Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Alejandro Canales.
One of the main concerns related to SARS-CoV-2 infection is the symptoms that could be developed by survivors, known as long COVID, a syndrome characterized by persistent symptoms beyond the acute phase of the infection. This syndrome has emerged as a complex and debilitating condition with a diverse range of manifestations affecting multiple organ systems. It is increasingly recognized for affecting the Central Nervous System, in which one of the most prevalent manifestations is cognitive impairment. The search for effective therapeutic interventions has led to growing interest in Mesenchymal Stem Cell (MSC)-based therapies due to their immunomodulatory, anti-inflammatory, and tissue regenerative properties.
- long COVID
- mesenchymal stem cells
- exosomes
- neurological sequelae
1. Introduction
COVID-19 is caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), a novel coronavirus that shares more than 96% of the genome sequence with SARS-CoV. This novel coronavirus exhibits clinical symptoms similar to those reported for SARS-CoV and MERS-CoV [1][2]. In June 2023, the World Health Organization (WHO) reported 767,750,853 confirmed cases of COVID-19 and 6,941,095 deaths worldwide. In México, the confirmed cases are more than 7 million and 330 thousand deaths since the first confirmed case on 28 February 2020 [3]. Currently, there is no effective cure for COVID-19 and recovery depends on the immunity of the individuals [4].
Although the mechanisms of Central Nervous System (CNS) infection remain unclear and highly debated, the neurological symptoms of COVID-19 have been described frequently in critically ill patients with comorbidities [5][6]. However, one of the main concerns about these symptoms is that they could be developed by survivors after recovery [5][6] or in patients with mild acute disease, as part of a syndrome defined by the WHO as post-COVID-19 or long COVID [7]. The prevalence, duration, and severity of these symptoms differ among patients [8] and cognitive impairment is one of the most prevalent deficits [9].
2. SARS-CoV-2 Neuroinvasiveness and Long COVID
The SARS-CoV-2 infection mechanism involves the spike glycoprotein (S) and the binding with the angiotensin-converting enzyme 2 receptor (ACE2). The protein binding, eased by specific proteases such as transmembrane serine protease 2 (TMPRSS2), makes the virus capable of invading the respiratory and gastrointestinal epithelial cells [10]. Nevertheless, the S can bind to other receptors, such as Neuropilin-1 (NRP1) and dipeptidyl peptidase 4 (DPP4), that facilitate alternative viral entry and transmission in the target cells [11][12]. Although the ACE2 receptor is mainly expressed in pneumocytes, enterocytes, and vascular endothelial cells, this receptor can also be found on glial cells and neurons in the brainstem, the paraventricular nucleus (PVN), nucleus tractus solitarius (NTS), and the rostral ventrolateral medulla making them a potential target for SARS-CoV-2 [13] with a subsequent CNS infection. Despite many published investigations, the mechanisms of viral infection of the CNS remain unclear and highly debated [14]. Two main pathways of virus entry into the CNS have been proposed (Figure 1). In the first instance, sensory or motor nerve endings are infected, along with the subsequent retrograde neuronal transport [1]. Supporting evidence demonstrates that SARS-CoV-2 can penetrate the brain upon intranasal infection, crossing the neural–mucosal interface in the olfactory mucosa, with a further spreading to defined neuroanatomical areas, including the primary respiratory and cardiovascular control center in the medulla oblongata [15]. This neuroinvasiveness pathway is supported by the localization of viral RNA or proteins in sites such as olfactory mucosa and olfactory sensory neurons (OSNs) [16].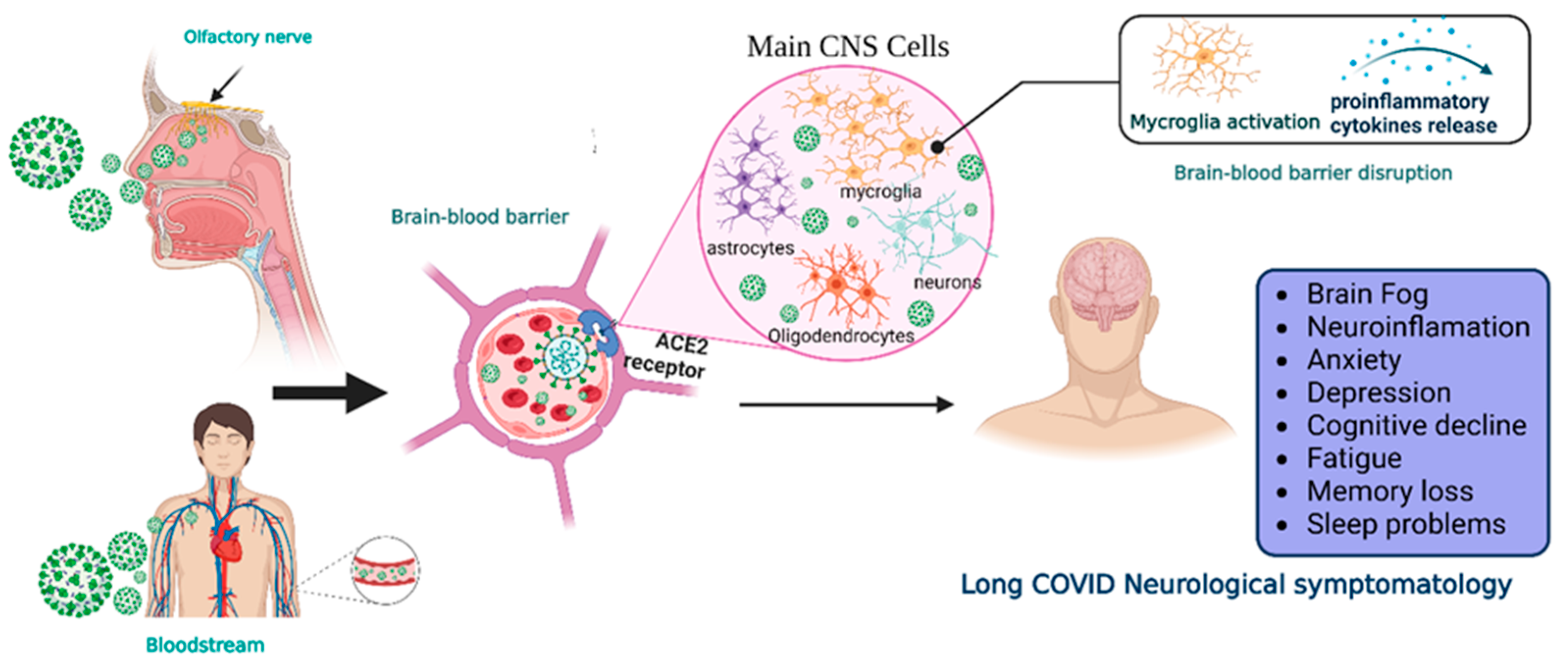
Figure 1. Neuroinvasiveness routes of SARS-CoV-2. Once the virus is in the respiratory system, it can reach the central nervous system by two main mechanisms.SARS-CoV-2 makes contact with the olfactory mucosa, reaching the olfactory nerves, and by transport through the nerve endings, it can travel and spread to the CNS. The other pathway is the hematogenous route, where the virus can reach the brain-blood barrier and, by transcytosis, infect the neuroepithelia and then the cells of the CNS. Viral RNA is present in neurons, astrocytes, oligodendrocytes, and endothelial cells. After the prolongated symptoms, people with long-COVID-19 develop neurologic sequalae. CNS = central nervous system, ACE2 = angiotensin-converting enzyme 2 receptor. Created with BioRender.com.
3. Current Landscape of MSC and MSC-Derived Exosomes in Long COVID
