructures revert to their rough surface topography. This, in turn, bestows the superhydrophobic surfaces with improved durability and sustainability.
The development of more durable and stretchable superhydrophobic surfaces has opened up various potential applications. The following sections provide an overview of some representative applications of stretchable superhydrophobic surfaces.
2. Strain Sensing
Strain sensing stands out as a widely embraced application for stretchable superhydrophobic surfaces. Over the last decade, scientists have dedicated significant efforts to creating diverse superhydrophobic surfaces that exhibit stretchability and ingeniously combined them with other functional materials and electronic components to construct innovative strain sensors suitable for a range of applications, including human activity monitoring, electronics, and robotics, etc.
[89,90,91,92,93][52][53][54][55][56]. This session will present some illustrative examples to explain how stretchable superhydrophobic surfaces work in strain sensing.
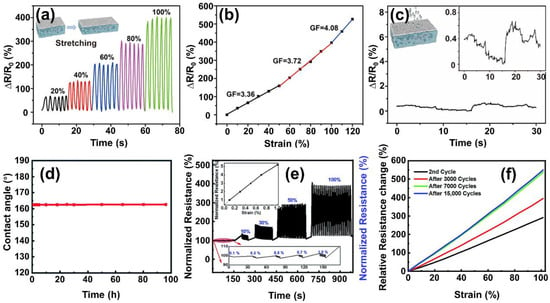
Recently, Wang et al. introduced a wearable strain sensor with an anti-liquid-interference feature, relying on a composite hydrogel that combines superhydrophobicity and conductivity
[94][57]. The sensor showcases a dual-layered design incorporating a conductive composite hydrogel. The arrangement encompasses an exterior layer composed of a composite film of silicone elastomer (Ecoflex) and silica microparticles, as well as an inner layer consisting of a polymer network of P(AAM-co-HEMA)-MXene-Ag hydrogel. The Ecoflex/silica microparticle composite film imparts superhydrophobic functionality to the wearable strain sensor, ensuring liquid-repellency stability during stretching and bending. The contact angle of water droplets on the sensor surface is around 153°, confirming the superhydrophobic feature of the Ecoflex/silica composite surface. The combined sensor demonstrates stretchability, leveraging the elasticity of both elastomer and hydrogel components. Superhydrophobic properties remain intact during stretching, with the water-contact angle consistently exceeding 150° across a strain range (ε) of 0–100%. Through monitoring human bodily movements and joint actions, the strain sensors exhibit a notable relative resistance change ∆R/R
0 (%) = (R − R
0)/(R
0 × 100%) (R
0 and R are the initial resistance and the real-time electrical, respectively) that escalates from 66% to 396% as strain increases from 20% to 100% (
Figure 1a). Different stretching states result in distinct gauge factors (GF): 3.36, 3.72, and 4.08 for strains of 0–60%, 60–100%, and 100–120%, respectively (
Figure 1b). The strain sensor’s resistance response at a tensile strain of 100% was also evaluated underwater. The outcomes indicate no divergence between resistance changes in water and air due to the sensor’s superhydrophobic outer layer that repels water interference. Furthermore, the sensor’s liquid repellency effectively shields against the influence of water droplets and flow. This protective feature safeguards the sensor from the impact of water droplets or flows while monitoring subtle human movements (
Figure 1c) such as speech, facial expressions, pulse, etc.
Figure 1. (
a) Resistance variation (ΔR/R
0) of the multifunctional wearable strain sensor with strains of 20%, 40%, 60%, 80%, and 100%; (
b) gauge factors of the multifunctional wearable strain sensor at different strain regions; (
c) change in resistance when the wearable sensor with superhydrophobic film was interfered with by the continuous water droplets, intermittent water droplets, and water flow
[94][57]; (
d) time-course evaluation of the contact angle on the water-repellent sensor; (
e) normalized resistance changes with increasing strain; and (
f) relative resistance changes with strain after particular cycles during cycling stability testing of the sensor
[95][58].
Kaneko et al. developed a superhydrophobic strain sensor capable of monitoring structural integrity by incorporating single-walled carbon nanotubes (SWCNTs) into a non-fluorinated superhydrophobic PDMS coating
[95][58]. The resulting surface, a combination of dual-scale clusters created by silica nanoparticles and the inherent properties of PDMS, exhibited superhydrophobic characteristics with a water-contact angle of 161°. The liquid repellency was impressively maintained even when stretched to a 100% strain for 100 h (
Figure 1d). Their group developed a Zn-Al sol-gel dispersant technology for SWCNT bundles, enabling effective anchoring of SWCNTs at a molecular level within the PDMS. This well-dispersed configuration facilitated good electrical contact between SWCNTs. The strain sensor demonstrated a linear increase in resistivity as the strain was applied, with the contact area decreasing. It displayed high sensitivity in detecting low strain levels, as little as 0.1%, up to 100% strain, with a highly linear and rapid response (
Figure 1e). Notably, the strain sensor maintained its high linearity and increased response even after stabilization for 7000 cycles (
Figure 1f), showcasing its reliability for infrastructure monitoring compared to previously reported sensors. Additionally, the PDMS/SWCNTs/PDMS sensor exhibited exceptional real-time responsiveness, detecting strain changes of less than 1° in aluminum and steel strips. Its low initial resistance allowed it to power an LED using a 3 V battery. Beneficial to its superhydrophobic property, the PDMS/SWCNTs/PDMS strain sensor demonstrated significant potential to function effectively under challenging practical conditions such as humid, acidic, saline, and alkaline environments.
