You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by John Nicholson and Version 2 by Sirius Huang.
The compound ytterbium trifluoride is used as a component of several dental materials. Ytterbium trifluoride is reported to be insoluble in water. Despite this, its presence is associated with fluoride release from dental materials. There is evidence that it reacts with the components of calcium trisilicate cements to form small amounts of a variety of compounds, including ytterbium oxide, Yb2O3, and calcium–ytterbium fluoride, CaYbF5. In nanoparticulate form, it has been shown to reinforce glass polyalkenoates and it also provides high contrast in X-ray images.
- ytterbium trifluoride
- structure
- properties
- solubility
- dental cements
- restorative materials
1. Ytterbium Compounds
Ytterbium forms a number of simple binary compounds [1]. Its most common oxidation state is +3, which is shown in compounds such as Yb2O3, YbF3, and YbCl3. It also forms reasonably stable compounds in the +2 oxidation state, for example the difluoride YbF2, a feature which is unusual for a lanthanide element.
The compound ytterbium (III) fluoride is the particular subject of this text. Its properties are listed in Table 1. Typically, for compounds of Yb3+, the trifluoride is colourless, i.e., white in appearance [1][2][3][1,4,5]. It has a single unpaired electron and any possible electronic transitions lie outside the region of the visible spectrum, hence the absence of colour.
| Property | Value |
|---|---|
| Appearance | White crystalline solid |
| Relative molecular mass | 230.04 |
| Melting point | 1157 °C |
| Boiling point | 2380 °C |
| Atomic radius | 176 pm |
| Solubility in water | Insoluble |
| Density | 8.17 g cm−1 |
Ytterbium trifluoride has the crystal structure shown in Figure 1 [4][8]. In this structure, the Yb3+ ions are in 8 coordination with the fluoride ions, which form a bicapped trigonal prism [2][4]. Because ytterbium is a heavy metal (atomic mass 173.04), it is able to absorb X-rays strongly [5][9]. This means that the presence of an ytterbium compound, typically the trifluoride compound, in a dental restorative material enhances the contrast in X-ray images. This is the reason why the substance has been used increasingly in a variety of such materials.
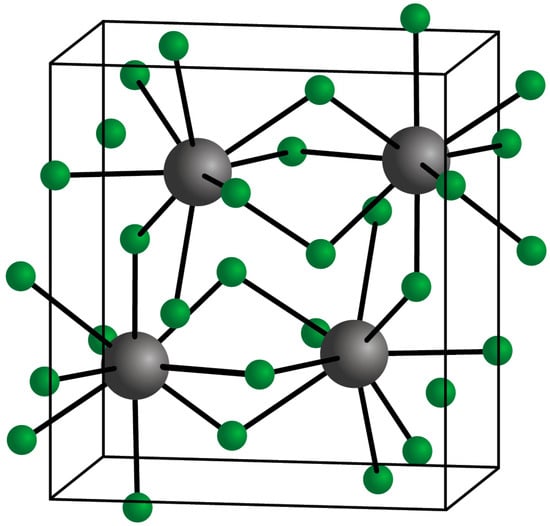
Figure 1.
Crystal structure of YbF
3
(Yb
3+
in grey; F
The question of the toxicity of YbF3 is important in the context of its use in dental materials. As a lanthanide, ytterbium has no role in the biochemistry of either animals or plants [7][11]. The suggestion has been made that lanthanide elements may show some degree of toxicity through competing with calcium in calcium-mediated biological reactions. However, any reported toxicity appears to be low. For example, a recent study found no obvious toxicity over 14 days following the exposure of rats to nanoparticles doped with Yb3+ ions [8][12]. Also, safety data sheets provided by companies typically claim that ytterbium fluoride lacks any significant toxicity [9][10][13,14]. In particular, this substance is reported not to be persistent, bio-accumulative, or toxic [9][13].
Those heavy metals that are unquestionably toxic are typically main group elements, such as mercury, lead, and thallium, rather than lanthanides [11][15]. They are also Lewis acids which are soft in Pearson’s Hard and Soft Acids and Bases (HSAB) scheme [12][16]. By contrast, lanthanides in general and ytterbium in particular are also Lewis acids but are hard. This scheme, which is based on the polarizability of the central metal ion in compounds, suggests that the most stable complexes of hard acids are those formed with hard bases, typically those based on oxygen donor atoms in the Lewis base [12][16]. Although there is some correlation between softness in the HSAB scheme and acute toxicity, possibly because of the ease with which soft acids can bind to sulphur-containing bases, such as enzymes, hardness alone is not a sufficient property to determine whether an element or ion is non-toxic or not.
