Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Vangelis D. Karalis and Version 2 by Catherine Yang.
Artificial intelligence has revolutionized the field of medicine and healthcare by providing innovative solutions to complex problems. One of the most important benefits of AI in clinical practice is its ability to investigate extensive volumes of data with efficiency and precision. This has led to the development of various applications that have improved patient outcomes and reduced the workload of healthcare professionals. AI can support doctors in making more accurate diagnoses and developing personalized treatment plans.
- artificial intelligence
- clinical practice
- machine learning
- neural networks
1. General
Artificial intelligence has revolutionized the field of medicine and healthcare by providing innovative solutions to complex problems [1][2][1,5]. There are various types of AI, including deep learning (DL), machine learning (ML), and natural language (Figure 12). DL is a subset of artificial intelligence that focuses on training neural networks to learn and make decisions in a manner similar to the human brain (Figure 23). DL algorithms are designed to learn and improve from experience automatically, without the need for explicit programming [3][4][5][24,25,26]. This ability to analyze large amounts of data and extract meaningful patterns has made DL a powerful tool in fields such as image recognition and autonomous driving.
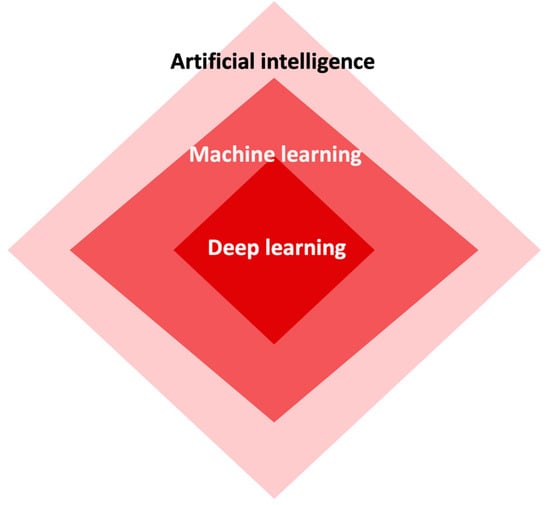
Figure 12.
The interconnectedness among artificial intelligence, machine learning, and deep learning.
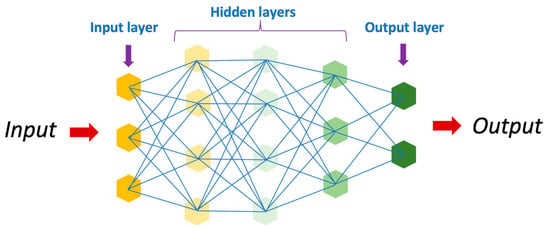
Figure 23.
Schematic representation of an artificial neural network.
Machine learning primarily focuses on advancing algorithms and models that empower computers to acquire knowledge and generate predictions or decisions autonomously without the need for explicit programming [6][2]. ML can be broadly classified into several categories, such as supervised, unsupervised, and reinforcement learning (refer to Table 1). In supervised learning, an algorithm learns from labeled data to make predictions or decisions [6][2]. This approach trains the algorithm on a dataset comprising input variables and their corresponding output variables. The goal is to enable the algorithm to understand the relationship between the input and output variables, thereby facilitating precise predictions for novel and unobserved data instances. Various supervised learning algorithms are commonly used, including linear regression, logistic regression, support vector machines, and decision trees.
Table 1.
A common classification of machine learning algorithms.
| Supervised | Unsupervised | Reinforcement |
|---|---|---|
| Linear regression | Principal component analysis | Q-learning |
| Logistic regression | K-means clustering | SARSA |
| Linear discriminant analysis | KNN (k-nearest neighbors) | Policy iteration |
| Decision trees | Hierarchal clustering | Monte Carlo tree search |
| Naive Bayes | Anomaly detection | Bellman equations |
| Support vector machines | Neural networks | Markov decision process |
Unsupervised learning represents a distinct subfield within machine learning, where the algorithm functions without the presence of labeled data [3][6][7][8][2,24,27,28]. Instead, its purpose is to autonomously identify patterns, structures, or relationships within the data. This learning type proves highly advantageous when a definitive target variable is absent or when the goal is to extract valuable insights from the data without predetermined predictions. Unsupervised learning algorithms include various methods, such as clustering algorithms like k-means and hierarchical clustering, as well as dimensionality reduction techniques like PCA and factor analysis.
The primary objective of reinforcement learning is to train autonomous agents to effectively make a series of decisions within a given environment, aiming to optimize the total cumulative reward obtained [9][29]. Unlike supervised learning, where the agent is provided with labeled data, or unsupervised learning, where the agent learns patterns and structures from unlabeled data, reinforcement learning operates on the principle of trial and error. Examples of reinforcement learning approaches include the value-based methods (e.g., Q-learning and SARSA), the policy-based methods (e.g., policy gradient and reinforce), and model-based methods (e.g., Monte Carlo tree search).
Natural language processing (NLP) is a field of study that centers on examining and understanding the interplay between computer systems and human language [6][2]. The field of study pertains to the advancement of algorithms and methodologies that facilitate machines in comprehending, interpreting, and producing human language in a manner that possesses significance and utility. NLP has become increasingly important in our digital age, as it allows computers to process and analyze vast amounts of text data, such as emails, social media posts, and news articles, to extract valuable insights and information.
One of the primary advantages of AI in clinical practice is its ability to rapidly and accurately analyze extensive volumes of data. This capability has given rise to a variety of applications that have not only improved patient outcomes but also lessened the workload on healthcare professionals [10][30].