Since the beginning of the COVID-19 pandemic, several authors suggested MSC and their derivates, including conditioned media, extracellular vesicles, and exosomes, as a promising therapy for SARS-CoV2 infection [45][46]. The hypothesis was that these therapies could induce an immunomodulatory response against CSs along with improved regeneration of damaged tissue and improved lung function, mainly through the secretion of bioactive molecules [47][48], as well as because of the positive results obtained from preclinical models of acute respiratory distress syndrome (ARDS) [49][50], influenza [51][52], and other respiratory virus infections [53] in which MSC or its derivates improved animal outcomes and survival rates, mitigated pulmonary and systemic inflammation, and evidenced safety [53][54]. After the release of the first study of a successfully treated COVID-19 patient with MSC in China [55], several pilot trials and case reports appeared in which MSC or MSC-derived treatments were administered alone or in addition to the COVID-19 standard treatment [56]. The preliminary results were favorable in critically ill patients with poor prognoses, showing that these therapies could restore oxygenation levels and lung function, and downregulate CSs. To date, more than 100 clinical trials registered on the clinicaltrials.gov website are exploring the effects of MSC and their derivates in COVID-19. The results of the finalized trials are published in the PubMed database and describe the administration of MSC from different origins, as well as MSC-derived exosomes, EVs, and their secretome [57][58][59][60]. The main endpoint of those trials was to demonstrate safety and tolerability. All trials concluded that these therapies are completely safe, and no severe adverse events were observed. Another secondary endpoint was the efficacy of MSC-based therapies, based on the survival rate, clinical and laboratory improvements, such as the control of CSs. However, these results were not satisfactory [57][60][61][62][63]. While MSC and MSC-derived therapy administration demonstrated beneficial effects in the trials that recruited severe or critically ill patients, the results of the effect of those therapies in patients with mild-moderate symptoms or with low clinical risk were inconclusive. This was mainly explained due to the small number of subjects enrolled in those trials. Therefore, additional clinical investigation is recommended [64][65]. In addition to the immunomodulatory activity, MSCs also enhance functional recovery by endogenous neurogenesis and the up-regulation of synaptic plasticity linked to releasing neurotrophic factors such as FGF, VEGF, NGF, NT-3, SDF-1, and BDNF [66][67][68]. Increasing levels of these neurotrophic factors activate several pathways promoting the survival, proliferation, and differentiation of neural precursor cells [68]. The co-culture of MSC with neural precursor cells increases the expression of proliferative markers as well as progenitors and neuronal markers. Furthermore, MSCs increase the expression of beta catenin and Ngn1, indicating that MSCs have a role in the commitment of the neuronal fate of neural precursor cells by increasing the Wnt signaling pathway [69]. Additionally, MSCs have the ability to induce axonal growth [70][71]. In a recent study, the injection of MSCs overexpressing FGF-21 corrected the abnormal TBI-induced dendritic morphology of immature newborn neurons [72]. Although the effectiveness of MSC therapy regarding genuine cell replacement remains limited considering the very limited MSC transdifferentiation, several studies support that the neuroprotective potential of MSCs relies on their secretome [66][73][74], a set of secreted bioactive molecules which are either dissolved in the cell medium or encapsulated within EVs [75]. This MSC-derived secretome stimulates endogenous self-repair processes, such as the proliferation and differentiation of neural stem cells, as well as neuron maturation and survival, resulting in positive outcomes [76][77][78]. In this line, preclinical studies support the use of MSC-derived exosomes in neural regeneration approaches [79][80][81][82]. Proteomic analysis of MSC-derived exosomes resulted in the identification of more than 900 proteins [83][84][85], including filamin-A, BDNF, vinculin, NGF, FGF, neuropilin-1, VEGF, neuroplastin, glia-derived nexin, DPYSL2, flotillin-1, ephrins, drebrin, neprilysin, teneurin-4, and stathmin, which induce neurogenesis and myelin formation, promote neurite outgrowth and branching, stimulate axonal growth and regeneration, and provide neuroprotection to injured neurons [77][86]. Moreover, their broad cytokine repertoire can efficiently inhibit the effector function of the inflammatory M1-like phenotype and induce the generation of the anti-inflammatory M2-like phenotype in microglial cells, as well as contribute to ameliorating cognitive alterations associated with inflammatory states [87][88]. Moreover, MSC-derived exosomes exhibit properties and cell functions without the controversial long-term fate of MSCs [89]. For instance, the MSC-derived exosomes exhibit a lower or no risk of mutagenicity, oncogenicity, and very low immunogenicity. For CNS targeting approaches, the main advantage of exosomes is their higher capacity to cross the BBB [90]. In addition, they have manufacturing advantages such as storage stability and more accessible transportation [45]. Therefore, the use of stem cell-derived exosomes has also been proposed as a treatment option for long COVID. On the way to developing and