Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Marco Fogante.
A cardiac lesion detected at ultrasonography might turn out to be a normal structure, a benign tumor or rarely a malignancy, and lesion characterization is very important to appropriately manage the lesion itself. The exact relationship of the mass with coronary arteries and the knowledge of possible concomitant coronary artery disease are necessary preoperative information.
- cardiac masses
- pseudomasses
- non-neoplastic lesions
1. Introduction
Cardiac lesions can be subdivided into pseudomasses and true masses. The latter can be categorized into non-neoplastic masses and neoplastic ones, which can be classified into benign and malignant, as reported in the 2021 WHO classification [1]. These lesions can be recognized as intracavitary, myocardial, valvular, pericardial lesions or as paracardiac masses involving atrioventricular groove or pericardiophrenic angles.
Cardiac ultrasonography is the first-line imaging modality, but when the nature of the lesion is not clearly understood or the precise localization and the relationship with surrounding tissues and coronary arteries need to be evaluated, computed tomography (CT) and magnetic resonance (MR) imaging are performed [2]. Moreover, the increasing use of coronary CT angiography to evaluate coronary artery disease results in more frequent observation of incidental findings [3]. PET/CT could be useful when a lymphoma or a malignant lesion is suspected, and sometimes it could determine false positive findings as it is the case of lipomatous hypertrophy of the inter-atrial septum in which there is metabolically active brown adipose tissue [4].
Many cardiac masses consist of pseudomasses, non-neoplastic lesions and benign tumors, and lesion characterization is very important to manage the patients appropriately. Large lesions with ill-defined borders, tissue heterogeneity, nodular pericardial involvement and multiplicity are suspicious findings for malignancy.
2. Pseudomasses
2.1. Normal Intracardiac Structures
Normal cardiac structures or embryologic remnants can sometimes raise suspicion of tumor or thrombus with echocardiography, especially if prominent, but are generally easily characterized with MR imaging or CT given the large field of view and high spatial resolution. Coumadin ridge or warfarin ridge or left atrial ridge is a normal elongated structure with a bulbous tip in the left atrium that lies between the left atrial appendage and left upper pulmonary vein (Figure 1). A prominent ridge can be mistaken for a thrombus or tumor, but it usually has an echogenicity that is similar to surrounding cardiac structures and shows mobility with underlying tissue. CT attenuation, MR signal intensity and contrast enhancement are like those of the normal surrounding atrial wall. Sometimes the coumadin ridge may be a substrate for paroxysmal atrial fibrillation. Rarely, tumors such as fibroelastoma and myxoma may originate from this ridge [5,6,7][5][6][7].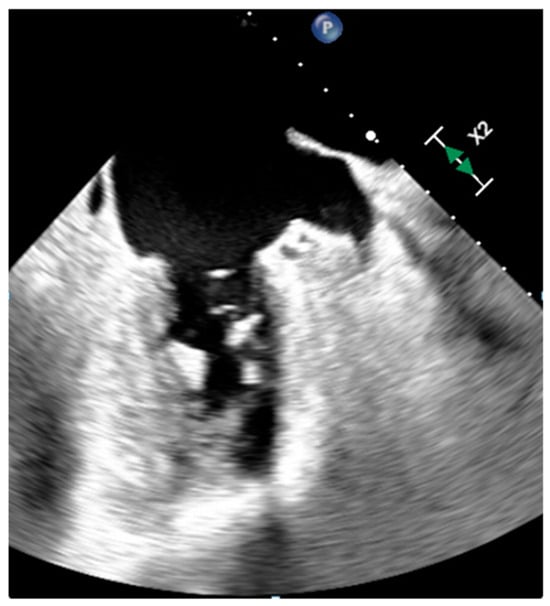
Figure 1. Warfarin ridge at transesophageal echocardiography.
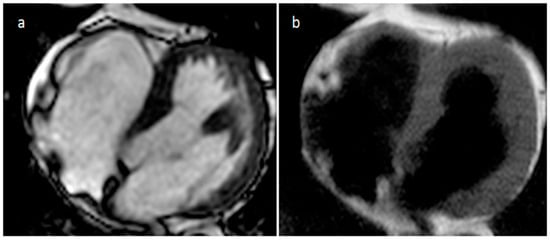
Figure 2. Axial cine MR sequences (a) and T1-weighted images (b) in a patient with prominent crista terminalis.
