You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Md Delwar Hossain.
Cladding is used as the outer layer in high-rise buildings to protect the building from weather conditions and enhance its visual appeal. Its primary function is to shield structures from environmental factors such as radiant sunlight and moisture, thereby preserving their structural integrity.
- biopolymer
- cladding materials
- manufacturing methods
1. Introduction
Cladding is used as the outer layer in high-rise buildings to protect the building from weather conditions and enhance its visual appeal. Its primary function is to shield structures from environmental factors such as radiant sunlight and moisture, thereby preserving their structural integrity [1,2][1][2]. However, escalating global energy consumption and environmental challenges have brought the necessity for energy efficiency in buildings to the forefront of architectural considerations [3]. With the construction sector accounting for more than 40% of worldwide energy usage and a significant contribution to greenhouse gas emissions, adopting sustainable building practices has never been more urgent [4]. The conventional array of cladding materials, encompassing metals, concrete, glass, plastics, and composites, has effectively provided protection; however, these materials often fall short in terms of sustainability and ecological impact [5]. While conventional synthetic polymer-based cladding materials have been widely used, their production from fossil fuels raises concerns about environmental sustainability, including greenhouse gas emissions and waste generation [6].
Conventional cladding materials are not sustainable. Polymer-based cladding materials are derived from non-renewable fossil fuels, which contribute to greenhouse gas emissions and global warming. They are also not eco-friendly, so they will persist in landfills and the environment for a long time, which is a threat to the environment. Additionally, they release toxic and harmful gases when on fire. Conventional cladding materials are also difficult to recycle, releasing harmful chemicals during disposal [7]. As the world seeks pathways toward a more sustainable future, the emergence of biopolymer-based materials offers a promising avenue for transforming the cladding landscape (Figure 1) [3,4,5][3][4][5].
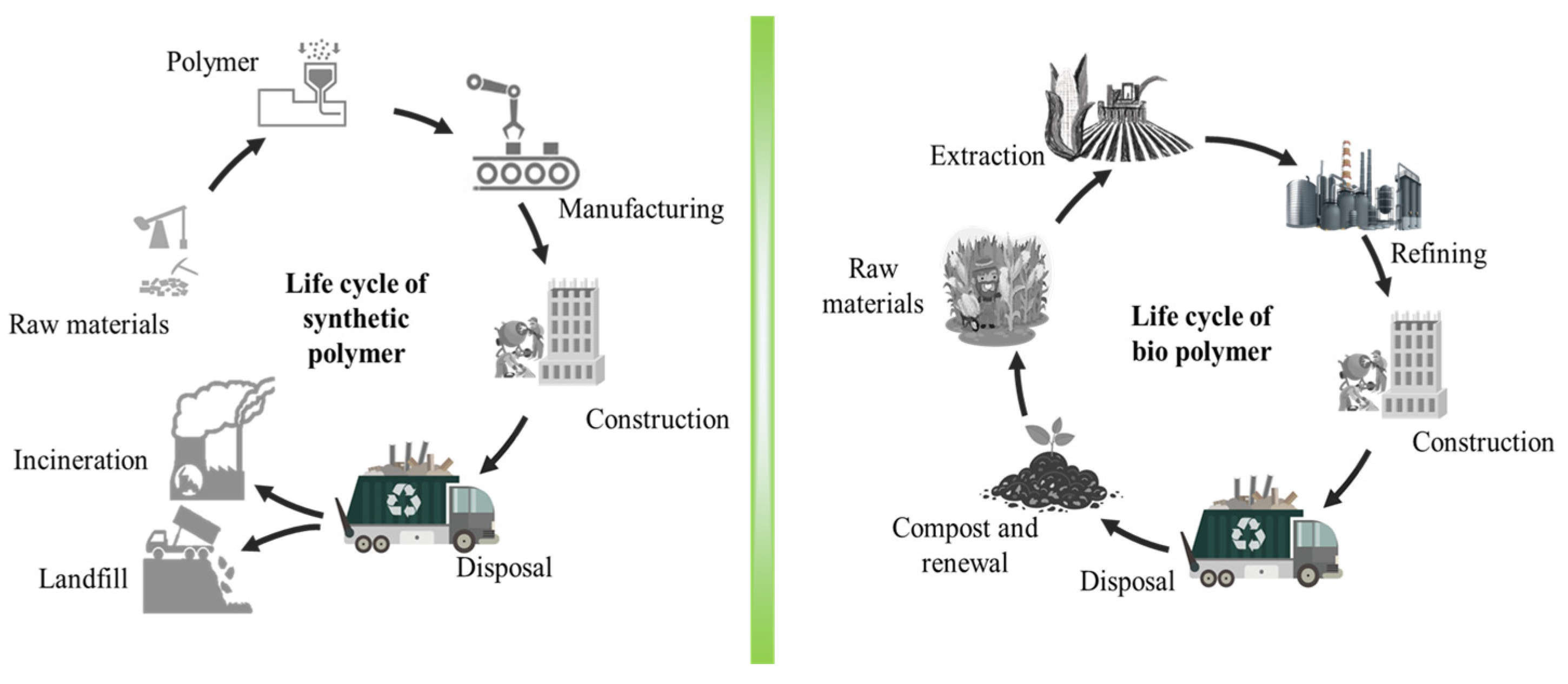
Figure 1.