optimizing a cell-based therapy for long COVID, several parameters need to be controlled [91][92]. Among the most important determinants of the success of MSC-based therapies in neuropathies is deciding on the optimal delivery route to ensure that the treatment will reach the CNS [93]. In this line, some key factors that will determine the efficacy of the delivery are delivery to the olfactory area as opposed to the respiratory region, the dose volume, the retention time at the nasal mucosal surface, penetration of nasal epithelia, and a reduction of compound metabolism in the nasal cavity. In this context, using nanoparticles, penetration enhancers, and matrices like hydrogels could improve the delivery to the brain via the nose-to-brain route.4. Administration Routes of MSC and MSC-Derived Exosomes for Neurological Diseases
One of the biggest challenges of developing therapies for the nervous system is the delivery of treatment due to natural barriers. In cell-based therapies, we must customize the delivery route according to the targeted disease and the patient’s circumstances [94]. If the MSCs need to be in the injury site to exert their effects, the optimized and accurate delivery of cells to the injured tissue is a major determinant of overall success [93][95][96]. However, when the distal effects of the MSC-derived secretome can relieve the pathology of the disease, it may not be necessary for the cells to be located at the injury site, and we can use systemic routes [97]. It is important to highlight that delivery route efficacy can vary depending on the disease and target tissue, thus, the amount of MSC derivates necessary in the parenchyma to achieve the expected biological effect must be considered [96]. Different delivery methods have been used before in clinical trials to reach the nervous system. The most prevalent route for MSCs in clinical trials for COVID-19 is the intravenous route (IV) [45]. However, these trials were not focused on a specific pathology but on controlling the severity of the disease. An analysis of the MSC clinical trials from 2004 to 2018 showed that the most used administration routes for neurological disorders were IV and intrathecal (IT), followed by intra-muscular (IM), intra-arterial (IA), and intra-osseous (IO), probably because these routes matched with the targeted tissue [92]. Another work considering 71 clinical trials that used MSC for neurodegenerative diseases found that the most used route was IT, followed by IV. Other methods included administration into the injury site by surgery [98]. The IT route is the second most popular delivery method for neurological disorders since it administers cells directly into the cerebrospinal fluid (CSF), covering the entire neuraxis. It infuses the MSCs into the subarachnoid space and allows for higher concentrations of cells to migrate to the lesion site compared with systemic administration [99]. This safe route does not require brain surgery, avoiding serious complications such as needle tract injury, infection, and bleeding and lowering the medical cost and psychological burden of surgical procedures [100]. To date, the IT administration of MSCs has shown efficacy for various neurological conditions, including multiple sclerosis, autism, traumatic brain injury, and more, without serious adverse effects, infections, clinical rejection, or tumors [101]. The Intranasal (IN) route of stem cell administration is an opportunity for the efficient delivery of stem cells directly to the brain parenchyma because it is a non-invasive, rapid absorption method that allows for the penetration of BBB [102]. It uses the olfactory and respiratory pathways and the nasal vasculature to enter the brain tissue [103]. Three transport steps are necessary for delivery to the nervous system after IN administration: across epithelial barriers, from the nasal mucosa to brain entry sites, and from those sites to the parenchyma [104]. IN-administered stem cells appear to cross the olfactory epithelium and enter the subarachnoid space crossing the cribriform plate via the fila olfactoria [105]. To date, only one clinical trial has proved the feasibility and safety of intranasally administered MSCs [106]. More studies are needed to better understand this administration route. Although the correct administration route is critical to reach the CNS, there are other approaches to guarantee the distribution of MSCs and MSC-derived exosomes in a determined zone. In this sense, diverse strategies, including formulation enhancement have been designed to achieve this goal.References
- Li, Y.; Bai, W.; Hashikawa, T. The Neuroinvasive Potential of SARS-CoV2 May Play a Role in the Respiratory Failure of COVID-19 Patients. J. Med. Virol. 2020, 92, 552–555.
- Santos-López, G.; Cortés-Hernández, P.; Vallejo-Ruiz, V.; Reyes-Leyva, J. SARS-CoV-2: Basic Concepts, Origin and Treatment Advances. Gac. Med. Mex. 2021, 157, 84–89.
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 14 November 2023).
- Loke, X.Y.; Imran, S.A.M.; Tye, G.J.; Wan Kamarul Zaman, W.S.; Nordin, F. Immunomodulation and Regenerative Capacity of MSCs for Long-COVID. Int. J. Mol. Sci. 2021, 22, 12421.