2. Cardiology
The application of sophisticated computational algorithms and machine learning techniques in the field of cardiology is commonly referred to as AI. This approach aims to analyze and interpret cardiac data in a more advanced and efficient manner. It involves the development of intelligent systems that can learn from data, make predictions, and offer valuable insights to assist in diagnosing, treating, and managing cardiovascular diseases.
At present, two distinct positions for AI exist in the domain of cardiovascular imaging [11][31]. Automation refers to the process of replacing human involvement in various tasks, including but not limited to image segmentation and the assessment of structural and functional parameters. Another significant aspect is the identification of insights that hold clinical significance. The majority of documented applications were primarily centered around the implementation of task automation. Furthermore, there have been reports on developing algorithms capable of acquiring cardiac measurements.
AI has significantly impacted various facets of cardiovascular imaging, covering the entire spectrum from initial data acquisition to the final reporting phase [12][13][32,33]. Examples of this impact include the use of AI in advancing computed tomography and magnetic resonance imaging techniques for measuring lumen diameter, recognizing coronary calcium score, and identifying obstructive coronary disease. Furthermore, AI has been instrumental in automating processes such as acquisition, segmentation, and report generation [14][15][34,35]. In contrast to the methodologies mentioned earlier, a notable concern arises regarding the substantial observer variability observed in the interpretation of echocardiograms. AI holds the potential to address this issue by mitigating inter-observer variability and enhancing diagnostic precision within the field of echocardiography.
In recent years, numerous studies have been conducted to investigate cardiomyopathy screening, with a particular focus on the utilization of AI in conjunction with electrocardiography (ECG) for enhanced diagnostic capabilities [16][17][36,37]. The feasibility of the joint use of AI/ECG screening for amyloidosis, cardiomyopathy, and dilated cardiomyopathy remains intact, even in cases of mild left ventricular dysfunction [18][19][38,39]. The application of AI/ECG in regular clinical practice has increased the identification of left ventricular systolic dysfunction. In imaging, AI is utilized to automatically evaluate the thickness and properties of the myocardium to distinguish between different types of cardiomyopathies [19][20][39,40]. However, there is currently a lack of research investigating the prognostic potential of this AI technology. AI is also being utilized in cardiomyopathy genomics, particularly for predicting the pathogenicity of genetic variants and determining their clinical relevance [21][22][23][41,42,43].
3. Surgery
The application of AI and ML models holds significant potential in the field of surgery. These models demonstrate promising applications in both the preoperative phase, accurately diagnosing pancreatic conditions, and the postoperative phase, evaluating prognosis and predicting complications [24][25][26][44,45,46]. AI has also proven beneficial in assisting bariatric surgeries. The increasing integration of AI technologies in various healthcare subspecialties has led to promising developments in their application within bariatric surgery [27][28][47,48]. The management of patients who are candidates for bariatric surgery is a complex subject. The evaluation process requires the involvement of a multidisciplinary team comprising professionals from various fields, including internists, psychiatrists, general surgeons, and anesthesiologists. Physicians across various medical specialties engage in the comprehensive assessment of patients before, during, and after surgical procedures, a task that presents considerable difficulties due to the intricate nature of individuals afflicted with obesity [29][49].
Numerous potential applications of AI exist during the intraoperative period. It has the potential to be utilized in the management of pharmacotherapy, hemodynamic optimization, neuromuscular block monitoring, and anesthesia depth assessment [30][50]. One of the most notable reports pertains to predicting the early distribution kinetics of propofol. Indeed, the volume of drug distribution in individuals with obesity is subject to modification. Specifically, there is an increase in blood volume and cardiac output, alongside alterations in plasma transport proteins. A study utilized AI to handle the induction phase’s kinetics effectively [31][51]. This was achieved through the utilization of a comprehensive pharmacokinetic dataset with high resolution. A comparative analysis was undertaken to evaluate the performance of a traditional four-compartment model, a recirculatory model, and a gated recurrent unit neural network. The study concluded that both a recirculatory model and a gated recurrent unit ANN demonstrated similar performance, surpassing a compartmental model in accurately representing high-resolution pharmacokinetic data of propofol [31][51].
In the same context, plastic surgeons frequently encounter clinical scenarios that lack definitive solutions. Achieving an ideal treatment approach necessitates utilizing a comprehensive decision model that effectively incorporates various influential factors, including clinical and demographic data. Before the advent of AI, the decision tree analysis technique was commonly used for constructing such models. The localization of significant anatomical landmarks in medical imaging plays a crucial role in preoperative planning and postoperative outcome evaluation [32][52]. Nevertheless, the current identification process is carried out either manually or by running the inserted auxiliaries, resulting in a time-consuming and imprecise procedure. In order to enhance the precision of landmark localization on the distal femur surface, scientists devised an algorithm that initially transformed three-dimensional images into three distinct sets of two-dimensional images [32][52]. Subsequently, the algorithm acquired the ability to recognize landmarks within these images and subsequently integrated these outcomes to accurately determine the spatial coordinates of the identified landmarks in three dimensions.
4. Anesthesiology
The application of AI has yielded remarkable outcomes in anesthesia and operating room management [33][34][53,54]. Throughout each phase of the perioperative process—specifically the preoperative [35][36][37][55,56,57], intraoperative [38][39][40][41][42][16,17,18,19,20], and postoperative phases [22][24][42,44]—distinct tasks can be executed using diverse techniques. The effectiveness of a neural network designed to identify esophageal intubation becomes unnecessary in the presence of continuous capnography [43][44][58,59]. In this case, a reliable clinical examination has revealed a previously concealed and highly detrimental complication. The use of video laryngoscopy requires the adjustment of an ML model designed to predict challenging intubation based on patient appearance. The expansion of airway management technology has resulted in an increased spectrum of acceptable outcomes in terms of laryngeal visualization.