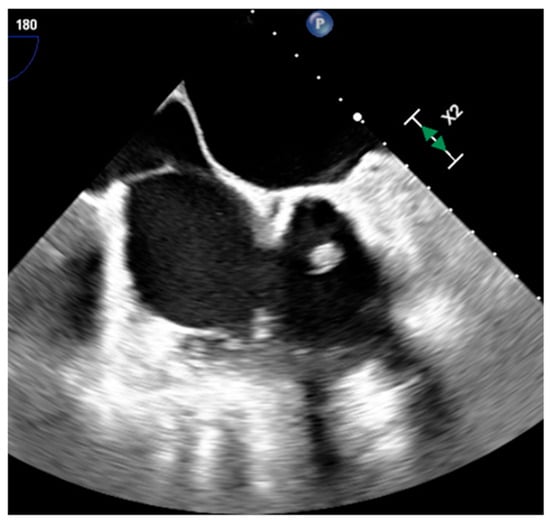
Figure 3. A prominent eustachian valve at transesophageal echocardiography.
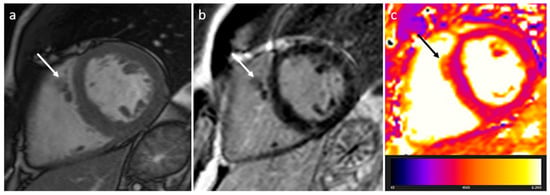
Figure 4. Right ventricle pronounced muscolar ridge which extends from the basal ventricular septum to the free wall on cine images (a), late gadolinium enhancement MR sequence (b), and T2 mapping image (c).
2.2. Lipomatous Hypertrophy of the Inter-Atrial Septum (LHIS or LHAS)
LHIS is a benign unencapsulated hyperplasia of lipocytes resembling brown fat in the fold of the septum secundum, sparing the fossa ovalis which forms part of the septum primum, producing the typical barbell or dumbbell appearance (Figure 5). Interatrial Septum (IS) thickness > 2 cm suggests the diagnosis of LHIS. Extensive LHIS can also involve the posterior wall of the right atrium and crista terminalis. Cardiac lipoma is encapsulated, affects the fossa ovalis and represents the main differential diagnosis. LHIS metabolically active brown adipose tissue can show 18F-FDG uptake during Positron Emission Tomography (Figure 6). LHIS is usually asymptomatic. However, sometimes it can cause atrial arrhythmias or, rarely, superior vena cava compression. Surgery is indicated if there are signs of superior vena cava syndrome, or if there are severe intractable arrhythmias [13,14,15][13][14][15].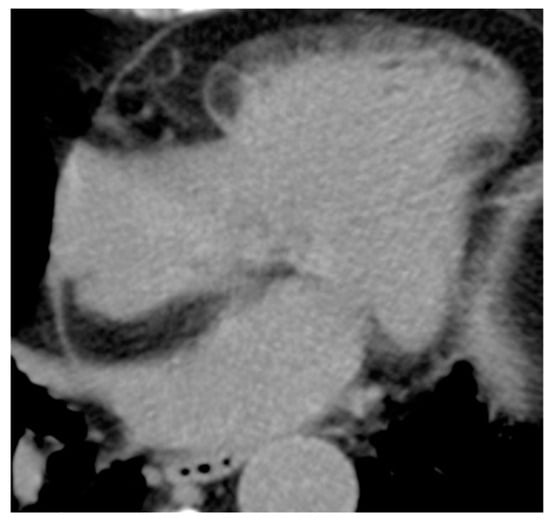
Figure 5. 81-year-old female patient with lipomatous hypertrophy of the interatrial septum in CT scan.
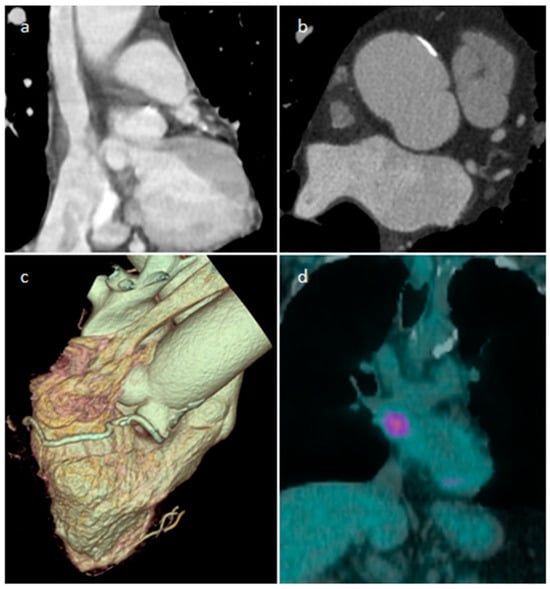
Figure 6. A 65-year-old male patient with lipomatous hypertrophy of the interatrial septum on coronal and axial CT images (a,b), volume rendering (c), which showed focal uptake of FDG in PET/CT scan (d).