Life cycle of synthetic polymer and biopolymer.
Biopolymers, derived from living organisms or their by-products, have garnered significant attention as a potential solution to the limitations of conventional synthetic polymers. These materials, including starch [8], cellulose [9], chitin [10], proteins [11], and lignin [12], exhibit attractive ecological attributes such as biodegradability, renewability, carbon sequestration potential, and notably low embodied energy requirements [1]. As such, biopolymer-based materials hold the potential to revolutionise cladding practices by providing environmentally friendly alternatives that align with the growing demand for sustainable construction materials [13] (Figure 2). Moreover, the integration of biopolymers into synthetic polymers can offer pathways to enhancing the biodegradability of materials. By blending synthetic polymers with biopolymer constituents like starches or infusing bioactive agents, the resulting composite materials facilitate the breakdown of the polymers into smaller components, contributing to the overall degradation process. Recently, biopolymers, like polylactic acid (PLA) [14], polyhydroxyalkanoates (PHAs) [15], starch-based polymers [16], cellulose [17], polyhydroxybutyrate (PHB) [18], and polybutylene succinate (PBS) [19], attract more attention in the biopolymer research field due to their biodegradable, waste disposal-friendly, and ecologically sustainable properties. However, most of the study mainly focused on packaging materials but rarely on building cladding products [20]. The intrinsic eco-friendly nature of biopolymer-based materials positions them not only as suitable for packaging material applications but also as sustainable cladding materials for future building uses.
Figure 2.
Different types of biopolymer-based materials have been explored in board cladding applications since they are great alternatives to conventional synthetic polymers. These biopolymer-based materials are great substitutes which offer a wide range of benefits such as biodegradability, renewable sourcing, reduced carbon footprint, enhanced environmental performance, etc. The most commonly used biopolymer-based materials used in cladding have been discussed in the following sub-sections.
3.1. Polylactic Acid (PLA)
2. Polylactic Acid (PLA)
Polylactic acid (PLA) is a biodegradable polymer that comes from renewable resources like corn starch, sugarcane, or other plant-based materials [22]. Its outstanding performance as a cladding material has gained it a great name and attention. It has excellent mechanical properties, low toxicity, and innate biodegradability [14]. PLA is a thermoplastic polymer made from lactic acid (Figure 3). Lactic acid is mainly produced by microbial fermentation of plant starch and is produced in two stereoisomeric forms of lactic acid, which are L-lactic acid and D-lactic acid [23]. The formulation of polylactic acid needs a monomer. Lactic acid is the monomer obtained through the polycondensation process [24]. PLA formulation also involves a catalyst, a solvent, and additives. The standard metal catalyst for polylactic acid is tin octoate [25]. PLA can be made from lactic acid monomer through two different methods, which are ring-opening polymerisation (ROP) and direct polycondensation (DP). While polycondensation directly connects lactic acid molecules, ROP breaks down lactide cyclic dimers [26]. The ultimate properties of polylactic acid depend on the stereoisomeric form of its production and the catalyst used during synthesis [27]. ROP creates large, well-structured, and crystalline polylactic acid molecules; however, lactic acid monomers directly react in the DP process, and water is removed by vacuum or azeotropic distillation. This process produces moderate-weight polylactic acid with lower cost and less environmental impact [28]. PLA is biodegradable under industrial composting conditions and has a lower carbon footprint compared to petroleum-based plastics.
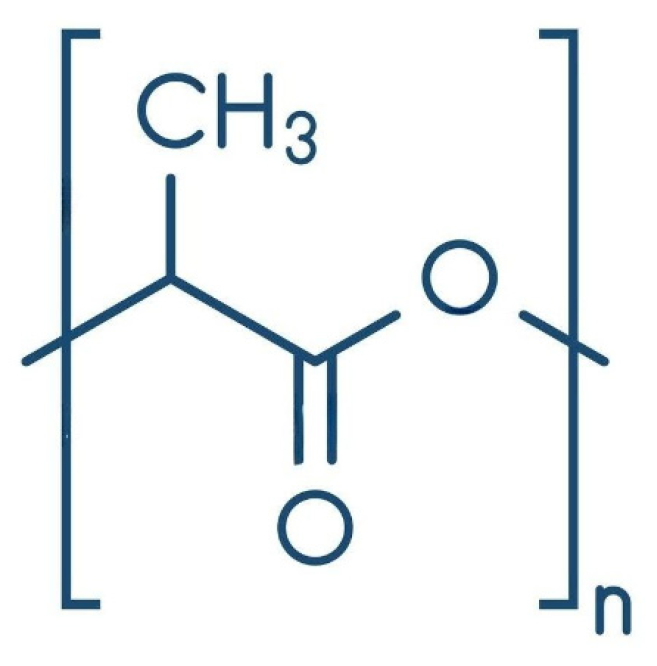
Figure 3.
3.2. Polyhydroxyalkanoates (PHAs)
3. Polyhydroxyalkanoates (PHAs)
Another fascinating biopolymer group is polyhydroxyalkanoates (PHAs), which are widely used in the cladding industry. PHAs show a wide range of excellent properties, including biodegradability, thermoplasticity, and mechanical strength [15]. Furthermore, the physical and chemical properties of PHAs can be tailored to cater to specific cladding requirements, making them a versatile option. PHAs are easily biodegradable in soil, ocean, and industrial composting conditions. They offer less reliance on fossil fuels and have less of an impact on the environment [15].