Since the 1950s, the concept of an algorithm autonomously regulating the depth of anesthesia using EEG recordings has been a subject of ongoing research. Anesthesiologists have explored this possibility for a considerable period, but it continues to be an active area of investigation.
5. Gastroenterology and Hepatology
The field of gastroenterology and hepatology is witnessing significant growth in the potential implementation of AI and ML techniques. In recent years, there has been a burgeoning body of research focusing on examining AI applications in various medical contexts, particularly involving the utilization of computer-aided diagnosis (CAD). These applications encompass the use of CAD in diagnosing premalignant and malignant gastrointestinal lesions, predicting treatment response in patients with inflammatory bowel disease, conducting histopathological analysis of biopsy specimens, assessing the severity of liver fibrosis in individuals with chronic liver disease, developing models for liver transplant allocation, and exploring other related areas [45][60].
The domain of esophageal cancer prevention and early detection shows significant potential for advancements through the utilization of AI. Substantial research advancements have been made in this field, with a notable portion of esophageal cancer research in the United States dedicated to investigating technologies, including those involving AI, aimed at enhancing the early detection and treatment of Barrett’s esophagus and esophageal adenocarcinoma [46][47][61,62].
AI possesses the capacity to assume a significant role in the decision-making process for the treatment of inflammatory bowel disease by accurately predicting treatment response at an earlier stage and providing guidance for personalized therapy selection. Within the field of inflammatory bowel disease, researchers have made advancements in the development of AI/ML computer vision tools. These tools have been specifically designed to assess the severity of diseases through endoscopic examination. The main goals of their study involve the differentiation of colitis from neoplasia and the distinction between sporadic adenomas and non-neoplastic lesions. AI algorithms have undergone training to forecast the response to treatment and assess the likelihood of disease recurrence [48][49][63,64]. There are numerous potential applications for AI and ML in the domain of hepatology. The objectives above encompass the assessment of hepatic fibrosis progression, the identification of non-alcoholic fatty liver disease, the recognition of individuals at risk for hepatocellular carcinoma development, and the enhancement of protocols for organ transplantation [50][51][65,66].
The prevention and control of colorectal cancer represent significant public health endeavors undertaken by gastroenterologists. The progress made in the field of ML has resulted in the utilization of computer vision techniques to assist in the detection of polyps during colonoscopy procedures. Empirical evidence has demonstrated the efficacy of CAD systems in enhancing the adenoma detection rate [52][53][54][55][67,68,69,70].
6. Pneumonology
AI, specifically the utilization of DL and ML algorithms for pattern recognition, holds significant promise for various applications within the field of pulmonary medicine. These applications encompass image analysis, decision-making processes, and the prediction of prognoses [2][56][57][5,6,7]. Lung cancer is a prevalent malignant neoplasm characterized by significant clinical morbidity and mortality rates [58][71]. Lung nodules are the prevailing imaging manifestations observed during the initial phase of lung cancer, posing challenges to manual film interpretation. AI recognition technology can conduct multi-parameter cluster analysis and streamline image processing, thereby assisting medical professionals in the early detection of lung cancer [59][72]. In recent years, reports have indicated that AI systems have demonstrated the capability to identify malignant pulmonary nodules by analyzing chest computed tomography (CT) images [60][73]. The model has been developed using DL technology, and AI is utilized for the analysis of CT films in order to support medical professionals in enhancing the accuracy of lung cancer screening. Another study constructed a predictive model by applying logistic regression analysis, integrating specific tumor markers into the model [61][74]. The study’s results demonstrated that the developed predictive model showed significantly better performance when compared to the basic combined detection strategy involving tumor markers.
Research has demonstrated that AI can potentially enhance surgical risk prediction, thereby facilitating the selection of the most optimal surgical approach [62][63][75,76]. An example of a cognitive computing system, IBM Watson for Oncology, utilizes AI techniques for data analysis and image conversion. Its primary objective is to assist medical professionals in efficiently identifying crucial information within patients’ medical records, presenting pertinent evidence, and facilitating the exploration of potential treatment options [64][77]. The application of deep neural networks in the identification of respiratory illnesses, specifically in chest radiographs and CT scans, has resulted in a noteworthy enhancement in diagnostic precision compared to subjective characteristics like tumor speculation, as well as objective characteristics such as shape and texture acquired through image analysis software [65][78].
7. Nephrology
The concept of progressive immunoglobulin refers to the gradual development and maturation of immunoglobulins and IgA nephropathy (IgAN) is an acknowledged etiology of renal failure. However, the ability of the nephrologist to anticipate the occurrence of kidney failure among patients at the time of diagnosis is challenging. Nevertheless, the capacity to discern these individuals would prove advantageous in terms of prognostication and treatment purposes. It has been postulated the existence of a function that establishes a relationship between clinical and biological parameters, such as age, sex, blood pressure, proteinuria, serum creatinine level, and anti-hypertensive treatments, at the time of IgAN diagnosis and the likelihood of developing progressive IgAN [66][79]. The researchers devised and executed the development of an ANN with the purpose of approximating the aforementioned function. The findings indicated that the ANN demonstrated superior accuracy in predicting the onset of progressive IgAN compared to experienced nephrologists [66][79]. Specifically, the ANN achieved correct predictions in 87% of cases, whereas the nephrologists achieved a lower accuracy rate of 69.4%. Furthermore, the ANN exhibited a higher sensitivity of 86.4% compared to the nephrologists’ sensitivity of 72%, indicating its ability to correctly identify true positive cases. Similarly, the ANN displayed a higher specificity of 87.5% compared to the nephrologists’ specificity of 66%, indicating its capacity to accurately identify true negative cases. These approaches can potentially be used in a wide range of progressive diseases, thereby aiding clinicians in the process of patient staging and management.