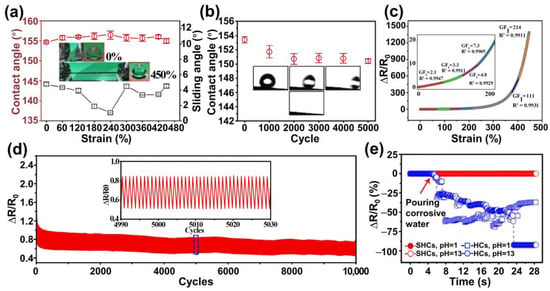
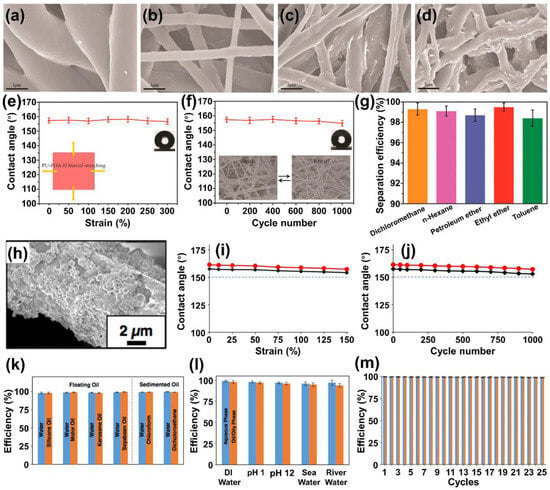
Taking advantage of a post-surface roughening process, Jia et al. engineered a coating-free superhydrophobic material by uniformly embedding MWCNTs in vulcanized silicone rubber (RTV)
[96][59]. The mixture of MWCNTs and RTV was allowed to cure in a paper box at ambient conditions, resulting in a composite surface with rough structures replicated from the paper mold. Subsequently, the surface was abraded with sandpaper to achieve a dual-scale roughness reminiscent of a lotus leaf, featuring continuous large protrusions densely covered with small features. The rough structure and the inherent hydrophobicity of RTV endowed the surface with superhydrophobic properties. Water droplets deposited on this surface maintained a spherical shape, boasting contact angles of approximately 155° and sliding angles of about 6°. Given the high flexibility of RTV and the homogeneous distribution and encapsulation of MWCNTs within the RTV matrix, the resulting superhydrophobic surface displayed impressive mechanical robustness. Water droplets retained their spherical morphology even when stretched within a 0–450% strain range. The water-contact angles (WCAs) and sliding angles (SAs) of droplets at different strain levels remained above 154° and below 8°, respectively (
Figure 2a). After subjecting the surface to 5000 cycles of stretching–releasing at a strain of 200%, the WCA still measured above 151°, with the SA remaining below 10° (
Figure 2b). The results demonstrated the remarkable stability of the superhydrophobic surface under dynamic, extensive, and long-term strains, ensuring its waterproofing protection throughout its sensing range and after repeated sensing cycles. The prepared composite surface exhibited robust dynamic durability when employed in strain-sensing applications. The signal shape and intensity of relative resistance (ΔR/R
0) remained almost identical over 10,000 stretching cycles at 50% strain (
Figure 2c). The average gauge factor (GF) ranged from 2.1 to 214 within a wide range, reaching up to 447%, indicating both high sensitivity and a broad sensing range for the sensor (
Figure 2d). Moreover, the stretchable superhydrophobicity ensured the sensing material maintained its sensing performance under harsh conditions such as strong acids, alkalis, artificial sweat, and everyday liquid contaminants (
Figure 2e). These exceptional properties resulted in remarkable dynamic durability, rendering the composite material suitable as a flexible and stretchable sensor capable of detecting a wide range of human motions in challenging environments.
Figure 2. Multifaceted robustness characterization of the superhydrophobic MRS material: (
a) water-contact angle and sliding angle variation while stretching the MRS materials stretched from 0 to 450% strain; (
b) water-contact angles after 0–5000 stretching cycles at 200% strain; (
c) linear fittings of the hierarchically structured MRS material; (
d) relative resistance under cyclic stretching from 0 to 50% strain over 10,000 cycles; and (
e) relative resistance changes of unabraded and superhydrophobic MRS materials at static states under different vigorous conditions: corrosive water
[96][59].
Moreover, Ding et al. achieved the creation of a flexible, conductive, and superhydrophobic porous film by blending thermoplastic polyurethane (TPU), carbon black (CB), and 1H,1H,2H,2H-perfluorodecyltriethoxysilane (PFDTS)
[97][60]. TPU served as the substrate for electrospinning, CB functioned as the conductive coating, and PFDTS was chosen to impart superhydrophobic properties. The resulting surface exhibited exceptional liquid repellency, boasting a water-contact angle of 179°. This film displayed impressive performance characteristics, including high repeatability and linear sensitivity within a 0–100% strain range. It featured a swift stretch response time of 200 ms and a rapid recovery time of 150 ms. Furthermore, a clear relationship between tensile strength and the peak value of the output current signal was observed over 2000 s, underscoring the strain sensor’s outstanding durability and reliability. Thanks to its high sensitivity, rapid response time, stability during stretching and releasing, and superhydrophobic properties, these conductive films are well-suited as wearable devices for monitoring human movements across various conditions.
3. Prevention of Corrosion
The
Cassie–
Baxter wetting status of water droplets on superhydrophobic surfaces significantly reduces the contact between water and the substrate, making it an effective strategy for preventing corrosion. The stretchability ensures that the liquid repellency is maintained when exposed to external deformation or stretching, expanding the superhydrophobic surface’s application area to curved and soft substrates. Gao et al. engineered wearable strain sensors with stretchability and anti-corrosive properties using a PDMS/CNT-decorated elastomer nanofiber composite
[98][61]. In their approach, ultrasonication was employed to decorate CNTs onto the surface of conductive thermoplastic PU nanofibers. Subsequently, PDMS was modified to the nanofiber surface to confer low surface energy, imparting superhydrophobic and anti-corrosion properties to the composite. The resulting surface exhibited outstanding water repellency, maintaining its superhydrophobic property even when subjected to various aqueous conditions (water, acid, base, and salt). After a 6 h immersion in an acidic solution, the contact angles remained stable and hovered around their initial values, demonstrating excellent anti-corrosion characteristics.
Liu et al. introduced a superhydrophobic composite film with exceptional stretchability, recyclability, and anti-acid corrosion by combining thermoplastic elastomers (TPE), inorganic nanoparticles, and fluorinated materials
[99][62]. TPE was chosen for its high stretchability and integrated with fumed silica nanoparticles to enhance film stability during formation and provide necessary surface roughness. Then, the stretchable superhydrophobic surface was achieved after modification with FAS-17 (heptadecafluoro-1,1,2,2-tetradecyl-trimethoxysilane). Among a series of screenings, the TPE/17.5% SiO
2 composite film exhibited optimal performance, whose water-contact angle was 151.8° ± 0.5° with a remarkable tensile elongation of up to 750%. The composite films maintained water-contact angles exceeding 151.0° and sliding angles below 10.0° under diverse tensile strains, showcasing remarkable superhydrophobicity. Even after 500 cycles of cyclic stretching, the composite films retained strong hydrophobicity with a contact angle of 148.5° ± 0.6° and a sliding angle of 11.0° ± 0.2°. The TPE/17.5% SiO
2 composite film exhibited robust anti-acid corrosion and retained its superhydrophobic state after 15 days of immersion. The inclusion of acid-resistant SiO
2 nanoparticles contributed to the film’s superior acid corrosion resistance and the augmentation of micro-nanostructures on the surface. The flexible and easy-to-apply characteristics of the superhydrophobic composite film suggest promising applications in various fields, including electronics, automotive, construction, decoration, and other equipment.