- Priyal; Sehgal, V.; Kapila, S.; Taneja, R.; Mehmi, P.; Gulati, N. Review of Neurological Manifestations of SARS-CoV-2. Cureus 2023, 15, e38194.
- Román-Montes, C.M.; Flores-Soto, Y.; Guaracha-Basañez, G.A.; Tamez-Torres, K.M.; Sifuentes-Osornio, J.; González-Lara, M.F.; de León, A.P. Post-COVID-19 Syndrome and Quality of Life Impairment in Severe COVID-19 Mexican Patients. Front. Public Health 2023, 11, 1155951.
- World Health Organization a Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 14 November 2023).
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An Overview. Diabetes Metab. Syndr. 2021, 15, 869–875.
- Natarajan, A.; Shetty, A.; Delanerolle, G.; Zeng, Y.; Zhang, Y.; Raymont, V.; Rathod, S.; Halabi, S.; Elliot, K.; Shi, J.Q.; et al. A Systematic Review and Meta-Analysis of Long COVID Symptoms. Syst. Rev. 2023, 12, 88.
- Zhao, Y.; Lukiw, W.J. SARS-CoV-2 Neuroinvasion, Inflammatory Neurodegeneration and Alzheimer’s Disease. Front. Cell. Neurosci. 2022, 16, 937961.
- Chapoval, S.P.; Keegan, A.D. Perspectives and Potential Approaches for Targeting Neuropilin 1 in SARS-CoV-2 Infection. Mol. Med. 2021, 27, 162.
- Masre, S.F.; Jufri, N.F.; Ibrahim, F.W.; Abdul Raub, S.H. Classical and Alternative Receptors for SARS-CoV-2 Therapeutic Strategy. Rev. Med. Virol. 2021, 31, 1–9.
- Xia, H.; Lazartigues, E. Angiotensin-Converting Enzyme 2: Central Regulator for Cardiovascular Function. Curr. Hypertens. Rep. 2010, 12, 170–175.
- Leng, A.; Shah, M.; Ahmad, S.A.; Premraj, L.; Wildi, K.; Li Bassi, G.; Pardo, C.A.; Choi, A.; Cho, S.-M. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells 2023, 12, 816.
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory Transmucosal SARS-CoV-2 Invasion as a Port of Central Nervous System Entry in Individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175.
- Bauer, L.; Laksono, B.M.; de Vrij, F.M.S.; Kushner, S.A.; Harschnitz, O.; van Riel, D. The Neuroinvasiveness, Neurotropism, and Neurovirulence of SARS-CoV-2. Trends Neurosci. 2022, 45, 358–368.
- Norouzi, M.; Miar, P.; Norouzi, S.; Nikpour, P. Nervous System Involvement in COVID-19: A Review of the Current Knowledge. Mol. Neurobiol. 2021, 58, 3561–3574.
- Reza-Zaldívar, E.E.; Hernández-Sapiéns, M.A.; Minjarez, B.; Gómez-Pinedo, U.; Márquez-Aguirre, A.L.; Mateos-Díaz, J.C.; Matias-Guiu, J.; Canales-Aguirre, A.A. Infection Mechanism of SARS-COV-2 and Its Implication on the Nervous System. Front. Immunol. 2020, 11, 621735.
- Erickson, M.A.; Rhea, E.M.; Knopp, R.C.; Banks, W.A. Interactions of SARS-CoV-2 with the Blood–Brain Barrier. Int. J. Mol. Sci. 2021, 22, 2681.
- Stefanou, M.-I.; Palaiodimou, L.; Bakola, E.; Smyrnis, N.; Papadopoulou, M.; Paraskevas, G.P.; Rizos, E.; Boutati, E.; Grigoriadis, N.; Krogias, C.; et al. Neurological Manifestations of Long-COVID Syndrome: A Narrative Review. Ther. Adv. Chronic Dis. 2022, 13, 204062232210768.
- Monje, M.; Iwasaki, A. The Neurobiology of Long COVID. Neuron 2022, 110, 3484–3496.
- Whittaker, A.; Anson, M.; Harky, A. Neurological Manifestations of COVID-19: A Systematic Review and Current Update. Acta Neurol. Scand. 2020, 142, 14–22.
- Acharya, A.; Kevadiya, B.D.; Gendelman, H.E.; Byrareddy, S.N. SARS-CoV-2 Infection Leads to Neurological Dysfunction. J. Neuroimmune Pharmacol. 2020, 15, 167–173.
- Desforges, M.; Le Coupanec, A.; Brison, E.; Meessen-Pinard, M.; Talbot, P.J. Neuroinvasive and Neurotropic Human Respiratory Coronaviruses: Potential Neurovirulent Agents in Humans. Adv. Exp. Med. Biol. 2014, 807, 75–96.
- Asadi-Pooya, A.A.; Simani, L. Central Nervous System Manifestations of COVID-19: A Systematic Review. J. Neurol. Sci. 2020, 413, 116832.
- Doyle, M.F. Central Nervous System Outcomes of COVID-19. Transl. Res. 2022, 241, 41–51.
- Chen, X.; Laurent, S.; Onur, O.A.; Kleineberg, N.N.; Fink, G.R.; Schweitzer, F.; Warnke, C. A Systematic Review of Neurological Symptoms and Complications of COVID-19. J. Neurol. 2021, 268, 392–402.
- van Kessel, S.A.M.; Olde Hartman, T.C.; Lucassen, P.L.B.J.; van Jaarsveld, C.H.M. Post-Acute and Long-COVID-19 Symptoms in Patients with Mild Diseases: A Systematic Review. Fam. Pract. 2022, 39, 159–167.