PHAs are a type of biopolymer that are naturally produced by bacteria. PHAs can be synthesised by large numbers of Gram-positive and Gram-negative bacteria from 75 different genres. PHAs can be produced through a variety of metabolic routes, depending on the particular microbe and its surrounding environment [30]. PHAs are a straight-chain polyester which is formed through the chemical ring-opening polymerisation of β-lactones [31]. Additionally, both Gram-positive and Gram-negative bacteria spontaneously make them under unfavourable circumstances, such as nutritional or physical stress [32]. The most common hydroxyalkanoates used for PHAs’ production are 3-hydroxybutyrate (3HB) and 3-hydroxyvalerate (3HV) [33,34][33][34]. PHAs are obtained from the microbial cells through different types of extraction techniques. In biological methods, the cell wall is disintegrated by enzymes or other microbes, allowing the separation of PHAs for retrieval. PHAs are purified using many techniques, such as precipitation, filtration, centrifugation, or chromatography. Their performance and properties depend on the purification [35,36][35][36].
3.3. Starch-Based Polymers (SBP)
4. Starch-Based Polymers (SBP)
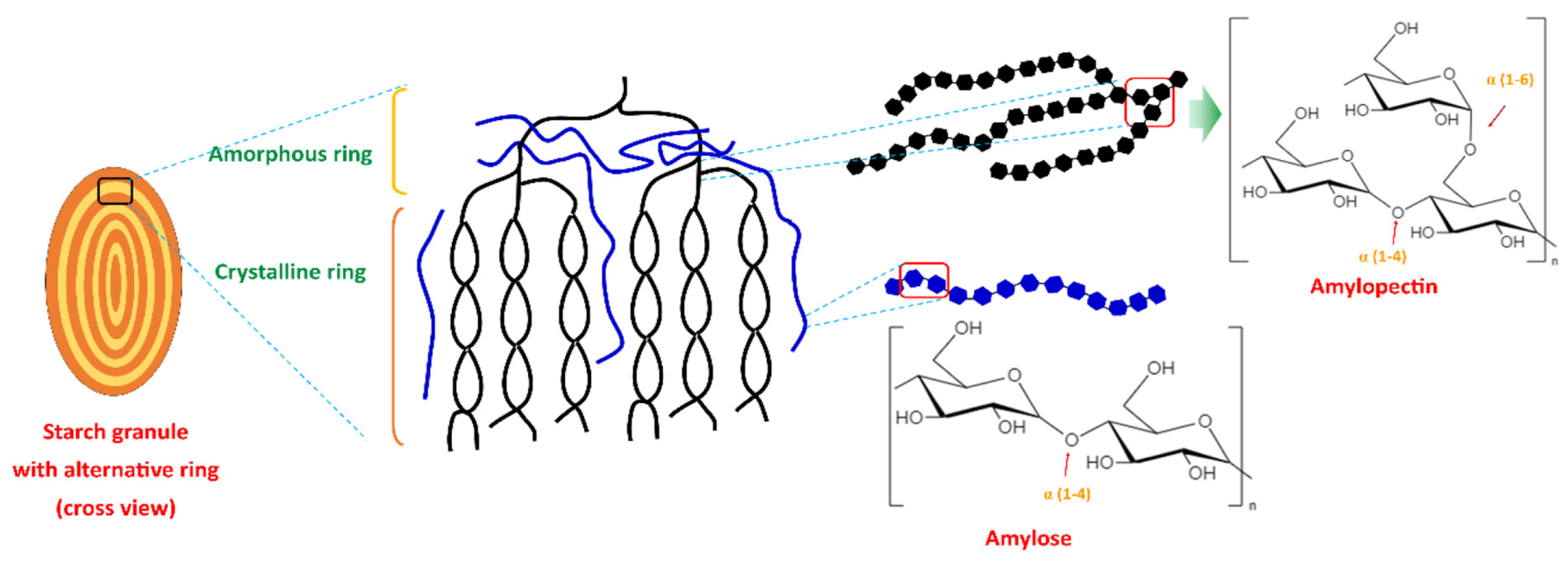
Starch-based polymers such as thermoplastic starch (TPS) are mainly derived from natural renewable sources like corn, potatoes, wheat, etc. (Figure 4). These polymers usually blend with other biodegradable polymers to enhance their properties, such as mechanical strength, processability, high dimensional stability, and moisture resistance. Starch-based polymers are biodegradable, renewable, and have a reduced carbon footprint compared to petroleum-based plastics, making starch-based polymers an appealing choice for cladding [16,37][16][37].

Figure 4.