3. Non-Neoplastic Masses
3.1. Thrombus
Thrombus is the most common cardiac mass. It is usually identified in the left atrium in patients with atrial fibrillation, atrial dilatation, mitral stenosis or previous mitral valve surgery (Figure 7). After myocardial infarction thrombus can be found adjacent to a left ventricle hypokinetic region (Figure 8). Occasionally, thrombi can be located in the RA or in vena cava, especially in patients with enlarged right chambers and with central venous lines. They are usually broad-based and move synchronously with the adjacent wall during the cardiac cycle. During CT a thrombus shows low attenuation on delayed phase acquisition, and it shows absence of iodine uptake on dual-energy CT iodine map. Cardiac MR is the most accurate imaging method in differentiating cardiac thrombi and tumors. T1- and T2-weighted signal characteristics vary depending on the age of a thrombus. Usually, thrombus does not show contrast enhancement. Late gadolinium enhancement (LGE) imaging can sometimes show a hypointense border and a brighter central zone. However, organized chronic thrombus may rarely enhance peripherally due to its fibrous content. Frequently patients are asymptomatic, and thrombus identification is necessary in order to start anticoagulation treatment and prevent possible embolic events [16,17,18][16][17][18].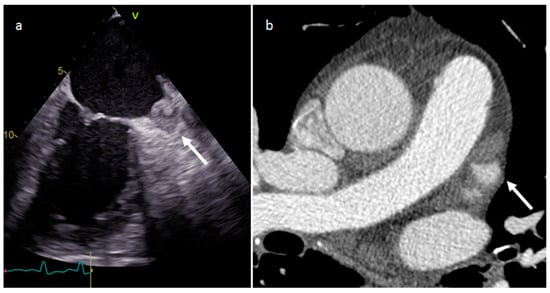
Figure 7. Left atrium appendage thrombus at transesophageal echocardiography (a) and CT (b).
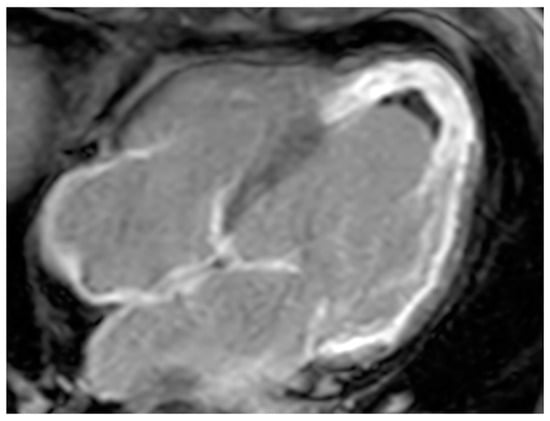
Figure 8. A 67-year-old female patient with left ventricle thrombus related to myocardial infarction on late gadolinium enhancement MR sequence.
3.2. Vegetations
Endocarditis is usually due to an infectious etiology and is characterized by leaflet destruction and often valvular incompetence (Figure 9). Either a native or prosthetic cardiac valve may be affected (Figure 10). Moreover, electrocatheters can be involved (Figure 11). The modified Duke criteria are used for diagnosis. Echocardiography is the first-line imaging modality for patients with suspected vegetations. Vegetations are highly mobile lesions, and usually do not enhance. However, peripheral rim enhancement on LGE imaging rarely can be observed. Moreover, adjacent myocardial enhancement could denote perivalvular abscess, irreversible myocardial damage, or fibrosis. CT and MR are very useful to evaluate complications. In fact, severe cases may be characterized by perivalvular extension with paravalvular abscesses, mycotic pseudoaneurysm or fistula formation. Moreover, total-body CT should be performed to evaluate distant septic embolization. Proper diagnosis is vital because prompt management with antibiotic administration and surgery is crucial. Sometimes small verrucous vegetations can be found in sterile endocarditis secondary to inflammation (Libman-Sacks endocarditis), such as in patients with systemic lupus erythematosus [19].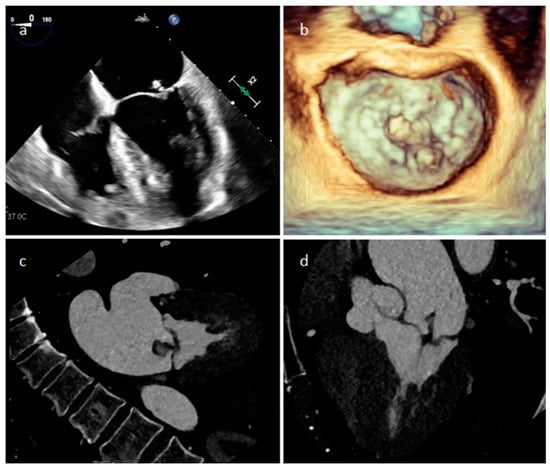
Figure 9. A mitral valve vegetation at 2D (a) and 3D (b) transesophageal echocardiography, and at CT (c,d).