In fact, in the cells of living systems, much use is made of the ion Ca2+ in key biological processes. The calcium ion is hard in the HSAB scheme [12][16]. This means that lanthanides, including ytterbium, may displace these ions in certain cellular functions, and compete with them for active binding sites in enzymes and other important bio-molecules [13][17]. Hence, lanthanides may impair the biological function of these substances, interfering with metabolism and showing toxicity [14][18], at least to an extent.
These results suggest that a degree of caution may be prudent in the handling of lanthanide compounds. On the other hand, there is also evidence that they are not particularly toxic. For example, one recent study showed that the presence of lanthanide elements in urban street dust caused no increase in cancer in populations exposed to these dusts [15][19], and no adverse effects have been reported in connection with the use of YbF3 in dental materials. Hence, YbF3 seems to be acceptable for its application in dentistry from a toxicological point of view [9][10][13,14].
2. Ytterbium Fluoride in Dental Materials
The main reason for incorporating ytterbium trifluoride into dental materials is to make them radiopaque. This means that the contrast between them and the natural tissues is enhanced in X-ray images. This allows the material to be readily distinguished from the surrounding oral structures, ensuring that the clinician can detect voids in repairs such as root fillings or direct restorations [16][20], and also marginal gaps and other defects at the interface with the tooth [17][18][19][21,22,23].
X-ray beams are absorbed by atoms of a high atomic number. In dentistry, the most widely used of these elements have been barium and strontium, typically added in the form a powdered speciality glass based on oxides of these elements [20][24]. Other heavy elements that have been used include bismuth, zirconium, and titanium [21][22][25,26]. However, use has increasingly been made of ytterbium in the form of YbF3 [17][23][21,27]. Particulate fillers based on any of these elements have the advantage that they can also improve the mechanical properties of the material when set [17][24][25][21,28,29]. Many of the studies involving such radiopaque fillers study not only their effect on X-ray attenuation, but also on their mechanical properties. In the case of YbF3, there has also been interest in the extent to which the materials can release fluoride ions into their surroundings [26][27][30,31].
It is worth noting that the majority of the elements employed in dental materials are relatively light. This includes carbon, hydrogen, and oxygen in the polymer phase and silicon and oxygen in the filler [28][32]. As such, their inherent radiopacity is low. The inclusion of fillers based on heavy elements overcomes this problem [29][33], and is therefore clinically beneficial.
The use of ytterbium fluoride in dental materials seems to have originated in the mid 1980s, when a patent was granted to what was then the Ivoclar company in Liechtenstein (now Ivoclar Vivadent) [30][34]. This concerned the use of such compounds in dental composite resins. The use was clearly aimed at improving the radiopacity of the composite resin, and YbF3 was only one of several lanthanide fluorides named in the patent. The others were the trifluorides of lanthanum, cerium, samarium, gadolinium, dysprosium, and erbium [30][34]. In fact, although this implies a wide range of possibilities, only four examples were described, two of which were based on ytterbium trifluoride. There were also some data provided to indicate that YbF3 had the best radiopacity of all the compounds tested [30][34].
Ivoclar Vivadent remains a major international manufacturer of dental materials, and their current brands of composite resin such as Tetric EvoCeram and Tetric EvoFlow still employ ytterbium trifluoride as the contrast agent for radiopacity [31][32][35,36]. Interestingly, these materials have been shown to release fluoride in small amounts [33][34][35][36][37][37,38,39,40,41], even though the only source of fluoride in the material is the YbF3 radiopacifying agent [31][32][35,36]. This suggests that the substance is not completely insoluble, despite what is claimed elsewhere in the literature.
The fluoride release experiments were carried out as follows [33][37]: Samples were suspended with a fluoride-free dental floss in 4 mL deionized water in a plastic tube at 37 °C. The whole assembly was covered to prevent water loss and stored for 24 h, after which the samples were washed with a 1 mL volume of deionised water that was combined with the original volume to give a total of 5 mL. The samples were then stored again in deionised water that was changed each day. After periods of 1 week and 4 weeks, the samples were again washed with a 1 mL volume of deionised water which was added to the final 4 mL storage volume, and the resulting solutions were analysed. Fluoride analysis was carried out with a calibrated ion-selective electrode. Prior to analysis, all storage solutions were diluted with TISAB (total ionic strength acid buffer) at a ratio of 10:1. This was to de-complex all of the fluoride and to ensure that it was all present as free F− ions.
Similar experimental procedures have been used in other studies [33][34][35][36][37,38,39,40], and this means that the results can be compared. Some representative values of fluoride released from composite resins in this type of experiment are shown in Table 2. In all cases, YbF3 was the only source of fluoride within the material.