Starch-based polymers are members of the biodegradable polymer family, and their source is from renewable natural resources like corn, potato, wheat, rice, and cassava [39]. Starch is a naturally occurring carbohydrate that is made of glucose units joined by α-1,4 and α-1,6 glycosidic bonds, and these two different forms of polymer chains are called amylose and amylopectin. Amylopectin contains α-1,4 glycosidic bonds, which are connected to the α-1,6 glycosidic bonds. On the other hand, amylose consists of a long straight chain containing only 1,4-glycosidic linkages [40,41][40][41]. Various types of physical or chemical modifications are applied to improve its properties [42]. Thermoplastic starch (TPS) is produced by mixing the starch-based polymer with other biopolymers, such as poly (lactic acid), poly (butylene adipate-co-terephthalate), poly(ε-caprolactone), and poly (vinyl alcohol). Adding these biopolymers enhances the mechanical properties, thermal stability, water resistance, and biodegradability of starch-based polymers. Thermoplastic starch can be reshaped through extrusion, injection, moulding, etc. [43]. Chemical alteration of starch-based biopolymers is another method to improve their properties, and it is normally achieved by reaction with the hydroxyl group in the starch molecule [44]. The resulting derivatives have different physicochemical characteristics in comparison with the original starch, while retaining their inherent biodegradability. Therefore, adding or changing some groups or chains on the hydroxyl parts can make different biodegradable starch-based materials that serve different purposes [43]. Compared to cellulose and other polysaccharides, starch has a diverse origin, different molecular weights, and distinct functional characteristics. Starch shows better processability and compatibility with other biopolymers. These qualities are highly relevant to its position within the biodegradable polymer sectors, highlighting its usefulness in supporting environmentally concerned enterprises [45].
3.4. Cellulose-Based Polymers (CBP)
5. Cellulose-Based Polymers (CBP)
Cellulose is one of the most common and natural polymers. It can be processed into various shapes and forms, like powders, films, gels, etc. [17]. Cellulose can also be chemically modified to produce cellulose-derivative biopolymers, such as cellulose acetate and cellulose nitrate, with desirable properties for cladding and various applications [17,46][17][46]. Plastic or synthetic polymers can be melted and reshaped, but cellulose cannot be melted or reshaped this way because of the strong connection between its molecules and hydrogen bonds [47]. Thermoplastics can be created by adding additional components to cellulose derivatives in a solvent state. However, this method can be costly because of using chemical agents such as cellulose ester [48]. There are different methods of making cellulose-based biopolymers, such as the hot-pressing process [49[49][50],50], directed deformation assembly process [51], and bacterial cellulose synthesis process [52]. In the hot-pressing process, cellulose molecular chains are guided to align in various directions but in a fixed dimension. As a result of this alignment, the cellulose undergoes a change in its shape and structure, ultimately leading to deformation [49,50][49][50]. The bacterial cellulose (BC) process is conducted with various bacterial species, such as acetobacter, agrobacterium, rhizobium, sarcina, and others. This complex procedure takes place under many environmental and growth circumstances, which help to produce the result [52]. It has excellent properties, such as biodegradability, biocompatibility, thermal stability, and good mechanical and barrier properties. Wood pulp, cotton, bacterial cellulose, etc., are the most common sources of cellulose-based polymers [46,53][46][53].
3.5. Polyhydroxybutyrate (PHB)
6. Polyhydroxybutyrate (PHB)
PHB is a biodegradable polymer produced from renewable and sustainable sources like food waste. It has a great capacity to break down in specific biological conditions, and these factors make it a strong substitute for artificial polymers such as PVC, PP, and PE [18]. However, the production cost of PHB is relatively higher than that of petrochemical-based plastics. Recent research has identified methods for lower PHB manufacturing costs, including increased bacterial strains, simplified fermentation, and improved recovery methods [54,55][54][55]. PHB is biosynthesised and accumulated by a number of specialised bacterial strains such as Alcaligenes eutrophus, Bacillus megaterium, Pseudomonas oleovorans, strains using a variety of organic substances that are found naturally and unutilised (wasted) as carbon sources. PHB can be blended or combined with other polymers or fillers like wood, metal, glass, etc, to improve its properties and performance, especially to form hybrid cladding systems [56,57,58][56][57][58]. The formulation of PHB can be divided into two main steps: the production of PHB by microorganisms and the extraction of PHB [59]. For the production of PHB, specific bacteria need to be selected and grown in a nutrient environment for 24 h at 37 °C. According to one report, around 5% of thriving bacteria was added to the 50 mL of modified mineral salt and left to grow for 72 h at 37 °C with gentle agitation (120 rpm) [60]. After the bacteria multiplied, these were separated from the liquid, the dried bacterial clumps were collected, and they were soaked in sodium hypochlorite to release the PHB materials. A filter was used to remove unwanted components from PHB material [61].
3.6. Polybutylene Adipate Terephthalate (PBAT)
7. Polybutylene Adipate Terephthalate (PBAT)
PBAT’s speciality lies in its ability to break down naturally in the environment, and the reason behind that is its ester linkage. The ester bond breaks down through the water and enzymes in the environment [62]. PBAT has good qualities of both synthetic and bio-based polymers. Although it is produced from regular petrochemicals such as purified terephthalic acid (PTA), butanediol, and adipic acid, it is biodegradable [19]. PBAT shows great water resistance properties, and its manufacturing process is much easier [63]. It has the perfect characteristics to create flexible films just like regular plastic materials [62]. However, PBAT is not strong enough for certain uses and is also more costly than other biopolymers [64].