In recent years, controllable wettability has played an essential role in both fundamental and industrial applications, effectively prolonging the lifetime of electronic devices under corrosive and humid environments
[100][63]. However, the hierarchical roughness and low-surface-energy substances that endow the system with super wettability are vulnerable and easily damaged under tensile or flexural strain, significantly limiting the practical application of the functional surfaces. To tackle these challenges, Ye et al. developed a stretchable material composed of carbon nanotubes and polydimethylsiloxane (CNTs/PDMS) using an atmospheric micro-plasma jet (μPJ) technique
[101][64]. They began by creating CNT membranes through vacuum filtration of a CNT suspension using polyvinylidene fluoride (PVDF) membranes. Next, they spin-coated polydimethylsiloxane precursors onto these CNT membranes and immersed them in deionized water after curing to facilitate the peeling of CNTs, resulting in stretchable CNTs/PDMS membranes. Finally, they employed a custom-made μPJ to pattern the surface, introducing the necessary roughness. This, combined with the inherent hydrophobicity of CNTs and the low surface energy of PDMS, achieved a stretchable superhydrophobic surface (
Figure 3a). Water droplets on this surface exhibited a contact angle of approximately 160° and a sliding angle below 3°. The water repellency of this material proved to be highly robust, with the contact angle experiencing only slight fluctuations within the range of 158° ± 3°, even after subjecting it to 1000 cycles of stretching and releasing (at a strain of 50%) (
Figure 3b). This exceptional performance was attributed to the synergistic effects of the chemical inertness of CNTs and the hierarchical surface structure. Notably, the superhydrophobic film demonstrated remarkable corrosion resistance, maintaining water-contact angles above 150° after exposure to open air and various corrosive liquids (ranging from pH 1 to 14) for 8 weeks (
Figure 3c). The stable
Cassie wetting state endows the resulting CNTs/PDMS surface with the potential for numerous practical applications in challenging environmental conditions.
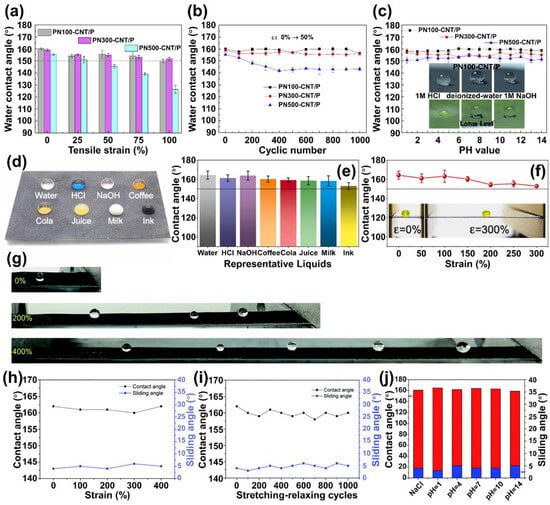
Figure 3. Durability of stretchable and conductive superhydrophobic CNT/PDMS films. WCAs recorded under tensile strain from 0% to 100% (
a); and underwent 1000 cyclic stretching from ε = 0% to ε = 50% (
b). (
c) WCAs of as-prepared films under corrosive liquids (pH value from 1 to 14). CNT, carbon nanotube; WCAs, water-contact angles
[101][64]; (
d) digital photographs of water, HCl (1 M) solution, NaOH (1 M) solution, coffee, cola, juice, milk, and ink droplets on superhydrophobic PTCCS nonwoven textile; and (
e) their corresponding WCAs. (
f) WCAs of PTCCS under increased strains from 0% to 300%
[102][65]; (
g) water droplets on the graphene composite at a strain of 0%, 200%, and 400%. (
h–
j) CAs and SAs of the graphene composite (
h) at various stretching strains; (
i) after various stretching–releasing cycles; and (
j) after immersing the composite in different corrosive liquids for 24 h, the red and blue colors represent contact angle and sliding angle, respectively
[103][66].
By utilizing a flexible network composed of carbon black nanoparticles and carbon nanotubes (CB/CNT) as well as titanium dioxide nanoparticles modified with perfluorooctyltriethoxysilane (PFOTS-TiO
2 nanoparticles), Dong et al. engineered and successfully fabricated a stretchable superhydrophobic surface with applications in health monitoring and pollution resistance
[102][65]. The process began with electrospinning styrene-ethylene-butylene-styrene (SEBS) to create a nonwoven fabric, followed by the coating of CB and CNT to form composite CCS fabrics. PFOTS and Degussa P25 TiO
2 were then spray-coated onto these CCS fabrics, ultimately realizing a stretchable superhydrophobic surface known as PTCCS. The flexible SEBS component, integrated with the adaptable CB/CNT network, was designed to accommodate stretching, with CB particles filling the voids between CNTs, effectively acting as bridges. It also ensured that the composite surface exhibited excellent stretchability. PFOTS, known for its high permeability and possessing bifunctional groups (R-SiO
3 and R-F), played a critical role by significantly reducing the surface energy and establishing strong layer adhesion. PFOTS also bridged the TiO
2 nanoparticles and the underlying matrix, enabling the rough structures formed by TiO
2 to adapt to external mechanical deformations. The PTCCS surface demonstrated remarkable liquid repellency, with various droplets such as coffee, cola, juice, yogurt, salt, acid, and alkali solutions, maintaining approximately spherical shapes and contact angles exceeding 150° (
Figure 3d,e). The results highlighted the surface’s excellent superhydrophobicity. Furthermore, this property remained robust even when subjected to mechanical deformation, as evidenced by a water-contact angle exceeding 150° on the PTCCS surface under high stretching conditions of up to a 300% strain (
Figure 3f). In addition to stretching, the PTCCS surface displayed resilience against mechanical wear. After subjecting the PTCCS surface to 1200-grit SiC sandpaper abrasion with a 100 g weight over a 10 cm distance for 50 cycles, the water-contact angle only experienced a slight reduction from 162° to 152°. Moreover, the PTCCS surface exhibited exceptional corrosion resistance. Following immersion in typical corrosive liquids, including HCl solution (pH = 1), 5 wt% NaCl, and NaOH solution (pH = 14), for 24 h, all samples maintained a water-contact angle greater than 150°. Additionally, the surface morphology and structure remained well-preserved, underscoring the outstanding chemical resistance of this superhydrophobic surface, and highlighting its substantial potential for practical applications.
Wang et al. devised a straightforward method to synthesize a superhydrophobic graphene composite through a dissolution and re-solidification process
[103][66]. They accomplished this by partially embedding perfluorosilane-coated graphene into thermoplastic polyurethane (TPU). This approach enhanced the mutual interaction between the components and provided the essential surface roughness. Thanks to the graphene’s high surface area and the exceptional tensile strength of TPU, the interface between water and the resulting composite maintained the
Cassie state. This was evident in the contact angles of water droplets (10 μL), which exceeded 155° and the sliding angles, which measured less than 10°, even when the material was subjected to stretching strains of up to 400% (
Figure 3g,h). Moreover, the superhydrophobicity of the surface persisted even after undergoing 1000 stretching–relaxation cycles with a 300% strain, demonstrating remarkable durability in both stretchability and superhydrophobicity (
Figure 3i). The micro-nano structure formed on the TPU played a vital role in the surface’s robust liquid repellency. This structure effectively traps air, preventing the penetration of water and corrosive substances. Consequently, the composite surface showcased impressive anti-corrosion properties. After immersion in corrosive liquids such as 3.5 wt% NaCl and aqueous solutions with pH values ranging from 1 to 14 for 24 h, followed by rinsing and drying at room temperature, the superhydrophobicity remained intact. Contact angles (CAs) remained above 150° while sliding angles (SAs) stayed below 10° (
Figure 3j). This exceptional corrosion resistance opens possibilities for applying the graphene composite in various practical scenarios.