Table 2.
Fluoride released after 1 day from two composite resins containing YbF
3
(standard deviations in parentheses).
Composite resins containing ytterbium trifluoride have also been shown to be capable of taking up fluoride when stored in aqueous solutions of an appropriate metal fluoride, such as sodium or potassium fluoride, or when exposed to a fluoridated gel [27][33][34][35][36][37][31,37,38,39,40,41]. There is no evidence that this is due to a complete reversal of the fluoride release reaction, and it may be that a small amount of fluoride ions simply becomes entrained within the surface layers of the composite resin. However, it is notable that the Tetric materials within each study have both released and taken up the lowest amounts of fluoride of all the materials studied [27][36][31,40], suggesting that there is a connection between the fluoride release and fluoride recharge effects.
Some composite resin materials have been formulated as fluoride-releasing materials by including speciality fluoride-containing glasses in the formulation alongside ytterbium trifluoride [38][39][42,43]. This means that the source of the fluoride is not clear. However, given the high levels released compared with materials that contain ytterbium trifluoride alone, it seems likely that most of the fluoride comes from the glass filler.
Another material that releases fluoride and contains two possible sources of this ion is the so-called “alkasite” material Cention N, also manufactured by Ivoclar Vivadent [40][44]. Its setting is based on a complicated blend of chemistry, though it is claimed to be mainly of the composite resin type [41][45]. It contains ytterbium trifluoride, but again, the principal role of this substance appears to be to confer radiopacity rather than to provide fluoride for release. The major fluoride-releasing component seems more likely to be the calcium-barium–aluminium fluorosilicate glass, which is also present as a major component of the filler phase in this material [40][44].
As well as in composite resin restorative materials, ytterbium trifluoride has been used in several polymer-based materials for use in endodontics for root-end sealing. There have been a number of studies published that demonstrate that YbF3 is an effective radiopacifier for these polymer-based materials. Because of where they are used, these materials need to have an appropriate level of radiopacity and, in 2001, the relevant ISO standard established 3 mm of aluminium to be the minimum radiopacity value for them. This is still the value stated in the current standard [42][46]. The American Dental Association further specified that endodontic sealers should be at least 2 mm of aluminium more radiopaque than either dentin or bone [43][47].
To determine this, most researchers employ the technique of evaluating the radiopacity of endodontic materials that was developed by Tagger and Katz [44][48]. In this technique, radiographic images of the material are recorded together with those of an aluminium step-wedge (penetrometer) whose thickness increases from 0.5 to 9.0 mm. The radiographic images are analysed using appropriate software that is able to measure the pixel density of the grey image and which gives values equivalent to the thickness of aluminium as the measure of the radiopacity of the sample [44][48].
An early study, carried out over 30 years ago, evaluated the effect of including ytterbium trifluoride in the root-end cover material Retroplast. This material, which is no longer available, was an adhesive composite resin. When first manufactured, it used silver to impart radiopacity, but in 1990, it was reformulated and the silver was replaced with ytterbium trifluoride [45][49]. In this case, the evaluation was performed clinically, and these studies showed that the substitution led to an improved material which not only had good radiopacity but also had better levels of complete healing than with silver [45][46][49,50].
Ytterbium trifluoride has been studied for use as a radiopacifier in an experimental epoxy system suitable for use as a root canal sealer for endodontics [47][51]. The study involved three different radiopaque additives with calcium tungstate and barium sulphate, as well as YbF3. All three additives were found to affect the mechanical and chemical properties of the sealer, and had acceptable radiopacity (Table 3). There was some indication that YbF3 was superior in terms of radiopacity, but the effect was small [46][50]. For all aspects of chemical behaviour (setting time, water sorption, solubility), both ytterbium trifluoride and barium sulphate were very similar. This is the ideal situation, as it is desirable that radiopacifying additives should not alter the chemical properties of the material. Calcium tungstate, by contrast, had a variety of adverse effects, lengthening the setting time and substantially increasing both the water sorption and solubility of the final material. Only in terms of its radiopacity was its performance equivalent to that of ytterbium trifluoride and barium sulphate. Overall, the key finding from this work is that ytterbium trifluoride can be used in epoxy systems to confer radiopacity, just as they have been with composite resin systems based on either bisGMA [13][17] or bisEMA [48][52].
| Property | CaWO | 4 | YbF | 3 | BaSO | 4 |
|---|---|---|---|---|---|---|
| Setting time/min | 501 | 388 | 385 | |||
| pH | 6.84 | 6.25 | 6.45 | |||
| Radiopacity/mm Al | 1.96 | 2.07 | 1.62 | |||
| Water sorption/μg/mm3 | 183.2 | 67.8 | 51.3 | |||
| Solubility/μg/mm3 | 200.1 | 10.7 | 13.2 |
As well as polymer-based materials, ytterbium fluoride has been used in cements to impart radiopacity. This includes both glass polyalkenoate cements (also known as glass-ionomers) and calcium trisilicate cements. These will now be considered in turn.