In the PBAT synthesis process, adipic acid and 1,4-butanediol are added to a stainless-steel reactor in a fixed mole ratio. Stirring is used to bring the reactants’ temperature up, and distillation is used to get rid of the water that is produced during the reaction [65]. After 1–2 h, tetrabutylorthotitanate (TBOT) is added to the mixture at room temperature. Under vacuum, the reaction temperature is elevated for 4h, and dimethyl-terephthalate (DMT), 1,4-butanediol, and TBOT are added in a specific ratio. The temperature needs to be maintained, and the process takes around 20h under a high vacuum [65].
3.7. Polybutylene Succinate (PBS)
8. Polybutylene Succinate (PBS)
Polybutylene succinate (PBS) is a semi-crystalline polymer which is produced through a direct reaction of succinic acid and 1,4-butanediol. It is environmentally friendly because it breaks down more rapidly than traditional petrochemical plastics and leaves no toxic materials behind [66]. However, polybutylene succinate (PBS) is much more expensive compared to petrochemicals such as polystyrene (PS), polyamides (PAs), polyethylene terephthalate (PET), and polyethylene (PE), because it involves special processing and combining succinic acid and 1,4-butanediol in the process [67].
Polybutylene succinate (PBS) can be produced in various ways, including polycondensation of succinic acid and 1,4-butanediol, where monomers come from fossil-based or renewable sources. This method has potential in the case of enhanced thermal and mechanical properties [68]. In the fermentation process, microorganisms can be used to make succinic acid. To create bio-based PBS, numerous microorganisms have been examined and put to the test [69]. In the chemical synthesis process, chemically synthesised aliphatic polyesters with high molecular weights can improve the properties of PBS. For instance, poly (butylene succinate-co-ethylene succinate) is synthesised through direct polycondensation in the presence of N35 catalyst [70].
3.8. Polycaprolactone (PCL)
9. Polycaprolactone (PCL)
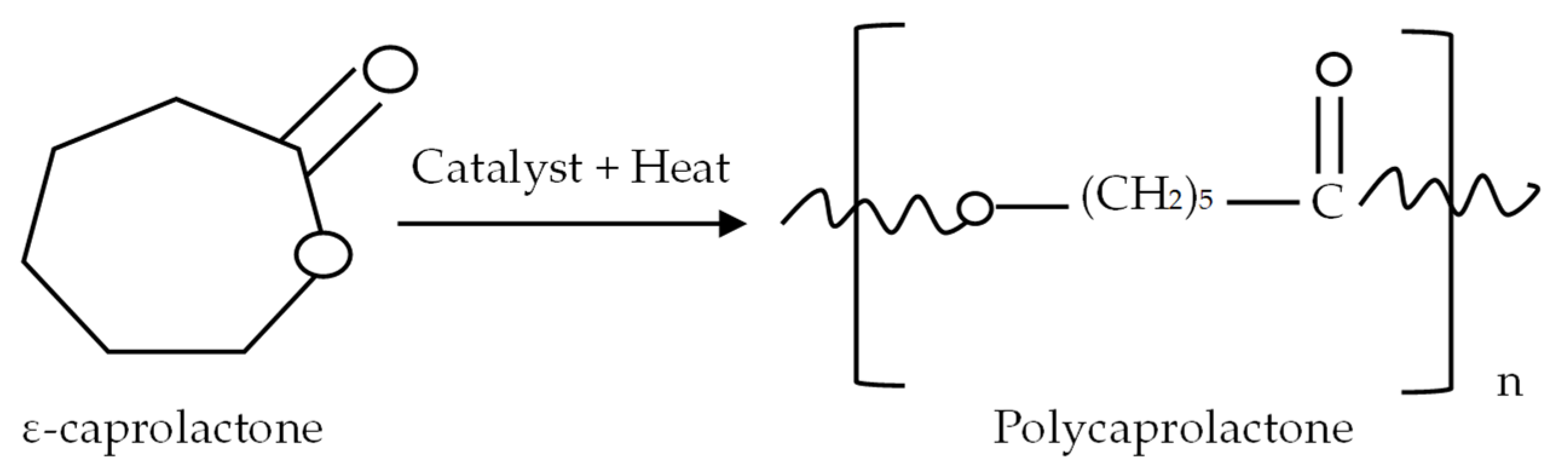
Polycaprolactone (PCL) is a member of the biodegradable synthetic polyesters group which has proven incredibly valuable in various applications [71]. In the 1930s, researchers produced polycaprolactone (PCL) polymer materials from the ε-caprolactone monomer for the first time with a cyclic polymerisation process facilitated by initiators (Figure 5). Polycaprolactone (PCL) is non-toxic in nature, which makes it safe for living organisms [72]. It has a great ability to break down naturally in the environment, and it also has excellent stability and compatibility with biological systems [73]. Additionally, PCL shows great crystallinity, which means it is in solid form at room temperature and is strong. It gets thick and sticky in its liquid state [74].

Figure 5.
PCL is mostly produced through a ring-opening polymerisation process by linking ε-caprolactone molecules together. This method was invented in the 1930s. Different types of catalysts are used in this process, such as stannous octoate, which is used to speed up the polymerisation process [75]. Different mechanisms, including anionic, cationic, coordination, and radical processes, influence the polymerisation process. These methods influence the resulting polymer, in terms such as molecular size, distribution, the composition of end groups, and the chemical structure [75]. Polycaprolactone (PCL) is a versatile and promising biopolymer, and mixing it with other polymers changes its properties [76]. It is a synthetic polymer that breaks down slowly in nature over time. It is also hydrophobic. Its interesting properties make it quite intriguing for the preparation of long-term implantable devices [77].