4. Oil–Water Separation
Stretchable superhydrophobic surfaces with inherent oleophilic properties have garnered attention for their potential application in environmental protection and resource conservation, particularly in the realm of oil–water separation, where oil possesses a lower surface tension than water, enabling it to permeate the surface while repelling water easily. The flexibility of the substrate effectively resists the impact of liquids, maintains superhydrophobicity under deformation caused by external pressure to maintain the efficiency of oil–water separation, and extends the lifetime of the surfaces. Inspired by the mechanical flexibility observed in nanofibrous biomaterials, Guo et al. fabricated a robust superhydrophobic membrane, PDMS/PVDF@KNFs, through a simple one-step approach devoid of incorporation with inorganic fillers
[104][67]. The combination of PDMS and PVDF micro/nanoparticles effectively lowered the surface energy and introduced hierarchical surface roughness by incorporating Kevlar nanofibrils (KNFs). The superhydrophobic membrane was realized by employing a single-step vacuum filtration process. When subjected to a mixture of dichloroethane and water (V
oil:V
water = 10:1), the resulting PDMS/PVDF@KNFs membrane enabled the oil to permeate without external pressure while water remained constrained at the surface. A remarkable oil purity of up to 99.9% was achieved upon oil collection, and the flow rate remained consistent even after 20 separation cycles, indicating the membrane’s high-efficiency separation performance and robust reusability. The static contact angle experienced minimal alteration during each cycle, fluctuating within a 3–6° range, affirming the membrane’s durability and superhydrophobic properties throughout the oil–water separation. Similarly, Gao et al. engineered a stretchable, superhydrophobic, and anti-ultraviolet nanofiber composite membrane
[105][68]. TiO
2 nanoparticles were anchored onto PU nanofiber surfaces via ultrasonication, followed by PDMS modification, lowered surface energy, and bolstered interfacial adhesion between TiO
2 nanoparticles and PU nanofibers. Due to its superior corrosion resistance, this composite membrane displayed remarkable potential in oil–water separation and oil–corrosive-solution separation. Dichloromethane–water mixture separation showcased the rapid penetration of dichloromethane and containment of the corrosive aqueous solution on the nanofiber membrane’s top surface due to its superhydrophobic and superoleophilic characteristics. The nanofibrous composite membrane demonstrated a substantial oil permeate flux and separation efficiency of 1964.7 L m
−2 h and 90.3%, respectively, during the initial separation and 1972.0 L m
−2 h with a sustained separation efficiency of about 92% during cyclic testing, underscoring its impressive recyclability. A meticulous study of the oil–water separation mechanism revealed that oil exhibited the
Wenzel state upon contact with the nanofiber composite membrane. It was augmented by a Laplace pressure gradient (produced by superoleophilicity) that facilitated its continuous penetration. Simultaneously, the superhydrophobic
Cassie–
Baxter state retained water on the nanofiber surface through repulsive forces, reinforcing the separation efficiency via Laplace pressure and gravity interplay.
Using a straightforward dip-coating technique, Li et al. constructed hierarchical superhydrophobic architectures on the surface of a three-dimensional copper foam (CF)
[106][69]. Surface modifications involved mussel-inspired polydopamine (PDA) and Ag nanoparticles onto the CF substrate, sequentially followed by n-dodecyl mercaptan (NDM) coupling. The incorporation of NDM coupling effectively reduced the surface energy, while the resulting CF-PDA-Ag-NDM exhibited heightened surface roughness through its hierarchical structures, thereby establishing a superhydrophobic property. To evaluate its oil–water separation capabilities, a hexane–water mixture (m
oil/m
water = 1) was employed. Gravity-driven, the hexane permeated the mesh while the water was blocked, remaining confined within the upper PMMA tube. Moreover, the superhydrophobic CF demonstrated a separation efficiency exceeding 95% for a range of oil–water mixtures (hexane–water, heptane–water, octane–water, dodecane–water, and sesame oil–water), notably reaching 98.3% for the dodecane–water mixture. Quantitatively, the maximum height and intrusion pressure (p) for water were 14.4 ± 0.9 cm and 1.4 ± 0.1 kPa, respectively, indicating that water could not traverse the foam’s structure under specific intrusion pressures.
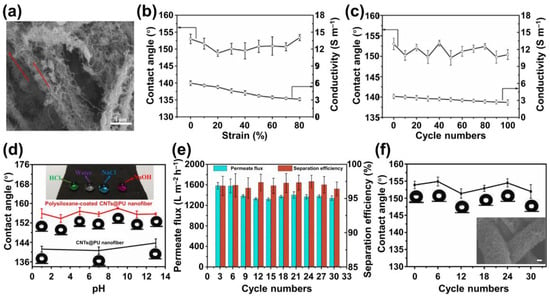
Huang et al. developed a flexible and stretchable superhydrophobic surface using a process involving ultrasonication-induced decoration of carbon nanotubes (CNTs) onto thermoplastic polyurethane (TPU) nanofibers, followed by the hydrolysis of methyltrichlorosilane (MTS) on the surface of the CNTs
[107][70]. Utilizing CNTs and polysiloxane derived from MTS, this approach conferred multi-scale physical structures onto the polymer nanofiber. It also imparted low surface energy, resulting in a
Cassie–
Baxter wetting state for water droplets deposited on the surface. Consequently, the material exhibited superhydrophobic properties, with a water-contact angle reaching 155°.
Due to the anchoring or embedding of polysiloxane-modified CNTs onto the TPU fiber, the resulting superhydrophobic surface retained the flexibility of the TPU nanofibers, enabling it to adapt to mechanical deformation. Tensile testing revealed that the TPU nanofibers and the polysiloxane-coated CNTs tended to reorient themselves at different strain levels. Nanofiber orientation became evident at a strain of 20% (Figure 4a), and this effect intensified as the strain increased to 80%. Simultaneously, the polysiloxane-coated CNTs aligned along the nanofiber axis during stretching and winding, maintaining the hierarchical structure on the nanofiber surface. It increased interfacial contact area and contributed to enhanced tensile strength. This adaptability allowed the prepared surface to stretch up to 370% at the break, with Young’s modulus of 2.35 MPa and tensile strength of 9.11 MPa. Remarkably, the surface’s superhydrophobicity was maintained throughout this process. Water droplets retained their spherical shape on the surface even when stretched to 20%, 50%, and 80% strains. The contact angles varied from 150° to 153°, while the contact angle fluctuated around 151° during stretching–releasing cycles (stretching at 50%) (Figure 4b,c).