- Xu, E.; Xie, Y.; Al-Aly, Z. Long-Term Neurologic Outcomes of COVID-19. Nat. Med. 2022, 28, 2406–2415.
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-Month Neurological and Psychiatric Outcomes in 236 379 Survivors of COVID-19: A Retrospective Cohort Study Using Electronic Health Records. Lancet Psychiatry 2021, 8, 416–427.
- Yong, S.J.; Liu, S. Proposed Subtypes of Post-COVID-19 Syndrome (or Long-COVID) and Their Respective Potential Therapies. Rev. Med. Virol. 2022, 32, e2315.
- Delgado-Alonso, C.; Valles-Salgado, M.; Delgado-Álvarez, A.; Yus, M.; Gómez-Ruiz, N.; Jorquera, M.; Polidura, C.; Gil, M.J.; Marcos, A.; Matías-Guiu, J.; et al. Cognitive Dysfunction Associated with COVID-19: A Comprehensive Neuropsychological Study. J. Psychiatr. Res. 2022, 150, 40–46.
- Hugon, J.; Msika, E.-F.; Queneau, M.; Farid, K.; Paquet, C. Long COVID: Cognitive Complaints (Brain Fog) and Dysfunction of the Cingulate Cortex. J. Neurol. 2022, 269, 44–46.
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long Covid-Mechanisms, Risk Factors, and Management. BMJ 2021, 374, n1648.
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-Acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169.
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020, 11, 1708.
- Tutal Gursoy, G.; Yuksel, H.; Mulkem Simsek, I.; Oral, S.; Erdogan Kucukdagli, F.; Karaman, A.; Akinci, E.; Bastug, A.; Guner, H.R.; Bektas, H. Neurological Presentations in Patients with COVID-19 in Cytokine Storm. Can. J. Neurol. Sci./J. Can. des Sci. Neurol. 2023, 50, 89–95.
- Jarrott, B.; Head, R.; Pringle, K.G.; Lumbers, E.R.; Martin, J.H. “LONG COVID”—A Hypothesis for Understanding the Biological Basis and Pharmacological Treatment Strategy. Pharmacol. Res. Perspect. 2022, 10, e00911.
- Thepmankorn, P.; Bach, J.; Lasfar, A.; Zhao, X.; Souayah, S.; Chong, Z.Z.; Souayah, N. Cytokine Storm Induced by SARS-CoV-2 Infection: The Spectrum of Its Neurological Manifestations. Cytokine 2021, 138, 155404.
- Díez-Cirarda, M.; Yus, M.; Gómez-Ruiz, N.; Polidura, C.; Gil-Martínez, L.; Delgado-Alonso, C.; Jorquera, M.; Gómez-Pinedo, U.; Matias-Guiu, J.; Arrazola, J.; et al. Multimodal Neuroimaging in Post-COVID Syndrome and Correlation with Cognition. Brain 2023, 146, 2142–2152.
- Díez-Cirarda, M.; Yus-Fuertes, M.; Sanchez-Sanchez, R.; Gonzalez-Rosa, J.J.; Gonzalez-Escamilla, G.; Gil-Martínez, L.; Delgado-Alonso, C.; Gil-Moreno, M.J.; Valles-Salgado, M.; Cano-Cano, F.; et al. Hippocampal Subfield Abnormalities and Biomarkers of Pathologic Brain Changes: From SARS-CoV-2 Acute Infection to Post-COVID Syndrome. EBioMedicine 2023, 94, 104711.
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 Is Associated with Changes in Brain Structure in UK Biobank. Nature 2022, 604, 697–707.
- Blomberg, B.; Cox, R.J.; Langeland, N. Long COVID: A Growing Problem in Need of Intervention. Cell Rep. Med. 2022, 3, 100552.
- Delgado-Alonso, C.; Díez-Cirarda, M.; Pagán, J.; Pérez-Izquierdo, C.; Oliver-Mas, S.; Fernández-Romero, L.; Martínez-Petit, Á.; Valles-Salgado, M.; Gil-Moreno, M.J.; Yus, M.; et al. Unraveling Brain Fog in Post-COVID Syndrome: Relationship between Subjective Cognitive Complaints and Cognitive Function, Fatigue, and Neuropsychiatric Symptoms. Eur. J. Neurol. 2023; Online ahead of print.
- Chrzanowski, W.; Kim, S.Y.; McClements, L. Can Stem Cells Beat COVID-19: Advancing Stem Cells and Extracellular Vesicles Toward Mainstream Medicine for Lung Injuries Associated with SARS-CoV-2 Infections. Front. Bioeng. Biotechnol. 2020, 8, 554.
- Zayed, M.; Iohara, K. Immunomodulation and Regeneration Properties of Dental Pulp Stem Cells: A Potential Therapy to Treat Coronavirus Disease 2019. Cell Transplant. 2020, 29, 096368972095208.
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451.
- Iyer, S.S.; Rojas, M. Anti-Inflammatory Effects of Mesenchymal Stem Cells: Novel Concept for Future Therapies. Expert Opin. Biol. Ther. 2008, 8, 569–581.
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286.
- Silva, J.D.; Lopes-Pacheco, M.; Paz, A.H.R.; Cruz, F.F.; Melo, E.B.; de Oliveira, M.V.; Xisto, D.G.; Capelozzi, V.L.; Morales, M.M.; Pelosi, P.; et al. Mesenchymal Stem Cells From Bone Marrow, Adipose Tissue, and Lung Tissue Differentially Mitigate Lung and Distal Organ Damage in Experimental Acute Respiratory Distress Syndrome*. Crit. Care Med. 2018, 46, e132–e140.
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Influenza Virus-Induced Acute Lung Injury in a Pig Model. Stem Cell Res. Ther. 2018, 9, 17.