Glass polyalkenoate cements (GPC) are acid-based cements that are set through a neutralization process [48][52]. They consist of a powder comprising a complex basic glass based on calcium and sodium alumino-silicates with added phosphate and fluoride compounds. These powders are reacted with an aqueous solution of polymeric acid, such as poly(acrylic acid) or acrylic-maleic acid copolymer [49][53]. Setting is fairly rapid and the final material is a hard, slightly translucent material. Setting can be augmented through the addition of a polymerizable system, typically a 2-hydroxyethyl methacrylate monomer together with an appropriate initiator. The latter materials are known as resin-modified glass polyalkenoates [49][53].
Ytterbium trifluoride in the form of nanoparticles has been studied as a possible radiopacifier in glass polyalkenoate cements [23][27]. This study compared the effects of nanoparticulate barium sulphate, and also examined the effects of both substances on their setting and working times, compressive strength (at 24 h), and hardness (also at 24 h). The cement into which they were incorporated was a commercial conventional glass polyalkenoate called Riva SC (SDI Ltd., Bayswater, Australia). Nanoparticles were added at levels of 1, 2, 5, 10, 15, and 25% (w/w), and were mixed with the glass powder. The modified mixtures were placed in capsules, closed, and mixed with poly(acrylic acid solution) on a vibratory mixer [23][27].
Ytterbium trifluoride had a greater effect on the working and setting time of the glass polyalkenoate than the barium sulphate. Representative data to demonstrate this are shown in Table 4. Compressive strengths were also reduced, but to a lesser extent with yttrium fluoride than with barium sulphate. For example, from an initial value of 160 MPa, the compressive strength at 24 h was reduced to about 132 MPa with 15% YbF3, but to about 95 MPa for 15% BaSO4. Hardness showed a more complex pattern, with a slight increase for low levels of either type of nanoparticle, but a substantial decrease at higher levels. There was little or no difference between the two additives for most levels of addition. The exception was at 25% loading, where cements containing BaSO4 showed such poor hydrolytic stability that the surface hardness could not be measured [23][27].
Table 4. Working and setting times for conventional glass polyalkenoate (GPA) cement with and without radiopacifying agents [23].
Working and setting times for conventional glass polyalkenoate (GPA) cement with and without radiopacifying agents [27].
| Cement | Working Time/s | Setting Time/s |
|---|---|---|
| GPA only (no additive) | 155.4 | 442.6 |
| GPA + 5% YbF3 | 64.6 | 213.0 |
| GPA + 5% BaSO4 | 132.4 | 376.0 |
| GPA + 15% YbF3 | 73.0 | 163.0 |
| GPA + 15% BaSO4 | 169.8 | 351.0 |
These results show that both of these additives interfere with the setting reaction of glass polyalkenoates. In the case of YbF3, it consistently shortened the working and setting times, showing that it accelerated the setting reaction. Exactly how it does so is not clear, but these results suggest that there was a distinct chemical reaction involving YbF3 and the other components of the cement, and that these led to additional hardening processes within the cement.
Lastly, ytterbium trifluoride has also been employed in experimental calcium trisilicate cements to make them radiopaque [50][54]. Calcium trisilicates are used in endodontics as root-end sealers [51][52][55,56]. The main material is known as MTA, and is similar to Portland cement [50][54]. Two main brands are currently available, namely ProRoot MTA, grey and white (Tulsa Dental Products, Tulsa, OK, USA), and MTA-Angelus (Angelus, Londrina, Brazil). There are also related products based on refined calcium trisilicate, where the detailed compositions vary from those of MTA, but the essential setting chemistry is the same. The main brand of this type of material is Biodentine (Septodont, St. Maur des Fausses, France). All of these calcium trisilicate materials have good sealing ability and, through their high pH, stimulate natural tissue repair at the root apex. They also show good biocompatibility in this application [53][54][57,58].