Figure 4. (
a) SEM images of the polysiloxane-coated CNTs@PU nanofiber mat under different strain 20%, the arrows demonstrated the stretching direction; (
b,
c) variation of the CAs and conductivity of the superhydrophobic mat (
b) under different strains; and (
c) during 100 uniaxial stretching cycles. (
d) Variation of the CAs of the CNTs@PU nanofiber mat and the polysiloxane-coated CNTs@PU membrane with the pH values of water droplets; (
e) permeate flux and separation efficiency of the oil–water separation using polysiloxane-coated CNTs@PU membrane during different cycles. (
f) Variation of CAs of polysiloxane-coated CNTs@PU membrane during different oil–water separation cycles
[107][70].
Thanks to the water-resistant and corrosion-resistant nature of the polysiloxane-coated CNTs, which form a protective shell layer, the stretchable superhydrophobic surface demonstrates excellent durability for use in harsh environments. When exposed to solution droplets with pH values of 1, 7, and 13, the contact angles remained consistent, ranging from 153° to 158°, regardless of the droplet’s pH level. Even after immersing the surface in acidic and alkaline solutions with pH values of 1 and 3 for 5 days, the contact angles still exceeded 150°, showcasing remarkable corrosion resistance (Figure 4d).
When the stretchable superhydrophobic surface was exposed to oil–water mixtures (chloroform and water, each phase was dyed for better visualization), separation could be easily achieved under gravity with high flux and efficiency. The oil rapidly permeated through the membrane and ultimately dripped into the beaker due to its superoleophilic properties. In contrast, water remained on the top surface of the membrane due to its superhydrophobic nature. Following separation, almost no residual chloroform was detected in the separated water. The surface could be reused after each oil–water separation. As depicted in Figure 4e, the oil permeation flux through the surface and the separation efficiency were monitored over multiple cycles. It was observed that the superhydrophobic surface exhibited a high oil permeation flux of approximately 1500 L m−2 s−1 in the first three cycles. Then, the flux stabilized around 1350 L m−2 s−1 over the subsequent 27 cycles, indicating good reusability. Furthermore, oil–water separation efficiency remained above 96% during all 30 cycles, demonstrating high cyclic separation performance. In addition to its recyclability in oil–water separation, the superhydrophobicity persisted throughout the separation cycles, with contact angles remaining nearly unchanged and staying around their initial value of 154° (Figure 4f). It affirms the durability of superhydrophobicity and its potential for practical applications in separating oil from various corrosive solutions.
Leveraging the flexibility of polymers, Chen et al. engineered a robust and stretchable superhydrophobic surface comprised of elastic polyurethane (PU) and chromatic polydiacetylenes (PDA) for highly efficient oil–water separation
[108][71]. In their method, PU and 10, 12-pentacosadiynoic acid (PCDA) were initially blended and electrospun to create a smooth, interconnected fibrous membrane (
Figure 5a). Subsequently, a brief UV irradiation was applied to initiate PCDA polymerization, forming PDA clusters on the electrospun fibers (
Figure 5b). These clusters resulted from the phase separation between PU and PDA and could be further enhanced by a heat treatment to increase surface roughness (
Figure 5c,d). The combination of the polymers’ low surface energy and the resulting hierarchical structures conferred superhydrophobicity on the surface, boasting a water-contact angle of 157° and superoleophilicity with a tiny oil-contact angle of 0°. The obtained superhydrophobic surface was also stretchable thanks to the in situ formed structure and the polymers’ elasticity. When subjected to biaxial mechanical strains within the 0–300% range, the water-contact angle barely changed (
Figure 5e). Furthermore, liquid repellency was preserved at around ~155° even after 1000 cycles of stretching and retracting (
Figure 5f). During the separation of a dichloromethane and seawater mixture, the oil swiftly permeated through the membrane under the influence of gravity while the water remained above. This remarkable permeation flux reached 6369.4 ± 37.7 L m
−2 h
−1, signifying an easily operable, low-energy-consuming process. Additionally, other oils with surface tensions below 30 mN m
−1, such as n-hexane, petroleum ether, ethyl ether, and toluene, could also be effectively separated from water mixtures, exhibiting separation efficiencies of 99.3%, 99.1%, 98.7%, 99.5%, and 98.4%, respectively (
Figure 5g). Moreover, after 12 separation cycles, the water-contact angle remained above 150°, underscoring the robust reusability of the superhydrophobic surface due to the advantages of the in situ growth approach. It ensured that the surface maintained a
Cassie–
Baxter wetting state for long-term applications.
Figure 5. SEM images of (
a) pure PU; (
b) PU-PCDA; (
c) PU-PDA; and (
d) PU-PDA-H FMs. (
e) WCA of PU-PDA-H FM under various strains; (
f) WCA of PU-PDA-H FM after 1000 stretching cycles; (
g) separation efficiency of PU-PDA-H FM for several oils
[108][71]; (
h) FESEM images of the fibrous substrate after deposition of the multilayer (10 bilayers) coating; (
i) change in static contact angle of beaded water (black) and oil (red) droplets on the SHM and UWSOM-during gradual increment of the tensile strain from 0% to 150%; (
j) changes in water (in the air, black) and oil (underwater, red) wettability during successive (1000 times) tensile deformations of SHM and UWSOM, respectively; (
k) simultaneous oil (orange) and aqueous (blue) phase separation efficiency; (
l) separation efficiency of both aqueous phase (blue) and oil phase (orange) under various severe settings; and (
m) ability of repetitive (minimum 25 times) oil (orange) and aqueous (blue) phase separation using the same lab-made prototype
[109][72].