AI models have been applied for various purposes, including predicting the rate of decline in glomerular filtration rate in individuals with autosomal dominant polycystic kidney disease, enhancing anemia management in hemodialysis patients, estimating an appropriate duration for dialysis to achieve the desired level of urea removal, determining the optimal dry weight in patients undergoing hemodialysis, and identifying specific pathogens responsible for bacterial infections in patients with Parkinson’s disease [67][68][69][70][80,81,82,83].
8. Urology
AI is predominantly used in the field of urology, particularly in the domain of genitourinary malignancies. In a study, AI was utilized to predict the outcomes of prostate biopsies, with a specific focus on prostate cancer. ML algorithms were applied to analyze recurrence-free probability and diagnostic evaluation for bladder cancer. There have been anecdotal reports concerning the staging and prediction of disease recurrence in cases of kidney and testis cancer. Recently, AI has found application in non-oncological diseases, specifically in areas such as stones and functional urology.
In recent decades, numerous scholarly investigations have examined the utilization of AI in the management of prostate cancer. These studies align with the contemporary paradigm of precision medicine and surgery [71][84]. Prostate cancer diagnosis encompasses a broad range of applications, which have experienced numerous advancements in recent years [72][85]. A seminal study was conducted in 1994 to determine the potential utility of ANN in predicting biopsy outcomes in males displaying abnormal prostate-specific antigen levels. Additionally, the study aimed to assess the effectiveness of ANN in predicting treatment outcomes following radical prostatectomy [72][73][85,86]. A study demonstrated the predictive accuracy of two distinct AI systems [74][87]. These systems were specifically designed using Vienna-based multicenter European referral database data. These AI systems aim to facilitate the early detection of prostate cancer in males. Another study found that a DL survival model exhibited the ability to predict the timeframe for urinary continence recovery after Robot-Assisted Radical Prostatectomy [75][88]. This prediction was achieved by incorporating Anatomical Pathology Markers (APMs) and patient-related factors. Furthermore, this particular model has successfully identified APMs of top surgeons that can effectively classify surgeons, surpassing the predictive ability of surgeon experience alone. The APMs were able to differentiate surgeons based on the quality of urinary continence recovery observed in their patients, distinguishing between those with superior and inferior outcomes.
Functional urology refers to the branch of urology that focuses on studying and managing the urinary tract. The exploration of AI potential applications has also extended to the domain of functional urology. A study compared an AI model and multiple linear regression in terms of their effectiveness in replacing preoperative urodynamic evaluation in women diagnosed with pelvic organ prolapse [76][92]. A total of 804 women diagnosed with pelvic organ prolapse were subjected to examination, revealing that both multivariate logistic regression and AI were determined to be less effective than urodynamic studies in evaluating urinary dysfunction. A kidney transplant is a surgical procedure in which a healthy kidney from a donor is transplanted into a recipient. Over the past few years, there has been a growing interest in utilizing AI predictive tools in kidney transplantation. Similarly, the potential application of AI in identifying risk factors and co-variates that contribute to the failure of renal transplantation has been explored [77][93]. The AI approach was compared with the traditional logistic regression model. The AI method demonstrated superior accuracy compared to logistic regression, as evidenced by data analysis from 378 patients.
9. Dermatology
Identifying skin diseases primarily relies on the apparent attributes exhibited by the lesions. However, dermatology encompasses a vast collection of over 2000 distinct types of dermatological diseases. Certain skin lesions associated with various diseases may exhibit similarities, posing challenges in accurate diagnosis and treatment [78][79][94,95]. Notably, there is a significant shortage of dermatologists, particularly in developing countries and remote regions, where increased medical resources, professional consultations, and clinical support are urgently needed [80][81][96,97].
The convergence of rapid iteration in big data, advancements in image recognition technology, and the global proliferation of smartphones present transformative potential for diagnosing and treating skin diseases [82][83][98,99]. AI, in particular, has the capacity to offer prompt diagnoses, facilitating a wider range of treatment options and enhancing accessibility, especially for marginalized regions and individuals with limited resources [84][100]. The integration of AI technology and algorithms has the potential to establish itself rapidly as a standard approach in the field of diagnosis and evaluation. The examination of the structure and form of a skin abnormality is a fundamental aspect of dermatological diagnosis. Advancements in AI have led to significant improvements in facial recognition and aesthetic analysis, rendering them more dependable [85][101].
The inception of AI in the field of dermatopathology can be traced back to 1987 when a text-based system known as TEGUMENT was utilized on a personal computer. The system was specifically developed with the purpose of classifying 986 histopathological diagnoses based on light microscopic images. It demonstrated a diagnostic accuracy of 91.8% compared to the assessments made by a qualified dermatopathologist [86][102]. During that particular time frame, the absence of necessary equipment and technologies for whole slide imaging led to the belief that the notion of human-independent image analysis was not viable. In recent years, the accurate classification of routine diagnoses by machine-based systems has become a tangible reality [87][103].
Distinguishing between malignant and benign lesions holds the highest importance for dermatopathologists due to the consequential divergence in therapeutic decisions. In this context, a study used a sample of 695 melanocytic neoplasms to distinguish between melanoma and nevus by means of classification [88][105]. The study included a comprehensive representation of all stages of melanoma, as well as various types of nevi. In the present investigation, it was observed that the convolutional neural network exhibited a statistically significant superiority over the pathologists in terms of accurately diagnosing nevi and melanoma through histopathological analysis. The observed discordance rate of 25–26% among dermatopathologists was found to be comparable to the aforementioned similarity.