References
- Hossain, M.D.; Hassan, M.K.; Yuen, A.C.Y.; He, Y.; Saha, S.; Hittini, W. Flame behaviour, fire hazard and fire testing approach for lightweight composite claddings—A review. J. Struct. Fire Eng. 2021, 12, 257–292.
- Hudson, F.S.; Sutrisna, M.; Chawynski, G. A certification framework for managing the risks of non-compliance and non-conformance building products: A Western Australian perspective. Int. J. Build. Pathol. Adapt. 2021, 39, 312–343.
- Hill, C.A.S. The environmental consequences concerning the use of timber in the built environment. Front. Built Environ. 2019, 5, 129.
- Balocco, C.; Grazzini, G.; Cavalera, A. Transient analysis of an external building cladding. Energy Build. 2008, 40, 1273–1277.
- Özdamar, E.G.; Murat, A.T.E.Ş. Rethinking sustainability: A research on starch-based bioplastic. J. Sustain. Constr. Mater. Technol. 2018, 3, 249–260.
- Sandak, A.; Sandak, J.; Brzezicki, M.; Kutnar, A.; Sandak, A.; Sandak, J.; Brzezicki, M.; Kutnar, A. Designing building skins with biomaterials. In Bio-Based Building Skin; Springer: Berlin/Heidelberg, Germany, 2019; pp. 65–97.
- Telmo, O. Polymers and the environment. In Polymer Science; IntechOpen,: London, UK, 2013; Chapter 1.
- Rong, L.; Chen, X.; Shen, M.; Yang, J.; Qi, X.; Li, Y.; Xie, J. The application of 3D printing technology on starch-based product: A review. Trends Food Sci. Technol. 2023, 134, 149–161.
- Aziz, T.; Haq, F.; Farid, A.; Kiran, M.; Faisal, S.; Ullah, A.; Ullah, N.; Bokhari, A.; Mubashir, M.; Chuah, L.F.; et al. Challenges associated with cellulose composite material: Facet engineering and prospective. Environ. Res. 2023, 223, 115429.
- Huang, K.X.; Zhou, L.Y.; Chen, J.Q.; Peng, N.; Chen, H.X.; Gu, H.Z.; Zou, T. Applications and perspectives of quaternized cellulose, chitin and chitosan: A review. Int. J. Biol. Macromol. 2023, 242, 124990.
- Yano, H.; Fu, W. Hemp: A Sustainable Plant with High Industrial Value in Food Processing. Foods 2023, 12, 651.
- Carvalho, V.R.; Costa, L.C.B.; Baeta, B.E.L.; Peixoto, R.A.F. Lignin-Based Admixtures: A Scientometric Analysis and Qualitative Discussion Applied to Cement-Based Composites. Polymers 2023, 15, 1254.
- Tusnim, J.; Hoque, M.E.; Biswas, M.C. Biopolymers in building materials. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 185–195.
- Grigoras, A.G. Natural and synthetic polymeric antimicrobials with quaternary ammonium moieties: A review. Environ. Chem. Lett. 2021, 19, 3009–3022.
- Muhammadi Shabina Afzal, M.; Hameed, S. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 2015, 8, 56–77.
- García-Guzmán, L.; Cabrera-Barjas, G.; Soria-Hernández, C.G.; Castaño, J.; Guadarrama-Lezama, A.Y.; Rodríguez Llamazares, S. Progress in starch-based materials for food packaging applications. Polysaccharides 2022, 3, 136–177.
- Yu, Z.; Ji, Y.; Bourg, V.; Bilgen, M.; Meredith, J.C. Chitin-and cellulose-based sustainable barrier materials: A review. Emergent Mater. 2020, 3, 919–936.
- Belgacem, M.N.; Gandini, A. (Eds.) Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2011.
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly (butylene succinate) (PBS): Materials, processing, and industrial applications. Prog. Polym. Sci. 2022, 132, 101579.
- Friedrich, D. How building experts evaluate the sustainability and performance of novel bioplastic-based textile façades: An analysis of decision making. Build. Environ. 2022, 207, 108485.
- Dahy, H. Biocomposite materials based on annual natural fibres and biopolymers–Design, fabrication and customized applications in architecture. Constr. Build. Mater. 2017, 147, 212–220.
- Gheorghita, R.; Anchidin-Norocel, L.; Filip, R.; Dimian, M.; Covasa, M. Applications of biopolymers for drugs and probiotics delivery. Polymers 2021, 13, 2729.
- Jeon, H.J.; Kim, M.N. Biodegradation of poly (L-lactide) (PLA) exposed to UV irradiation by a mesophilic bacterium. Int. Biodeterior. Biodegrad. 2013, 85, 289–293.
- Filachione, E.M.; Costello, E.J. Lactic esters by reaction of ammonium lactate with alcohols. Ind. Eng. Chem. 1952, 44, 2189–2191.
- Jiménez, A.; Peltzer, M.; Ruseckaite, R. (Eds.) Poly (Lactic Acid) Science and Technology: Processing, Properties, Additives and Applications (No. 12); Royal Society of Chemistry: London, UK, 2015.