Utilizing a layer-by-layer approach, Das et al. presented the creation of amine-reactive and covalently cross-linked multilayers composed of chemically reactive polymeric nanocomplex (CRPNC) and aminographene oxide (AGO) nanosheets. These multilayers were modified with octadecylamine to yield a stretchable superhydrophobic surface for oil–water separation
[109][72]. Starting with a fibrous polyurethane (PU) substrate, they alternately deposited CRPNC and AGO layers to generate a multi-layered structure impeding essential surface roughness (
Figure 5h). Subsequent covalent chemical modification with hydrophobic octadecylamine endowed the surface with low surface energy. The resulting surface displayed remarkable superhydrophobicity, boasting a water-contact angle of 157° and a sliding angle of 5°. Due to active amine groups within the multilayer structure, the surface could be modified with glucamine to achieve an underwater superoleophobic state, featuring an advancing oil-contact angle of 161° and a sliding angle of 4°. Even when mechanically stretched to a strain of 150%, the prepared surface maintained its liquid repellency, with both water and oil droplets assuming a spherical morphology and contact angles exceeding 150° (
Figure 5i). Impressively, the super liquid repellency remained unchanged even after undergoing 1000 cycles of stretching–releasing at a tensile strain of 150% (
Figure 5j). Combining the superhydrophobic and underwater superoleophobic surfaces in a prototype oil–water separation device made simultaneous separation of water and oil phases possible. In the case of a water and kerosene (light oil) mixture, kerosene selectively permeated through the superhydrophobic surface. In contrast, the water phase adhered to the underwater superoleophobic surface and penetrated it, ultimately collecting in a separate container. This process achieved the parallel separation and collection of oil and aqueous phases. Notably, no accumulation of any liquid phase was observed on the top of the utilized surfaces, confirming the high separation efficiency, consistently exceeding 98%. Furthermore, the efficiency of separation extended to various oil phases, including dichloromethane (heavy oil), vegetable oil, silicone oil, motor oil, and chloroform, when mixed with an aqueous phase. In all cases, the separation efficiency remained consistently high, exceeding 98%. The surface prepared in this manner was also suitable for separating oil and aqueous phases from three-phase oil–water mixtures comprising light oil, an aqueous phase, and heavy oil, as shown in
Figure 5k. In such mixtures, where the top layer consisted of kerosene, the middle layer contained deionized water, and the bottom layer was dichloromethane, the heavy and light oil phases selectively traversed the superhydrophobic surface. In contrast, the aqueous phase was effectively filtered through the underwater superoleophobic surface. The separated oils and deionized water collection efficiency consistently exceeded 98% (
Figure 5l). Moreover, the surface’s stretchability allowed for maintaining high-efficiency oil–water separation even after undergoing 1000 stretching cycles with a tensile strain of 150%. Furthermore, the separation process could be successfully repeated for a minimum of 25 cycles (
Figure 5m), endowing the surface with the potential for parallel and selective separation of various oil–water mixtures repetitively, regardless of variations in surface tension, density, viscosity of the oil phase, or chemical complexity in the aqueous phase.
5. Anti-Icing
Another potential application for stretchable superhydrophobic surfaces lies in anti-icing technology, which stems from the advantageous combination of low adhesion and minimal contact area between water droplets and superhydrophobic surfaces. A superhydrophobic surface hampers heat transfer and significantly delays the nucleation of droplets deposited on the surface at low temperatures. When tilting the surface at an angle more significant than the water droplet’s rolling-off angle, the droplets release from the surface without nucleation, exhibiting anti-icing properties. However, conventional superhydrophobic surfaces are often delicate and prone to destruction from external forces. Thus, stretchable superhydrophobic surfaces with robust mechanical stability and adaptable surface wettability emerge as viable alternatives for durable anti-icing applications to resist the influence of external pressure, stretching, twisting, etc., to maintain the efficiency of anti-icing in harsh environments. Wang et al. devised an anti-icing stretchable superhydrophobic surface by sequentially applying 2,2,6,6-tetramethylpiperidinyl-1-oxyl (TEMPO)-oxidized cellulose-silica (TOC-SiO
2) and PDMS onto cotton fabric
[110][73]. Water droplets on the surface exhibited a static contact angle of 158°, which can be retained even after 150 bending cycles. To evaluate anti-icing behavior, they deposited 40 µL water droplets on the superhydrophobic surface and untreated cotton fabric at −10 °C. On untreated cotton fabric, droplets froze in 6 min, whereas on the superhydrophobic surface, freezing was delayed to 11 min, a 5 min extension, indicating an outstanding anti-icing performance. Additionally, the anti-icing effect persisted after 8 freeze-thaw cycles, suggesting the potential for repetitive anti-icing applications. In another work, Liu et al. achieved anti-icing stretchable superhydrophobic surfaces by using direct laser writing on the top silicone rubber layer of a silicone rubber (SR)/MWCNTs/laser-induced graphene (LIG)/SR composite strain sensor
[111][74]. The composite surface acquired micro-nano convex structures through laser engraving, yielding outstanding superhydrophobicity. Deposited water droplets exhibited a static contact angle of 155° and a sliding angle of 5°. The surface wettability was proven to be durable, maintaining a contact angle of around 152° and a sliding angle of about 8° even after 2500 stretching–releasing cycles performed at a 200% strain. To address the vulnerability of polymer-based conductive composite strain sensors to ice accumulation, the anti-icing performance of the SR/MWCNTs/LIG/SR composite strain sensor was evaluated by measuring icing delay times at various low temperatures. Results revealed that the icing time for 6 µL water droplets deposited on the laser-induced superhydrophobic surface could be extended to 36, 17, 8, and 4 min at temperatures of −5, −10, −15, and −20 °C, respectively. This underscores the excellent deicing capability, effectively enhancing sensor applicability in low-temperature environments.
Recently, Yu et al. introduced a robust anti-icing composite surface based on modified PU elastomer and silica nanoparticles (SiO
2)
[112][75]. Initially, fluorinated silica nanoparticles (SiO
2-F) were blended with modified PU elastomer (PUE) at a SiO
2-F concentration of 8 wt%. Using a spray gun, the resultant mixture was sprayed onto an aluminum alloy substrate. After allowing the sprayed surface to cure at room temperature for 2 h to evaporate the organic solvent, followed by an 8 h cross-linking process at 80 °C, a stretchable, superhydrophobic, and anti-icing surface is achieved. Modification of PUE with dihydroxydodecyl-terminated PDMS endowed the surface with low surface energy, while the SiO
2-F nanoparticles were densely clustered to create micro-nanostructures. The synergy between the low surface energy and micro/nanostructure formation contributed to the composite surface’s superhydrophobicity. A 4 µL water droplet deposited on the surface exhibited a static contact angle of 162° and a sliding angle of 2°, indicating the excellent liquid repellency of the as-prepared surface. Capitalizing on the elasticity of PUE, the composite surface demonstrated stretchability up to a strain of 385% while maintaining the superhydrophobic characteristics. The anti-icing property was evaluated through an ice shear adhesion experiment, mimicking the real-world behavior of moving ice blocks. Low ice adhesion is indicative of superior anti-icing performance. The unmodified aluminum panel exhibited the highest ice adhesion strength at 235.36 kPa. Upon application of the PUE coating, the ice adhesion strength decreased to 68.04 kPa. Remarkably, the superhydrophobic SiO
2-PUE surface exhibited an ultra-weak ice adhesion strength of 14.33 kPa, over an order of magnitude lower than the bare aluminum. Accumulated ice on the superhydrophobic surface readily detached under its weight or natural wind forces
[113][76]. The as-prepared superhydrophobic surface showcased extraordinary anti-icing capabilities, positioning it as a promising candidate for widespread anti-icing surface applications in the aerospace and automotive industries.