In another research study, the objective was to assess the precision of a DL algorithm in diagnosing three dermatopathological conditions through the utilization of whole-slide imaging [89][106]. The study’s findings indicated that the AI system demonstrated high accuracy, correctly classifying several types of carcinomas. In contrast to the straightforward binary classification involved in diagnosing melanoma and distinguishing it from pigmented nevi, the diagnosis of non-melanoma skin cancers presents a more challenging task. This challenge stems from the intricate categorization of these conditions and the inclusion of various benign and malignant diseases, along with inflammatory dermatoses, within the differential diagnoses.
10. Orthopedics
Supervised ML can be applied to classify individuals into pain phenotypes based on brain MRI, considering the high prevalence of long-standing pain in the UK, estimated to be between 30% and 50% [90][108]. The absence of tissue pathology that corresponds to pain, as well as the dependence on self-reported measures for subgroup classification, pose a significant challenge in identifying the neural correlates of pain and provide a comprehensive overview of ML applications used in the context of chronic pain, which encompasses pain conditions beyond musculoskeletal disorders [91][109]. The authors specifically highlight using ML techniques to classify individuals into distinct pain phenotypes based on predictive models.
Another study established a correlation between frontal plane knee biomechanics and the ability to predict the risk of knee injuries [92][110]. In this study, inertial sensor data were used to categorize the performance of single-leg squats based on the extent of knee valgus [93][111]. The study sample consisted of 14 participants, and a total of 140 images were analyzed. Additionally, the researchers sought the opinions of three expert raters regarding the potential risk associated with the observed performances. Supervised learning was applied to perform classification among three distinct classes, namely “poor”, “moderate”, and “good”. The study’s findings indicate that the accuracy levels were observed to be significantly high when performing a 2-class classification task. However, when the complexity of the classification task was increased to a 3-class classification, the accuracy levels experienced a notable reduction of approximately 30%. There is a scarcity of instances where unsupervised learning techniques have been utilized within the domain of musculoskeletal research. According to a study, the chronic pain challenge assesses the likelihood of chronic pain based on assigned weights for various health behaviors [94][112]. The study included both supervised and unsupervised methods to demonstrate the precise prediction of pain levels, as measured by the visual analog scale and the Oswestry Disability Index. These predictions are made based on the corresponding scores for depression, nutrition, and physical activity. Nevertheless, although this emphasizes the potential of ML to categorize the risk of chronicity using patient-reported data, the effectiveness of unsupervised learning by itself has not been confirmed.
11. Neurology
Neuroimaging plays a pivotal role in clinical practice and scientific inquiry, facilitating the examination of the brain in various states of well-being and pathology. Similar to several other domains, neuroimaging is enhanced by the utilization of sophisticated analysis methodologies in order to harness imaging data effectively to investigate the brain and its functionality. In recent times, ML has made significant contributions. Additionally, it has played a crucial role in the prompt identification of acute conditions like stroke and in monitoring imaging changes over time. As our capacity to visualize and examine the brain progresses, so does our comprehension of its complex interconnections and their significance in making therapeutic decisions.
Despite being in the early stages of development, AI’s utilization in neuro-oncology exhibits considerable potential. It is highly probable that AI algorithms will enhance our comprehension of brain tumors and play a pivotal role in fostering advancements in the field of neuro-oncology. The field of neuro-oncology has experienced a growing emphasis on the integration of molecular markers for the purpose of guiding therapeutic interventions [95][113]. AI algorithms have demonstrated notable efficacy in the noninvasive identification of significant molecular markers from diagnostic imaging, exhibiting remarkable accuracy. In various institutional datasets, AI algorithms have successfully determined the mutational status of several markers [96][97][114,115]. Moreover, it has been demonstrated that algorithms based on AI can effectively identify investigational molecular markers, even in smaller cohorts of patients [98][116].
The utilization of AI for the analysis of diagnostic imaging has proven to be beneficial in the clinical management of brain tumors. The utilization of AI to automate labor-intensive tasks holds great potential in the field of neuro-oncology. Multiple studies have demonstrated the efficacy of DL techniques in identifying brain metastases measuring in the millimeter range through MRI imaging. Furthermore, it has been observed that comparable DL models have demonstrated significant efficacy in the automated segmentation of tumors, thereby enhancing the efficiency of radiation therapy treatment planning [99][100][101][117,118,119]. AI has demonstrated potential in accurately differentiating various central nervous system malignancies without the need for invasive procedures, achieving comparable results to those of expert neuroradiologists [102][103][120,121]. The extensive application of these AI algorithms could prove to be highly beneficial in resource-constrained environments that lack access to specialized neuroradiologists.
12. Gynecology
Despite encountering various obstacles, the integration of AI in obstetrics and gynecology has exhibited remarkable progress. The utilization of AI in various domains has proven to be highly effective in addressing persistent issues related to diagnosis and treatment. According to a study, AI has the potential to enhance knowledge and provide assistance to medical professionals in the fields of gynecology and obstetrics [104][122]. The latest applications of AI models in gynecology involve identifying endometrial carcinoma, in vitro fertilization, uterine sarcoma, cervical intraepithelial neoplasia, and advancing anticancer medication [105][106][123,124].