- Taib, N.A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Bin Julaihi, M.R.M.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213.
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly (lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: A review. RSC Adv. 2021, 11, 17151–17196.
- PLA (Polylactic Acid): Definition, Applications, and Different Types. Available online: https://www.xometry.com/resources/materials/what-is-pla (accessed on 8 August 2023).
- Galindo, S.; Ureña-Núñez, F. Enhanced surface hydrophobicity of poly (lactic acid) by Co60 gamma ray irradiation. Rev. Mex. Física 2018, 64, 1–7.
- Reddy, C.S.K.; Ghai, R.; Kalia, V. Polyhydroxyalkanoates: An overview. Bioresour. Technol. 2003, 87, 137–146.
- Riley, R.S.; Day, E.S. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449.
- Tomizawa, S.; Hyakutake, M.; Saito, Y.; Agus, J.; Mizuno, K.; Abe, H.; Tsuge, T. Molecular weight change of polyhydroxyalkanoate (PHA) caused by the PhaC subunit of PHA synthase from Bacillus cereus YB-4 in recombinant Escherichia coli. Biomacromolecules 2011, 12, 2660–2666.
- Grigore, M.E.; Grigorescu, R.M.; Iancu, L.; Ion, R.M.; Zaharia, C.; Andrei, E.R. Methods of synthesis, properties and biomedical applications of polyhydroxyalkanoates: A review. J. Biomater. Sci. 2019, 30, 695–712.
- Dalton, B.; Bhagabati, P.; De Micco, J.; Padamati, R.B.; O’Connor, K. A review on biological synthesis of the biodegradable polymers polyhydroxyalkanoates and the development of multiple applications. Catalysts 2022, 12, 319.
- Li, J.; Zhang, X.; Udduttula, A.; Fan, Z.S.; Chen, J.H.; Sun, A.R.; Zhang, P. Microbial-Derived Polyhydroxyalkanoate-Based Scaffolds for Bone Tissue Engineering: Biosynthesis, Properties, and Perspectives. Front. Bioeng. Biotechnol. 2021, 9, 763031.
- Guimarães, T.C.; Araújo, E.S.; Hernández-Macedo, M.L.; López, J.A. Polyhydroxyalkanoates: Biosynthesis from alternative carbon sources and analytic methods: A short review. J. Polym. Environ. 2022, 30, 2669–2684.
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An overview on starch-based sustainable hydrogels: Potential applications and aspects. J. Polym. Environ. 2022, 30, 19–50.
- Gamage, A.; Thiviya, P.; Mani, S.; Ponnusamy, P.G.; Manamperi, A.; Evon, P.; Merah, O.; Madhujith, T. Environmental Properties and Applications of Biodegradable Starch-Based Nanocomposites. Polymers 2022, 14, 4578.
- Marichelvam, M.K.; Jawaid, M.; Asim, M. Corn and rice starch-based bio-plastics as alternative packaging materials. Fibers 2019, 7, 32.
- Nawaz, H.; Waheed, R.; Nawaz, M.; Shahwar, D. Physical and chemical modifications in starch structure and reactivity. Chem. Prop. Starch 2020, 9, 13–35.
- Mary, S.K.; Koshy, R.R.; Arunima, R.; Thomas, S.; Pothen, L.A. A review of recent advances in starch-based materials: Bio nanocomposites, pH sensitive films, aerogels and carbon dots. Carbohydr. Polym. Technol. Appl. 2022, 3, 100190.
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375.
- Surendren, A.; Mohanty, A.K.; Liu, Q.; Misra, M. A review of biodegradable thermoplastic starches, their blends and composites: Recent developments and opportunities for single-use plastic packaging alternatives. Green Chem. 2022, 24, 8606–8636.
- Bao, J.; Xing, J.; Phillips, D.L.; Corke, H. Physical properties of octenyl succinic anhydride modified rice, wheat, and potato starches. J. Agric. Food Chem. 2003, 51, 2283–2287.
- Mischnick, P.; Momcilovic, D. Chemical structure analysis of starch and cellulose derivatives. Adv. Carbohydr. Chem. Biochem. 2010, 64, 117–210.
- Shen, J.; Liang, J.; Lin, X.; Lin, H.; Yu, J.; Yang, Z. Recent progress in polymer-based building materials. Int. J. Polym. Sci. 2020, 2020, 8838160.
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018, 564, 562–586.
- Li, B.; Konecke, S.; Wegiel, L.A.; Taylor, L.S.; Edgar, K.J. Both solubility and chemical stability of curcumin are enhanced by solid dispersion in cellulose derivative matrices. Carbohydr. Polym. 2013, 98, 1108–1116.
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206.
- Wang, Q.; Cai, J.; Zhang, L.; Xu, M.; Cheng, H.; Han, C.C.; Kuga, S.; Xiao, J.; Xiao, R. A bioplastic with high strength constructed from a cellulose hydrogel by changing the aggregated structure. J. Mater. Chem. A 2013, 1, 6678–6686.
- Guan, Q.F.; Yang, H.B.; Han, Z.M.; Ling, Z.C.; Yu, S.H. An all-natural bioinspired structural material for plastic replacement. Nat. Commun. 2020, 11, 5401.
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994.
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the cellulose-based textiles: Raw materials and technologies. Front. Bioeng. Biotechnol. 2021, 9, 608826.