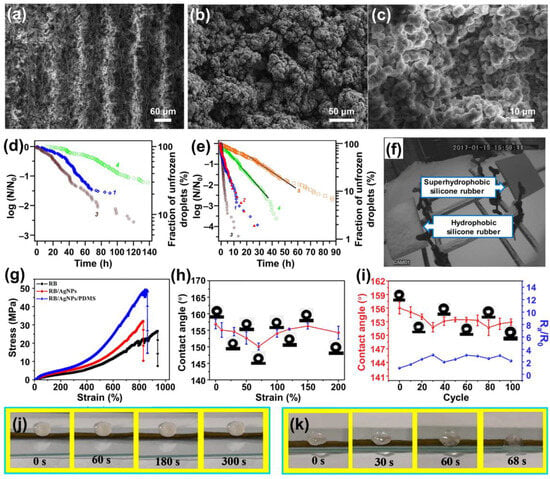
Emelyanenko et al. introduced a straightforward approach for creating a robust superhydrophobic coating tailored for outdoor applications, even in harsh conditions
[114][77]. Initially, they used laser technology to texture commercial composite silicone rubber, followed by a UV-ozone treatment to instill hydrophilicity on the surface. Subsequently, they covalently grafted methoxy-{3-[(2,2,3,3,4,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctyl)-oxy]-propyl}-silane onto the treated surface, reducing the surface energy through immersion and heating processes (
Figure 6a–c). The resulting surface exhibited exceptional water repellency, as evidenced by static contact angles of up to 170° for 15 μL water droplets, with sliding angles of around 4° for 10 μL droplets. Combining the textured structure and fluorooxysilane treatment significantly enhanced the surface’s anti-icing capabilities. Approximately a quarter of the droplets deposited on the surface remained unfrozen for 140 h at −10 °C and 20 h at −18 °C, prolonging the freezing delay time to approximately 2.5 times that of untreated hydrophobic and solely textured superhydrophobic samples (
Figure 6d,e). Additionally, the freezing of an 0.5 M NaCl aqueous solution under the same temperature and humidity conditions was also delayed, with 25% of the test droplets remaining in a liquid state after 40 h of exposure at T = 18 °C, further demonstrating the outstanding anti-icing properties of the prepared superhydrophobic surfaces (
Figure 6e, curve 5). Outdoor experiments underscored the effectiveness of the fluorooxysilane coating in improving the mechanical stability of the textured surface structure and reducing surface energy. As a result, the superhydrophobic surface exhibited remarkable resistance to severe outdoor conditions, such as snow and ice accumulation, even at temperatures well below 0 °C. Snow could slide effortlessly along the superhydrophobic surface for 6 h at T = −1 °C without adhesion (
Figure 6f). In calm or low wind speeds, snowfall led to the formation of a snow cup, which was spontaneously removed from the superhydrophobic surface when the snow layer thickness exceeded a critical value. No snow accumulation occurred during heavy snowfall when the wind velocity exceeded 3–5 m/s. Bouncing droplet experiments also confirmed superior icing resistance at temperatures equal to or below −17 °C. Although the effectiveness of droplet rebound decreased at lower temperatures, the accumulated ice remained significantly less than that on normal hydrophobic and superhydrophobic surfaces. These superhydrophobic silicone rubber surfaces, enriched with enhanced texture, open up significant opportunities for outdoor applications in challenging conditions.
Figure 6. (
a–
c) SEM images of the laser-textured surface of silicone rubber at different magnifications. The scale bars are 30 (
a); 10 (
b); and 1 μm (
c). (
d,
e) Time dependences for the fraction of unfrozen droplets at: −10 °C (
d); and at −18 °C (
e) on different substrates, 1-nontreated silicone rubber, 2-silicone rubber after chemisorption of fluorooxysilane, 3-laser-treated silicone rubber, 4-silicone rubber with texture reinforcement by fluorooxysilane, 5-the corresponding dependence for the ensemble of brine droplets on the surface of sample 4. (
f) The behavior of samples 1 (hydrophobic silicone rubber) and 4 (superhydrophobic silicone rubber) during snow. T = −1 °C, wind velocity 2 m/s, RH = 90%
[114][77]; (
g) stress–strain curves of RB, RB/AgNPs, and RB/AgNPs/PDMS; (
h) CAs of the composite under different strains; and (
i) the composite’s Ra/R
0 and CAs during cyclic stretching–releasing under a strain of 50%. (
j,
k) Deicing performance of RB/AgNPs/PDMS: (
j) without applied voltage; and (
k) with a voltage of 4 V
[115][78].
Moreover, Wang et al. presented a superhydrophobic, fluorine-free rubber composite achieved by encapsulating a rubber band with silver (Ag) nanoparticles, followed by a modification with polydimethylsiloxane (PDMS)
[115][78]. The presence of Ag nanoparticles created surface roughness, while the PDMS binding layer significantly reduced surface energy and greatly enhanced the interfacial adhesion among Ag nanoparticles. This combination resulted in a surface with exceptional superhydrophobicity, self-cleaning capabilities, resistance to corrosion, and excellent electrical conductivity. The static contact angle of 5 µL water droplets deposited on the surface could reach a maximum of 156°. The integration of Ag nanoparticles and PDMS also led to a significant increase in Young’s modulus and tensile strength of the composite, measuring 9.0 and 42.8 MPa, respectively, compared to the values for pure rubber band (5.4 and 24.2 MPa, respectively). Additionally, the composite surface exhibited remarkable elongation at break, reaching 901.5% (
Figure 6g). Even under a substantial strain of 200%, the superhydrophobicity was maintained, and water droplets on the surface retained their spherical shape (
Figure 6h). After undergoing 100 cyclic stretching–releasing tests at a 50% strain, the water-contact angles remained above 150° (
Figure 6i), demonstrating the reusability and durability of the surface. The inclusion of Ag nanoparticles endowed the superhydrophobic composites with an effective Joule heating effect, which could achieve a saturation temperature of approximately 21.5 °C at an input voltage of 2 V. This temperature increased to 35 and 53 °C at 3 and 4 V, respectively, providing the surface with excellent de-icing properties.
Figure 6j,k shows that ice placed on the unheated rubber band barely melts and remains on the surface even after 5 min at ambient temperature (
Figure 6j). In contrast, when a voltage of 4 V is applied to the composite, the ice begins to melt. It rolls off the material surface within 68 s (
Figure 6k), expanding its potential in de-icing and water removal applications.
6. Droplet Manipulation
Droplet manipulation in an open environment is another application of stretchable superhydrophobic surfaces. As it is open to the atmosphere, individual droplets can be treated directly without the risk of clogging
[116][79]. Droplet manipulation has attracted great interest, from fundamental biochemical analysis to clinical and diagnostics
[117][80]. General hydrophilic and hydrophobic surfaces usually have large contact-angle hysteresis and contact line pinning, resulting in droplet losses and contamination. In contrast, superhydrophobic surfaces use re-entrant or overhang micro-nanostructures to provide upward Laplace pressure to prevent the droplet wetting downward, presenting large contact-angle and small contact-angle hysteresis, which can efficiently avoid surface contamination and reagent loss. Anisotropic or directional wetting behavior on superhydrophobic surfaces can be realized by fabricating specific surface structures, which means that the surfaces can control the shape and motion of droplets actively, with the help of light, heat, electricity, etc., or in a passive manner, based on the requirements of multiple superhydrophobic surfaces with specific adhesions
[118,119][81][82]. However, the external fields have difficulties combining the intrinsic structures, while the passive approach based on wetting has a firm application limitation. Based on this, stretchable superhydrophobic surfaces have become an efficient platform for droplet manipulation since they can employ mechanical actuation to regulate the adhesion or transfer of droplets
[18,120,121][18][83][84].