- Loy, H.; Kuok, D.I.T.; Hui, K.P.Y.; Choi, M.H.L.; Yuen, W.; Nicholls, J.M.; Peiris, J.S.M.; Chan, M.C.W. Therapeutic Implications of Human Umbilical Cord Mesenchymal Stromal Cells in Attenuating Influenza A(H5N1) Virus—Associated Acute Lung Injury. J. Infect. Dis. 2019, 219, 186–196.
- Khoury, M.; Cuenca, J.; Cruz, F.F.; Figueroa, F.E.; Rocco, P.R.M.; Weiss, D.J. Current Status of Cell-Based Therapies for Respiratory Virus Infections: Applicability to COVID-19. Eur. Respir. J. 2020, 55, 2000858.
- Rogers, C.J.; Harman, R.J.; Bunnell, B.A.; Schreiber, M.A.; Xiang, C.; Wang, F.-S.; Santidrian, A.F.; Minev, B.R. Rationale for the Clinical Use of Adipose-Derived Mesenchymal Stem Cells for COVID-19 Patients. J. Transl. Med. 2020, 18, 203.
- Liang, B.; Chen, J.; Li, T.; Wu, H.; Yang, W.; Li, Y.; Li, J.; Yu, C.; Nie, F.; Ma, Z.; et al. Clinical Remission of a Critically Ill COVID-19 Patient Treated by Human Umbilical Cord Mesenchymal Stem Cells. Medicine 2020, 99, e21429.
- Shu, L.; Niu, C.; Li, R.; Huang, T.; Wang, Y.; Huang, M.; Ji, N.; Zheng, Y.; Chen, X.; Shi, L.; et al. Treatment of Severe COVID-19 with Human Umbilical Cord Mesenchymal Stem Cells. Stem Cell Res. Ther. 2020, 11, 361.
- Fathi-Kazerooni, M.; Fattah-Ghazi, S.; Darzi, M.; Makarem, J.; Nasiri, R.; Salahshour, F.; Dehghan-Manshadi, S.A.; Kazemnejad, S. Safety and Efficacy Study of Allogeneic Human Menstrual Blood Stromal Cells Secretome to Treat Severe COVID-19 Patients: Clinical Trial Phase I & II. Stem Cell Res. Ther. 2022, 13, 96.
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754.
- Zhu, Y.-G.; Shi, M.-M.; Monsel, A.; Dai, C.-X.; Dong, X.; Shen, H.; Li, S.-K.; Chang, J.; Xu, C.-L.; Li, P.; et al. Nebulized Exosomes Derived from Allogenic Adipose Tissue Mesenchymal Stromal Cells in Patients with Severe COVID-19: A Pilot Study. Stem Cell Res. Ther. 2022, 13, 220.
- Zarrabi, M.; Shahrbaf, M.A.; Nouri, M.; Shekari, F.; Hosseini, S.-E.; Hashemian, S.-M.R.; Aliannejad, R.; Jamaati, H.; Khavandgar, N.; Alemi, H.; et al. Allogenic Mesenchymal Stromal Cells and Their Extracellular Vesicles in COVID-19 Induced ARDS: A Randomized Controlled Trial. Stem Cell Res. Ther. 2023, 14, 169.
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity after Stroke in Rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715.
- Monsel, A.; Hauw-Berlemont, C.; Mebarki, M.; Heming, N.; Mayaux, J.; Nguekap Tchoumba, O.; Diehl, J.-L.; Demoule, A.; Annane, D.; Marois, C.; et al. Treatment of COVID-19-Associated ARDS with Mesenchymal Stromal Cells: A Multicenter Randomized Double-Blind Trial. Crit. Care 2022, 26, 48.
- Bowdish, M.E.; Barkauskas, C.E.; Overbey, J.R.; Gottlieb, R.L.; Osman, K.; Duggal, A.; Marks, M.E.; Hupf, J.; Fernandes, E.; Leshnower, B.G.; et al. A Randomized Trial of Mesenchymal Stromal Cells for Moderate to Severe Acute Respiratory Distress Syndrome from COVID-19. Am. J. Respir. Crit. Care Med. 2023, 207, 261–270.
- Kaffash Farkhad, N.; Sedaghat, A.; Reihani, H.; Adhami Moghadam, A.; Bagheri Moghadam, A.; Khadem Ghaebi, N.; Khodadoust, M.A.; Ganjali, R.; Tafreshian, A.R.; Tavakol-Afshari, J. Mesenchymal Stromal Cell Therapy for COVID-19-Induced ARDS Patients: A Successful Phase 1, Control-Placebo Group, Clinical Trial. Stem Cell Res. Ther. 2022, 13, 283.
- Karyana, M.; Djaharuddin, I.; Rif’ati, L.; Arif, M.; Choi, M.K.; Angginy, N.; Yoon, A.; Han, J.; Josh, F.; Arlinda, D.; et al. Safety of DW-MSC Infusion in Patients with Low Clinical Risk COVID-19 Infection: A Randomized, Double-Blind, Placebo-Controlled Trial. Stem Cell Res. Ther. 2022, 13, 134.
- Wang, F.; Tang, H.; Zhu, J.; Zhang, J.H. Transplanting Mesenchymal Stem Cells for Treatment of Ischemic Stroke. Cell Transplant. 2018, 27, 1825–1834.
- Cui, Y.; Ma, S.; Zhang, C.; Cao, W.; Liu, M.; Li, D.; Lv, P.; Xing, Q.; Qu, R.; Yao, N.; et al. Human Umbilical Cord Mesenchymal Stem Cells Transplantation Improves Cognitive Function in Alzheimer’s Disease Mice by Decreasing Oxidative Stress and Promoting Hippocampal Neurogenesis. Behav. Brain Res. 2017, 320, 291–301.