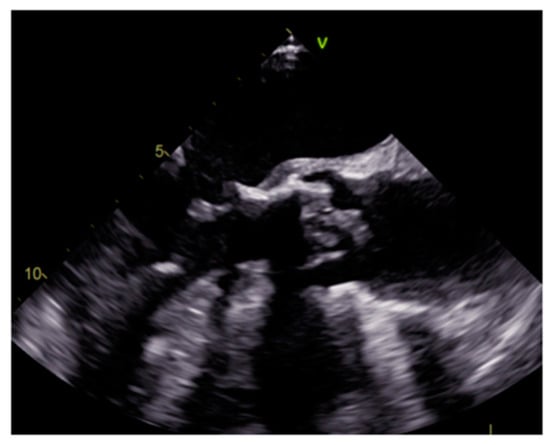
Figure 10. Prosthetic aortic valve infective endocarditis with vegetations at transesophageal echocardiography.
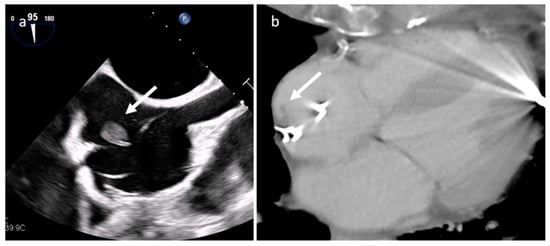
Figure 11. Electro-catheter endocarditis at transesophageal echocardiography (a) and CT (b).
3.3. Mitral Annular Calcification and Its Caseous Degeneration
Mitral annular calcification is an immobile mass, generally located in the inferolateral portion of the mitral anulus, caused by annular fibers calcium deposits, and rarely it can affect the whole annulus and press the myocardium (Figure 12). Imaging modalities show typical calcium deposition, and the fibrotic rim in which the calcified core is enclosed can show a peripheral LGE imaging hyperenhancement [20]. Rarely, this lesion can evolve into caseous degeneration (or liquefaction necrosis), and in this case the lesion core contains a mixture of cholesterol, fatty acids, and amorphous eosinophilic infiltrate with a surrounding envelope that enfolds lymphocytes, macrophages, and multiple necrotic areas with calcifications [21]. It is usually asymptomatic. However, symptoms can be caused by related complications such as mitral stenosis or regurgitation, infective endocarditis, and embolization. The central fluid proteinaceous and fatty content can show high signals on both T1- and T2-weighted sequences. Calcifications around and within the lesion are hypointense on MRI imaging but are better evaluated with CT. Peripheral LGE imaging hyperenhancement consistent with a fibrous cap is observed.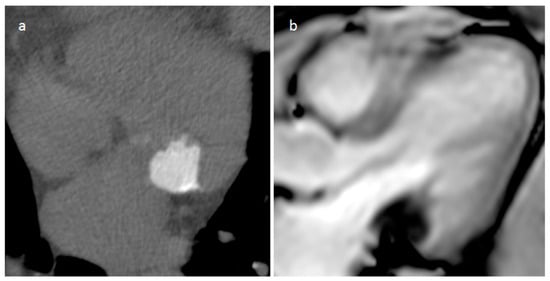
Figure 12. A 74-year-old female patient with mitral annular calcification on precontrast-phase CT image (a) and cine sequence (b).