Calcium trisilicate sets through a hydration reaction [55][59]. Two phases are involved, namely alite (Ca2SiO5) and belite (β-Ca2SiO4), and the initial product of reaction is a poorly crystalline phase comprising calcium hydroxide in calcium silicate hydrate. The latter substance corresponds approximately to Ca3Si2O7 [56][60]. It undergoes further condensation reactions that form short silicate chains, and these are responsible for improving the strength of the set cement [57][61]. The presence of Ca(OH)2 in the cement causes it to be alkaline [58][59][62,63], a feature which stimulates cell growth in vivo. It also provides a component capable of reacting with ytterbium trifluoride.
It is worth considering the behaviour of ytterbium trifluoride in calcium trisilicate cements in some detail. Typical cement formulations were those used in the previously mentioned study of the inclusion of YbF3 at 30% [50][54], and the details of these compositions are shown in Table 5.
Table 5.
Experimental calcium trisilicate cements with added YbF
| Material | Composition |
|---|---|
| Cement 1 | Calcium silicate 30%, calcium carbonate 40%, YbF3 30% |
| Cement 2 | Calcium silicate 17.5%, nano-HA 35%, gypsum 17.5%, YbF3 30% |
| Cement 3 | 70% Portland cement, 30% YbF3 |
The properties of the various modified cements are shown in Table 6 [50][54]. These cements all had pH values that were well on the alkaline side, and they also had adequate radiopacity. As the results in the table show, all three cements released reasonable amounts of fluoride and the modified Portland cement had quite a considerable fluoride release. Cements also released small amounts of ytterbium, presumably as Yb3+ ions [49][53]. In reporting this, the authors raise some concern about its toxicity, describing it as “potentially cytotoxic”.
Table 6.
Properties of experimental calcium trisilicate cements modified by adding 30% YbF
| Property | Cement 1 | Cement 2 | Cement 3 |
|---|---|---|---|
| Setting time/min | 6 | 24 | 20 |
| pH | 9.38 | 9.23 | 9.43 |
| Radiopacity/mm Al | 5.45 | 4.90 | 5.75 |
| F− release/μg/L | 8.79 | 44.87 | 74.47 |
| Yb release/μg/cm2 | 0.073 | 0.16 | 1.35 |
However, no evidence of any cytotoxicity was observed in the biological studies reported [50][54]. Rather, both osteoblast and osteoclast cells were found to grow in close contact with all three experimental cements, and both types of cell were found to be completely normal in size and shape when they did so. This shows that the small amount of Yb3+ released was insufficient to cause any adverse biological effects.
All three cements were examined with both FTIR spectroscopy and X-ray diffraction, and these techniques showed that there were several reaction products of ytterbium fluoride within the cement, notably ytterbium (III) oxide and calcium ytterbium fluoride, CaYbF5. Calcium hydroxide and calcium silicate hydrates were also present [50][54]. There was also evidence of a substance corresponding to Ca17Yb10F64 in Cement 1. The formation of this substance was held to be the reason that this cement released the smallest amounts of Yb3+ and F− ions [49][53]. The authors suggest that a similar substance was formed in Cement 2, but with some of the fluoride ions replaced by hydroxide ions. This, they suggest, leaves free fluoride ions which are capable of being released by the cement. The authors do not comment on the possible calcium–ytterbium-fluoride species in Cement 3, but presumably there is a similar effect, which would explain the even greater release of fluoride from this material.
Whatever the details of the chemistry, the feature that is apparent about the ion release is that much more fluoride is released than ytterbium. This suggests that the originally present YbF3 undergoes some sort of dissociation in order for the two constituent elements to be released separately; an observation that suggests that the compound has a degree of solubility in the aqueous medium found within the setting cement.
The simpler ytterbium compounds reported are able to rise from reactions of the ytterbium trifluoride with calcium hydroxide formed within the cement. These reactions are as follows:
2YbF
3
+ 3Ca(OH)
2
→ Yb
2
O
3
+ 3CaF
2
+ 3H
2
O
2YbF
3
+ Ca(OH)
2
→ CaYbF
5
+ YbF(OH)
2
Both reactions imply that YbF3 is acidic, at least to some extent. However, one paper, in describing its inclusion in a dental composite resin, incorrectly claims that it is alkaline [60][64]. In fact, ytterbium trifluoride is a Lewis acid, as we have seen and as are other simple compounds of ytterbium (III) [1]. Both ytterbium (III) chloride [61][65] and ytterbium (III) triflate [62][66] are known to be Lewis acids, a property that underpins their widespread use as catalysts in organic chemistry. Typically, metal fluorides are stronger Lewis acids than their equivalent metal chlorides [63][67], which suggests that YbF3 is likely to have considerable Lewis acidity. This provides part of the driving force for the reactions with calcium hydroxide shown above, which occur within the cement.