- Bao, X.; Wei, J.; Feng, M.; Lu, S.; Li, G.; Dou, W.; Ma, W.; Ma, S.; An, Y.; Qin, C.; et al. Transplantation of Human Bone Marrow-Derived Mesenchymal Stem Cells Promotes Behavioral Recovery and Endogenous Neurogenesis after Cerebral Ischemia in Rats. Brain Res. 2011, 1367, 103–113.
- Oh, S.H.; Kim, H.N.; Park, H.-J.; Shin, J.Y.; Lee, P.H. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Neuronal Differentiation by Enhancing the Wnt Signaling Pathway in an Alzheimer’s Disease Model. Cell Transplant. 2015, 24, 1097–1109.
- Martins, L.F.; Costa, R.O.; Pedro, J.R.; Aguiar, P.; Serra, S.C.; Teixeira, F.G.; Sousa, N.; Salgado, A.J.; Almeida, R.D. Mesenchymal Stem Cells Secretome-Induced Axonal Outgrowth Is Mediated by BDNF. Sci. Rep. 2017, 7, 4153.
- Lin, L.; Lin, H.; Bai, S.; Zheng, L.; Zhang, X. Bone Marrow Mesenchymal Stem Cells (BMSCs) Improved Functional Recovery of Spinal Cord Injury Partly by Promoting Axonal Regeneration. Neurochem. Int. 2018, 115, 80–84.
- Shahror, R.A.; Linares, G.R.; Wang, Y.; Hsueh, S.-C.; Wu, C.-C.; Chuang, D.-M.; Chiang, Y.-H.; Chen, K.-Y. Transplantation of Mesenchymal Stem Cells Overexpressing Fibroblast Growth Factor 21 Facilitates Cognitive Recovery and Enhances Neurogenesis in a Mouse Model of Traumatic Brain Injury. J. Neurotrauma 2020, 37, 14–26.
- Mitsialis, S.A.; Kourembanas, S. Stem Cell–Based Therapies for the Newborn Lung and Brain: Possibilities and Challenges. Semin. Perinatol. 2016, 40, 138–151.
- Harrell, C.R.; Volarevic, A.; Djonov, V.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes as New Remedy for the Treatment of Neurocognitive Disorders. Int. J. Mol. Sci. 2021, 22, 1433.
- Teixeira, F.; Salgado, A. Mesenchymal Stem Cells Secretome: Current Trends and Future Challenges. Neural Regen. Res. 2020, 15, 75.
- Reza-Zaldivar, E.; Hernández-Sapiéns, M.; Gutiérrez-Mercado, Y.; Sandoval-Ávila, S.; Gomez-Pinedo, U.; Márquez-Aguirre, A.; Vázquez-Méndez, E.; Padilla-Camberos, E.; Canales-Aguirre, A. Mesenchymal Stem Cell-Derived Exosomes Promote Neurogenesis and Cognitive Function Recovery in a Mouse Model of Alzheimer’s Disease. Neural Regen. Res. 2019, 14, 1626.
- Reza-Zaldivar, E.E.; Hernández-Sapiéns, M.A.; Minjarez, B.; Gutiérrez-Mercado, Y.K.; Márquez-Aguirre, A.L.; Canales-Aguirre, A.A. Potential Effects of MSC-Derived Exosomes in Neuroplasticity in Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 317.
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536.
- Vizoso, F.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852.
- Luarte, A.; Bátiz, L.F.; Wyneken, U.; Lafourcade, C. Potential Therapies by Stem Cell-Derived Exosomes in CNS Diseases: Focusing on the Neurogenic Niche. Stem Cells Int. 2016, 2016, 5736059.
- Cantinieaux, D.; Quertainmont, R.; Blacher, S.; Rossi, L.; Wanet, T.; Noël, A.; Brook, G.; Schoenen, J.; Franzen, R. Conditioned Medium from Bone Marrow-Derived Mesenchymal Stem Cells Improves Recovery after Spinal Cord Injury in Rats: An Original Strategy to Avoid Cell Transplantation. PLoS ONE 2013, 8, e69515.
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Paracrine-Mediated Neuroprotection and Neuritogenesis of Axotomised Retinal Ganglion Cells by Human Dental Pulp Stem Cells: Comparison with Human Bone Marrow and Adipose-Derived Mesenchymal Stem Cells. PLoS ONE 2014, 9, e109305.
- Kupcova Skalnikova, H. Proteomic Techniques for Characterisation of Mesenchymal Stem Cell Secretome. Biochimie 2013, 95, 2196–2211.
- Skalnikova, H.; Motlik, J.; Gadher, S.J.; Kovarova, H. Mapping of the Secretome of Primary Isolates of Mammalian Cells, Stem Cells and Derived Cell Lines. Proteomics 2011, 11, 691–708.
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692.
- Ma, X.; Huang, M.; Zheng, M.; Dai, C.; Song, Q.; Zhang, Q.; Li, Q.; Gu, X.; Chen, H.; Jiang, G.; et al. ADSCs-Derived Extracellular Vesicles Alleviate Neuronal Damage, Promote Neurogenesis and Rescue Memory Loss in Mice with Alzheimer’s Disease. J. Control. Release 2020, 327, 688–702.
- Yang, Y.; Ye, Y.; Su, X.; He, J.; Bai, W.; He, X. MSCs-Derived Exosomes and Neuroinflammation, Neurogenesis and Therapy of Traumatic Brain Injury. Front. Cell. Neurosci. 2017, 11, 55.