- Gurieff, N.; Lant, P. Comparative life cycle assessment and financial analysis of mixed culture polyhydroxyalkanoate production. Bioresour. Technol. 2007, 98, 3393–3403.
- Yu, P.H.; Chua, H.; Huang, A.L.; Lo, W.; Chen, G.Q. Conversion of food industrial wastes into bioplastics. Appl. Biochem. Biotechnol. 1998, 70, 603–614.
- Rajan, K.P.; Thomas, S.P.; Gopanna, A.; Chavali, M. Polyhydroxybutyrate (PHB): A standout biopolymer for environmental sustainability. In Handbook of Ecomaterials; Springer: Berlin/Heidelberg, Germany, 2019; p. 4.
- Vasudevan, M.; Natarajan, N. Towards achieving sustainable bioplastics production and nutrient recovery from wastewater—A comprehensive overview on polyhydroxybutyrate. Biomass Convers. Biorefinery 2022, 1–20.
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908.
- Wongmoon, C.; Napathorn, S.C. Optimization for the efficient recovery of poly (3-hydroxybutyrate) using the green solvent 1, 3-dioxolane. Front. Bioeng. Biotechnol. 2022, 10, 1086636.
- Paul, S.; Sasikumar, S.C.; Balakumaran, M.D. Optimization, purification and characterization of polyhydroxybutyrate (PHB) produced by Bacillus cereus isolated from sewage. Int. J. Chem. Tech. Res. 2017, 10, 884–904.
- Mahitha, G.; Madhuri, R. Purification and characterization of polyhydroxybutyrate produced from marine bacteria. Int. J. Sci. Eng. Res 2015, 6, 71–75.
- The Biodegradable Polymer PBAT Is Hitting the Big Time. 2022.Acs.org. 2022. Available online: https://cen.acs.org/business/biobased-hemicals/biodegradable-polymer-PBAT-hitting-big/99/i34 (accessed on 19 July 2023).
- Moustafa, H.; Guizani, C.; Dufresne, A. Sustainable biodegradable coffee grounds filler and its effect on the hydrophobicity, mechanical and thermal properties of biodegradable PBAT composites. J. Appl. Polym. Sci. 2017, 134.
- Park, H.Y.; Kim, S.S.; Kim, S.G.; Seo, K.H. Modification of physical properties of PBAT by using TPS. Int. Proc. Chem. Biol. Environ. Eng. 2012, 46, 67–71.
- Zhao, P.; Liu, W.; Wu, Q.; Ren, J. Preparation, mechanical, and thermal properties of biodegradable polyesters/poly (lactic acid) blends. J. Nanomater. 2010, 2010, 287082.
- Barrino, F.; De La Rosa-Ramírez, H.; Schiraldi, C.; López-Martínez, J.; Samper, M.D. Preparation and Characterization of New Bioplastics Based on Polybutylene Succinate (PBS). Polymers 2023, 15, 1212.
- Liu, L.; Yu, J.; Cheng, L.; Qu, W. Mechanical properties of poly (butylene succinate) (PBS) bio composites reinforced with surface modified jute fibre. Compos. Part A Appl. Sci. Manuf. 2009, 40, 669–674.
- Xu, J.; Guo, B.H. Microbial succinic acid, its polymer poly (butylene succinate), and applications. In Plastics from Bacteria: Natural Functions and Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 347–388.
- AL-Oqla, F.M.; Omari, M.A. Sustainable bio composites: Challenges, potential and barriers for development. In Green Bio Composites: Manufacturing and Properties; Springer: Berlin/Heidelberg, Germany, 2017; pp. 13–29.
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.; Lee, C.H. A review on properties and application of bio-based poly (butylene succinate). Polymers 2021, 13, 1436.
- Bikiaris, D.N.; Papageorgiou, G.Z.; Achilias, D.S.; Pavlidou, E.; Stergiou, A. Miscibility and enzymatic degradation studies of poly (ε-caprolactone)/poly (propylene succinate) blends. Eur. Polym. J. 2007, 43, 2491–2503.
- Krasowska, K.; Heimowska, A.; Morawska, M. Environmental degradability of polycaprolactone under natural conditions. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2016; Volume 10, p. 00048.
- Abbah, S.A.; Lam, C.X.; Hutmacher, D.W.; Goh, J.C.; Wong, H.K. Biological performance of a polycaprolactone-based scaffold used as fusion cage device in a large animal model of spinal reconstructive surgery. Biomaterials 2009, 30, 5086–5093.
- Lu, H.F.; Zhang, K.; Yi, J.L.; Wei, A.C. Study on mechanical properties of polycaprolactone modified cement-based material. Int. J. Concr. Struct. Mater. 2022, 16, 24.
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly (∊-caprolactone) fiber: An overview. J. Eng. Fibers Fabr. 2014, 9, 155892501400900309.
- Raina, N.; Pahwa, R.; Khosla, J.K.; Gupta, P.N.; Gupta, M. Polycaprolactone-based materials in wound healing applications. Polym. Bull. 2021, 79, 7041–7063.
- Archer, E.; Torretti, M.; Madbouly, S. Biodegradable polycaprolactone (PCL) based polymer and composites. Phys. Sci. Rev. 2021, 8, 4391–4414.
More