The integration of AI technology into ultrasonography has the capacity to enhance the adoption of medical ultrasound in various clinical environments, facilitating its broader application by healthcare professionals. Therefore, the utilization of AI in the field of ultrasonography for prenatal care has the potential to assist medical professionals in efficiently prioritizing and accurately diagnosing the anatomical structures of pregnant individuals. In certain medical applications such as obstetric pelvic and echocardiography ultrasonography, where visual analysis and measurement play a crucial role, the utilization of video clips can provide a comprehensive set of structured data. This enables spatiotemporal analysis and enhances the advantages of ANNs [107][125]. A study investigated the efficacy of AI algorithms in ultrasonic diagnosis for pregnant patients with brain tumors. They specifically focused on evaluating the accuracy rate of this diagnostic approach [108][126]. The diagnostic accuracy achieved through the utilization of AI was recorded at 94.50%. Another research study was conducted, which involved a prospective and descriptive approach. The study focused on a sample of approximately 244 pregnant women in their first pregnancy trimester. The registered female participants were specifically queried regarding their utilization of iron, folic acid, potassium iodide, and multivitamin supplements throughout their pregnancies. The utilization of an ANN model that incorporates variables related to pregnancy checks, intake of iodized salt, iodized supplements, and iodine-rich foods can be used to predict iodine deficiency during the early stages of pregnancy. This predictive model can assist experts in making a more feasible diagnosis [109][127]. In their study, Sakai et al. utilized a newly developed DL-based explainable graph chart diagram representation to aid in fetal cardiac ultrasound screening. This screening process is known to have a relatively low rate of detecting congenital heart disease during the second-trimester stages, primarily due to the challenges associated with mastering the technique [110][128]. Consequently, the utilization of AI in the second and third trimesters of pregnancy for diagnostic purposes, specifically using diagram representation, enhances screening performance. The accuracy rate for experts increases from 96% to 97.50%, while non-experts improve from 82% to 89% [111][129].
13. Ophthalmology
The utilization of AI in diagnosing and managing ocular disease has become increasingly popular due to research findings emphasizing its potential to enhance personalized medicine and improve healthcare outcomes [112][130]. Numerous AI algorithms are currently under development for managing patients diagnosed with diabetes mellitus [113][131].
Due to advancements in the management of diabetes mellitus, there has been an enhancement in the monitoring of patients, resulting in a higher incidence of diabetic retinopathy and diabetic macular edema. The primary cause of significant visual impairment and blindness among individuals of working age is the presence of diabetic macular edema that has not been diagnosed or treated [114][132]. Hence, it is imperative to conduct extensive screening for diabetic retinopathy on a large scale in order to identify potentially detrimental alterations at an early phase, thereby facilitating effective management and treatment strategies.
Considering the prevailing patterns of population growth and the significant incidence of diabetic retinopathy and diabetic macular edema within the community, it is inevitable that automated screening and diagnosis will become increasingly prevalent in ophthalmic healthcare settings. Efforts have been made to explore automated retinal screening techniques for the diagnosis of diabetic retinopathy to enhance patient management and mitigate the societal impact. Various AI, ML, and DL methodologies have been used for the automated diagnosis and grading of diabetic retinopathy. The most efficacious automated systems have been developed based on comprehensive investigations conducted within the last three years. Recent research on diabetic retinopathy has shown that AI techniques have exhibited significant accuracy, sensitivity, and specificity in identifying and diagnosing diabetic retinopathy [115][133].
Automated application systems have the potential to enhance doctors’ comprehension of diabetic retinopathy predictions and enhance the practicality of intelligent diagnostic models in real-world clinical settings [115][133]. Based on the aforementioned studies, it was observed that the automated analysis of retinal images exhibited a high level of accuracy, validity, sensitivity, and specificity in detecting diabetic retinopathy. Furthermore, the diagnostic performance of AI techniques was deemed clinically acceptable and demonstrated high reproducibility when applied to the validation data set.
Age-related macular degeneration is a chronic ocular condition that is recognized as a prominent contributor to visual impairment [116][134]. Prognostications AI algorithms exist to generate personalized predictions in age-related macular degeneration. These algorithms can make predictions regarding the presence of drusen beneath the retina in individuals with age-related macular degeneration. The AI algorithms offer automated detection capabilities for identifying drusen, fluid, and geographic atrophy in relation to age-related macular degeneration lesions. These algorithms leverage fundus images and spectral-domain optical coherence tomograph to enhance the diagnosis and treatment [117][135]. The utilization of AI in the automated detection of drusen holds promise for enhancing the diagnostic capabilities of ophthalmologists in the early and efficient assessment of fundus images [118][136]. The application of AI techniques in diagnosing and grading age-related macular degeneration has been extensively explored. Recent studies have shown that these automated approaches exhibit notable efficacy, demonstrating high accuracy, sensitivity, and specificity levels in detecting age-related macular degeneration [119][137].
Glaucoma, which ranks as the second most prevalent factor leading to visual impairment on a global scale, is distinguished by the gradual degeneration of retinal ganglion cells and the permanent depletion of axons within the optic nerve. The timely identification and management of glaucoma is of paramount significance in the prevention of preventable visual impairment. AI techniques have demonstrated exceptional efficacy in efficiently classifying glaucomatous and healthy eyes. Ophthalmologists have the ability to utilize these automated results as a reference point, enabling them to enhance their decision-making process within clinical practice. The utilization of automated AI applications has demonstrated significant efficacy and holds promise in addressing the imminent challenge of diabetic retinopathy, age-related macular degeneration, and glaucoma screenings in both developed and developing nations [120][138].
14. Pediatrics
Imaging techniques play a paramount role in the management of pediatric neurologic, neurosurgical, and neuro-oncological conditions [121][139]. Multi-parametric MRI techniques are gaining popularity, particularly when combined with radiogenomic analyses that establish connections between imaging features and molecular biomarkers associated with diseases. Nevertheless, incorporating this approach into regular clinical practice continues to be challenging. AI techniques can model extensive datasets related to childhood neurologic disease, including radiologic, biological, and clinical data. This capability enables the integration of such information into prognostic modeling systems at an early stage. Consequently, AI techniques offer a viable solution to address this issue [121][139].