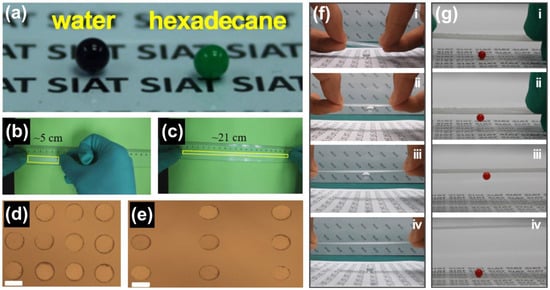
By a combination of soft replication and post-1H,1H,2H,2H-perfluorodecyltrichlorosilane modification, Wu et al. achieved an ethylene-vinyl-acetate (EVA)-based stretchable superhydrophobic surface for non-loss droplet transfer
[122][85]. Benefiting from the T-shaped replica surface structure, the as-prepared surface demonstrated a significant liquid repellency with the deposited water and hexadecane droplets presenting contact angles of around 158° and 159°, respectively (
Figure 7a). The EVA’s intrinsic elasticity endowed the surface with excellent stretchability, which was able to stretch up to more than 400% without destroying the isolated surface structure and liquid repellency (
Figure 7b–e). However, the stretching process can effectively regulate the space between the adjacent T-shape pillars, resulting in an apparent change of the solid fraction of the superhydrophobic surface, thus controlling the adhesion between the droplets and the surface for non-loss droplet manipulation. As shown in
Figure 7f,g, water and oil droplets were initially deposited on a PDMS-based liquid-repellent surface. They were easily lifted by the as-prepared surface due to the high surface adhesion derived from the large solid fraction. The adhesion is strong enough to move droplets freely. The adhesion force was significantly reduced when the EVA superhydrophobic surface stretched to ~350%, when the surface could no longer overcome the droplet gravity, resulting in the release of droplets in a non-loss manner.
Figure 7. Ethylene-vinyl-acetate-based stretchable superhydrophobic surface: (
a) excellent superlyophobic and transparency performance. (
b,
c) Stretchability of superhydrophobic surface: (
b) before; and (
c) after stretching. (
d,
e) Profiles of microstructures: (
d) before; and (
e) after stretching for 200%. 10 μm for all scale bars. (
f,
g) Demonstration of the nearly lossless water (
f); and ethylene glycol (
g) droplet transfer using the stretchable superhydrophobic surface
[122][85].
Taking advantage of the stretchability of the superamphiphobic surface, Butt et al. realized the programmable coalescence of droplets on the surface
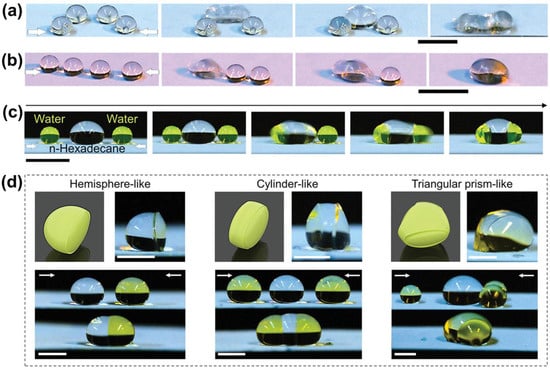
[52][86]. The stretching–releasing process enabled control of the spaces for droplets located on the surface from a separated status to a contact and coalescing status, which made it possible to realize a programmable coalescence of a sequence of droplets. As illustrated in
Figure 8a,b, trapezoidal-arranged water droplets and linearly arranged n-hexadecane droplets were deposited on a 100% stretched superamphiphobic surface for controlled coalescence. Once the substrate was gradually released, the distance between droplets changed accordingly. The trapezoidal arrangement enabled the middle droplets to coalesce first, and subsequently coalescence with the left- and right-hand sides, respectively (
Figure 8a). While the droplets were linearly arranged along the stretching direction, the releasing resulted in the merge of droplets in a particular order (
Figure 8b). The controllable coalescence of droplets was suitable for miscible and immiscible liquids. As presented in
Figure 8c, a dumbbell-like merged droplet is formed after a controlled coalescence by depositing one n-hexadecane and two water droplets at appropriate distances. Since the liquids in this case are immiscible, an apparent interface was observed between these two different droplets. The programmable droplet manipulation indicates a possible step-by-step reaction process regulated by liquid-repellent surfaces. Taking sodium alginate aqueous solution and iron chloride (FeCl
3) aqueous solution as a typical system, with the combination of coalescence between sodium alginate aqueous solution droplets and various numbers of FeCl
3 aqueous solution droplets and the gelation of sodium alginate catalyzed by FeCl
3, the authors successfully synthesized hydrogels with different morphologies on a superhydrophobic surface. Once the droplets of different solutions were attached, the iron ions rapidly diffused into the alginate drop. They initiated the gelation to convert the alginate droplet quickly to a hydrogel while the FeCl
3 droplets remained liquid. After removing the liquid droplets, alginate hydrogels with hemispherical, cylinder-like, and triangular prism-like morphologies were obtained (
Figure 8d), demonstrating the potential of using stretchable superhydrophobic surfaces to study the reactions or interactions of multidroplets.
Figure 8. Programmable manipulation of drop coalescences and synthesis of asymmetric hydrogels: (
a,
b) programmable coalescence of (
a) four water drops (15 µL) with a trapezoidal arrangement; and (
b) four n-hexadecane drops (10 µL) with a linear arrangement. Scale bars: 5 mm. (
c) Coalescence of one n-hexadecane drop (colorless) and two water drops (light green) on the NFSS surface. Scale bar: 5 mm: (
d) fabrication of asymmetric hydrogels with specific shapes. One alginate sodium aqueous solution drop (colorless) and different numbers (from left to right: 1, 2, and 3) of FeCl
3 aqueous solution drops (yellow) are used in the hydrogel fabrication process. Scale bars: 2 mm. The arrows represent the releasing of the stretched substrate
[52][86].
Beyond the previously discussed applications, stretchable superhydrophobic surfaces show promise for various other utilizations, including multifunctional sensing
[123[87][88],
124], anti-bioadhesion
[125[89][90],
126], deicing
[115[78][91],
127], droplet manipulation
[128[92][93],
129], and other fields
[130,131,132,133,134][94][95][96][97][98].