- Ding, M.; Shen, Y.; Wang, P.; Xie, Z.; Xu, S.; Zhu, Z.Y.; Wang, Y.; Lyu, Y.; Wang, D.; Xu, L.; et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochem. Res. 2018, 43, 2165–2177.
- Shi, J.; Zhao, Y.-C.; Niu, Z.-F.; Fan, H.-J.; Hou, S.-K.; Guo, X.-Q.; Sang, L.; Lv, Q. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles in the Treatment of Human Diseases: Progress and Prospect. World J. Stem Cells 2021, 13, 49–63.
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from Mesenchymal Stem/Stromal Cells: A New Therapeutic Paradigm. Biomark. Res. 2019, 7, 8.
- Grumet, M.; Sherman, J.; Dorf, B.S. Efficacy of MSC in Patients with Severe COVID-19: Analysis of the Literature and a Case Study. Stem Cells Transl. Med. 2022, 11, 1103–1112.
- Kabat, M.; Bobkov, I.; Kumar, S.; Grumet, M. Trends in Mesenchymal Stem Cell Clinical Trials 2004–2018: Is Efficacy Optimal in a Narrow Dose Range? Stem Cells Transl. Med. 2020, 9, 17–27.
- Masterson, C.H.; Curley, G.F.; Laffey, J.G. Modulating the Distribution and Fate of Exogenously Delivered MSCs to Enhance Therapeutic Potential: Knowns and Unknowns. Intensiv. Care Med. Exp. 2019, 7, 41.
- Kim, S.Y.; Chrzanowski, W. Stem Cell Delivery Systems and Devices—Spraying. In Stem Cell-Based Therapy for Lung Disease; Springer International Publishing: Cham, Switzerland, 2019; pp. 241–253. ISBN 978-3-030-29403-8.
- Jamshidi, E.; Babajani, A.; Soltani, P.; Niknejad, H. Proposed Mechanisms of Targeting COVID-19 by Delivering Mesenchymal Stem Cells and Their Exosomes to Damaged Organs. Stem Cell Rev. Rep. 2021, 17, 176–192.
- Lee, N.K.; Yang, J.; Chang, E.H.; Park, S.E.; Lee, J.; Choi, S.J.; Oh, W.; Chang, J.W.; Na, D.L. Intra-Arterially Delivered Mesenchymal Stem Cells Are Not Detected in the Brain Parenchyma in an Alzheimer’s Disease Mouse Model. PLoS ONE 2016, 11, e0155912.
- Kean, T.J.; Lin, P.; Caplan, A.I.; Dennis, J.E. MSCs: Delivery Routes and Engraftment, Cell-Targeting Strategies, and Immune Modulation. Stem Cells Int. 2013, 2013, 732742.
- Van den Bos, J.; Ouaamari, Y.E.; Wouters, K.; Cools, N.; Wens, I. Are Cell-Based Therapies Safe and Effective in the Treatment of Neurodegenerative Diseases? A Systematic Review with Meta-Analysis. Biomolecules 2022, 12, 340.
- Deng, L.; Peng, Q.; Wang, H.; Pan, J.; Zhou, Y.; Pan, K.; Li, J.; Wu, Y.; Wang, Y. Intrathecal Injection of Allogenic Bone Marrow-Derived Mesenchymal Stromal Cells in Treatment of Patients with Severe Ischemic Stroke: Study Protocol for a Randomized Controlled Observer-Blinded Trial. Transl. Stroke Res. 2019, 10, 170–177.
- Kim, H.; Na, D.L.; Lee, N.K.; Kim, A.R.; Lee, S.; Jang, H. Intrathecal Injection in a Rat Model: A Potential Route to Deliver Human Wharton’s Jelly-Derived Mesenchymal Stem Cells into the Brain. Int. J. Mol. Sci. 2020, 21, 1272.
- Barmada, A.; Sharan, J.; Band, N.; Prodromos, C. Serious Adverse Events Have Not Been Reported with Spinal Intrathecal Injection of Mesenchymal Stem Cells: A Systematic Review. Curr. Stem Cell Res. Ther. 2023, 18, 829–833.
- Patel, M.M.; Patel, B.M. Crossing the Blood–Brain Barrier: Recent Advances in Drug Delivery to the Brain. CNS Drugs 2017, 31, 109–133.
- Inoue, T.; Sugiyama, M.; Hattori, H.; Wakita, H.; Wakabayashi, T.; Ueda, M. Stem Cells from Human Exfoliated Deciduous Tooth-Derived Conditioned Medium Enhance Recovery of Focal Cerebral Ischemia in Rats. Tissue Eng. Part A 2013, 19, 24–29.
- Lochhead, J.J.; Thorne, R.G. Intranasal Delivery of Biologics to the Central Nervous System. Adv. Drug Deliv. Rev. 2012, 64, 614–628.
- Galeano, C.; Qiu, Z.; Mishra, A.; Farnsworth, S.L.; Hemmi, J.J.; Moreira, A.; Edenhoffer, P.; Hornsby, P.J. The Route by Which Intranasally Delivered Stem Cells Enter the Central Nervous System. Cell Transplant. 2018, 27, 501–514.
- Baak, L.M.; Wagenaar, N.; van der Aa, N.E.; Groenendaal, F.; Dudink, J.; Tataranno, M.L.; Mahamuud, U.; Verhage, C.H.; Eijsermans, R.M.J.C.; Smit, L.S.; et al. Feasibility and Safety of Intranasally Administered Mesenchymal Stromal Cells after Perinatal Arterial Ischaemic Stroke in the Netherlands (PASSIoN): A First-in-Human, Open-Label Intervention Study. Lancet Neurol. 2022, 21, 528–536.
More