In certain applications within the field of pediatric neuroradiology, ANNs have demonstrated notable efficacy in a focused manner. This concept is most effectively demonstrated through the utilization of ventricular size determination to categorize children into either a normal or hydrocephalic group. In a recent study, an analysis was performed on hydrocephalus and controls [122][140]. They achieved an accuracy score of 94.6% for hydrocephalus and 85.6% for controls using a training set of T2-weighted MRI images from around 399 children. Previous studies have reported comparable achievements in the field of pediatric hydrocephalus through the implementation of evolutionary modifications in ANN methodologies [123][141].
The application of a support vector machine for the categorization of children into normal or hypoxic-ischemic brain injury groups, based on the measurement of corpus callosum widths, yielded a classification accuracy of 95% [124][142]. Another study utilized a comparable methodology to examine a group of adolescents who had experienced traumatic brain injury. Specifically, they utilized edge-density imaging and support vector machines to classify the participants into two categories: normal and mild traumatic brain injury [125][143]. The aforementioned method, which achieved a precision rate of 94%, demonstrated superior performance compared to neurocognitive testing in this aspect [4][25]. Support vector machines have demonstrated successful classification of various magnetic resonance imaging abnormalities in the fetal brain. These abnormalities encompass functional connectivity, brain maturity, and severe fetal abnormalities. The classification accuracies achieved by support vector machines in these studies range from 79% to 84% [126][144].
Support vector machines (SVMs) have been utilized in magnetic resonance imaging texture analysis to examine brain tumors. This machine learning application aims to quantitatively analyze imaging data to generate an image texture that is generally imperceptible to human visual perception [127][145]. Texture analysis in clinical practice is advantageous for clinicians because it can incorporate comprehensive imaging data of the entire tumor. This approach takes into consideration the presence of intra-tumor heterogeneity, which may not be adequately represented by a single biopsy site or even multiple biopsy sites [127][145].
15. Hematology
AI has been used to examine various types of medical data, including hematopathological, radiographic, laboratory, genomic, pharmacological, and chemical data. The purpose of using AI in these analyses is to enhance the accuracy and effectiveness of diagnosis, outcome prediction, and treatment planning and to expand our understanding of benign and malignant hematology.
Recent advancements in CNN-based models have shown the ability to effectively differentiate between various types of leukocytes on peripheral smears, indicating their potential for automating routine pathology practices [128][148]. Ongoing research is being conducted in the field of automated interpretation of bone marrow specimens [129][149]. CNNs have also exhibited their usefulness in characterizing qualitative and quantitative variations within specific cell lineages, such as the morphology of erythrocytes and the textural alterations observed in sickle cell disease [130][150]. The aforementioned achievements encompass the differential diagnosis of various diseases, as evidenced by the capacity of models to accurately diagnose acute myeloid leukemia, distinguish between different causes of bone marrow failure, and function as a screening tool for lymphoma in settings with limited resources [131][151].
AI has been applied in various domains to enhance diagnostic processes’ dependability, convenience, and efficacy. Previous studies have shown that CNN methods have proven effective in diagnosing multiple myeloma solely using mass spectrometry data obtained from peripheral blood [132][133][152,153]. Personalized models have been shown to possess a high diagnostic capability when distinguishing between challenging conditions, such as different causes of bone marrow failure, is difficult. This is achieved by integrating patient demographics, laboratory data, and fundamental genetic information. Previous studies have also utilized similar methods to differentiate between peripheral leukemia and lymphoma [134][154].
The task of prognosis is widely recognized as challenging, and even commonly used clinical prognostication tools exhibit notable variability within different risk categories [135][155]. AI, possessing advanced capabilities in processing nonlinear and intricate data, promises to deliver more sophisticated and individualized prognostications. The aforementioned methodologies have been used within the field of benign hematology to enhance the accuracy of risk assessment for central catheter thrombosis. These methodologies have successfully identified individuals with a low risk of developing thrombosis [135][155]. AI has been utilized to categorize patients undergoing hematopoietic stem cell transplants into low and high-risk groups for acute graft-versus-host disease. This classification has important implications for making informed decisions regarding the administration of immunosuppressive treatments to these individuals [136][156]. Previous studies have also been conducted in the field of autologous transplants for multiple myeloma. AI has been utilized in the field of malignant hematology to enhance the initial assessment of risk stratification for acute myeloid leukemia and myelodysplastic syndromes [137][138][157,158]. In post-treatment scenarios, where minimal residual disease is considered a negative prognostic factor, AI has exhibited the capability to attain performance comparable to that of humans. This achievement has the potential to simplify and establish a consistent approach to handling and analyzing this kind of data [139][159].
16. Intensive Care Unit
ML models have been applied within the intensive care unit (ICU) setting to anticipate pathologies such as acute kidney injury, identify symptoms such as delirium, and suggest appropriate therapeutic interventions such as vasopressors and fluid administration in cases of sepsis. The timely identification and management of sepsis is of paramount importance due to its potential to significantly decrease mortality rates. Although the management of early sepsis involves source control and the administration of broad-spectrum antibiotics, the detection of sepsis during this phase of the illness poses considerable challenges [139][159]. Identifying sepsis becomes increasingly feasible as the condition advances, while the treatment poses considerable challenges. Due to the diverse nature of sepsis, the existing diagnostic and prognostic methods pose a significant challenge in early sepsis detection and accurate prognosis estimation. This difficulty further complicates determining an appropriate treatment strategy for individual patients [140][160]. AI prediction models have the potential to provide significant value for patients diagnosed with sepsis. AI models possess the capacity to enhance the timely identification of individuals requiring antibiotic treatment. Certain AI prediction models appear to outperform existing diagnostic methods; however, these models exhibit notable limitations, such as including predictor variables like blood pressure in the present sepsis definition. The assessment of AI models’ performance is exaggerated in this context. These models exhibit limited generalizability. The existence of unresolved concerns has resulted in a significant disparity between the advancement of algorithms and their practical implementation in clinical settings.
The growing utilization of Electronic Health Records within the ICU is driving the dissemination of data science and ML techniques in the critical care setting. Hemodynamic data derived from monitors, infusion data obtained from infusion pumps, and respiratory data collected from ventilators generate substantial data volumes. These datasets can be compared to other sources of big data, such as omics data encompassing genomics or proteomics. A study devised a computational model utilizing reinforcement learning techniques to propose optimal treatment strategies dynamically for adult ICU patients [141][161].
17. Diagnostic Methods
AI has the capability to fundamentally transform the methodologies applied in disease diagnosis and treatment. Through the examination of patient data, including medical history, symptoms, and test results, AI algorithms can provide clinicians with more precise and tailored diagnoses for individual patients. This has the potential to facilitate the identification of diseases at an earlier stage and enhance the efficacy of treatment strategies. Additionally, AI can assist in identifying potential drug interactions and adverse reactions, ensuring that patients receive the safest and most effective treatments.
Ultrasound (US) has gained widespread global adoption as a primary imaging modality in various clinical domains, owing to the continuous advancements in ultrasonic technologies and the established digital health infrastructure. Breast cancer is widely recognized as a prevalent form of cancer among women globally and continues to be the second most significant contributor to cancer-related mortality [142][163]. The predominant utilization of DL in breast US, as observed in the surveyed literature, pertains to diagnosing and categorizing breast masses [143][164]. The utilization of DL techniques for the analysis of abdominopelvic imaging has various applications within the United States. A significant portion of these applications have been specifically directed towards the examination of the liver [144][165]. Their research revealed that this approach exhibited superior accuracy compared to two-dimensional shear wave elastography and certain biomarkers in the evaluation of advanced fibrosis and cirrhosis in patients infected with the hepatitis B virus. In a study, the authors devised a CNN approach to predict the METAVIR score, a semi-quantitative measure of liver fibrosis [145][166]. The training dataset consisted of several thousands of US images obtained from two tertiary academic referral centers. This approach demonstrated a notable level of precision in forecasting the METAVIR score, surpassing radiologists’ diagnostic capabilities in identifying liver fibrosis. In their study, Ta et al. developed a computer-aided diagnosis system for the classification of malignant and benign focal liver lesions using contrast-enhanced ultrasound cine recordings [146][167]. The researchers found that the accuracy of this method was comparable to that of an expert reader.
Deep learning algorithms have been utilized in various applications, including identifying muscle diseases [147][168], determining cone positioning, and segmentation of muscle imaging [148][169]. The diagnostic accuracy for neuro-muscular diseases was improved by using a CNN-based method, which enhanced the assessment and classification of inflammatory muscle diseases [147][168]. The progress in the field of AI/ML tools for the interpretation of imaging is experiencing rapid acceleration. This can be attributed to several factors, including the availability of extensive digitized image datasets, the accessibility of open-source algorithms, advancements in computing power, the emergence of cloud services, and the continuous development of DL techniques [149][170].
The utilization of automation in tasks frequently performed by radiologists, such as identifying rib fractures and lung nodules using CT scans, reassessing pleural effusion size via sequential chest radiographs, or conducting mammographic screening, shows potential as a favorable strategy. This has the potential to enable radiologists to dedicate additional time to more advanced interpretive tasks that may not be amenable to automation, as well as participate in endeavors such as multidisciplinary team meetings. The utilization of AI in the triage procedure demonstrates a notable ability to efficiently assign priority to critical cases that require immediate reporting. These cases may include CT scans that unveil the presence of pulmonary embolism, chest radiographs that indicate pneumothorax, or head CTs that reveal hemorrhage. The aforementioned methodology possesses the capacity to reduce patient morbidity and expedite the duration of hospitalization in emergency departments. The utilization of DL systems in synthetic MRI enables the post-processing and reconstruction of MR image data, reducing image acquisition time without significant deterioration in image quality. This advancement can potentially enhance efficiency, decrease expenses, and enhance accessibility [150][171].
CNNs are a fundamental element within networks utilized in orthopedic and musculoskeletal radiology [151][172]. In recent times, the concept of generative adversarial networks (GANs) has been introduced. GANs are models designed to generate novel data closely resembling the original dataset. These models comprise two distinct networks that engage in a competitive game-like training process. A total of 12 enhanced GAN models incorporating CNNs have been successfully utilized in the domain of radiographic images [152][173].
Extensive research has been conducted in the field of oral and maxillofacial radiology to investigate the potential of AI in the diagnosis of various conditions. The aforementioned conditions encompass dental caries, periodontal disease, osteosclerosis, odontogenic cysts, and tumors, as well as ailments that impact the maxillary sinus or temporomandibular joints.
AI has been used in the field of dentistry for image analysis, encompassing a range of tasks, including tooth segmentation and localization, assessment of bone quality for osteoporosis, determination of bone age through hand-wrist radiographs, and localization of cephalometric landmarks [151][172]. DL systems utilizing CNN architectures have been successfully applied in the domain of dentistry. Notably, a novel system has been developed that incorporates both three-dimensional cone beam computed tomography images and two-dimensional images [151][172].
The application of DL techniques has been used to identify and categorize teeth within both cone beam computed tomography images and panoramic images. The utilization of classification systems for teeth enables dentists to make clinical decisions and streamline their charting process by using automated computer-aided design outputs. These outputs facilitate the automatic completion of digital patient records [153][174].