3.4. Pericardial Cyst
A pericardial cyst is usually congenital in etiology, but sometimes it is acquired following pericarditis. It is often placed in the right pericardiophrenic angle (70%), and less frequently in the left one (25%) (Figure 13). Sometimes intracystic septations or enhancing walls can be detected. It is usually asymptomatic, and imaging is useful to evaluate lesion stability. However, rarely larger cysts can determine right cardiac chambers compression, and patients may present with congestive heart failure symptoms or retrosternal pain. In these cases, surgery could be indicated [22].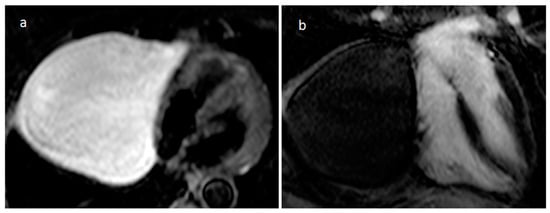
Figure 13. A 70-year-old female patient with a large right pericardiophrenic angle pericardial cyst on axial T2 STIR (a) and four-chamber view late gadolinium enhancement (b) MR imaging.
3.5. Coronary Artery Aneurism (CAA)
CAA may be mistaken for mass at cardiac ultrasonography, particularly if there is extensive peripheral thrombus. A saphenous vein coronary artery bypass grafting aneurysm can be detected 10–15 years after surgery, and it can be completely thrombosed. CT or MRI allows confident diagnosis as well as delineation of the size and extent of the aneurysm (Figure 14). Usually asymptomatic, rarely it can cause myocardial ischemia or infarction, fistula formation or can evolve into rupture. A coronary artery aneurysmal dilatation can also be found in the setting of a long-standing coronary artery fistula, as a reduced vascular resistance and increased flow caused by an abnormal communication of the coronary artery with a pulmonary artery, cardiac chamber, coronary sinus, or cardiac vein determine a dilatation of the involved artery. The right coronary artery is more frequently involved, and a left-right shunt occurs in most lesions. Coronary CT angiography is useful to evaluate the size of the dilatation, the presence of a concomitant thrombus, and the fistula anatomy [23].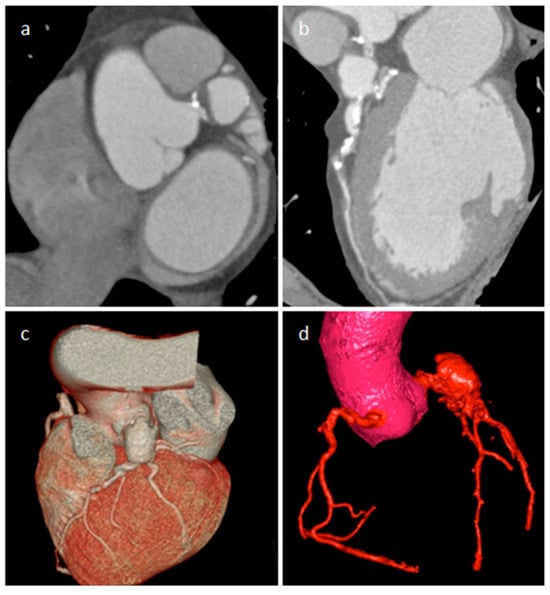
Figure 14. A 65-year-old male patient with a partially thrombosed giant aneurysm of the left main coronary artery on short-axis view (a), multiplanar reconstruction (b) and volume rendering (c,d) CT images.
3.6. Cardiac Chambers Aneurysm and Pseudoaneurysm
Aneurysm and pseudoaneurysms of the ventricles or atria may occur as complications of cardiac surgery, or secondary to infarction, trauma, or infection (more frequently endocarditis), and they could be occasionally mistaken for a focal mass [23]. CT and MRI are very useful to define the origin of these blood-filled structures (Figure 15).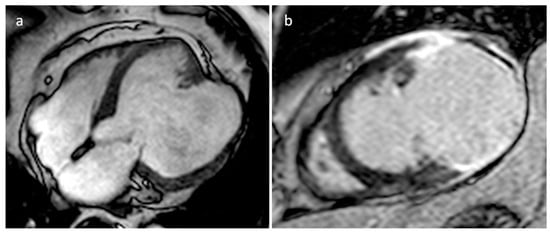
Figure 15. A 67-year-old male patient with huge left ventricle aneurysm after myocardial infarction on four-chamber cine sequence (a) and axial late gadolinium enhancement MR sequence (b).
3.7. IgG4-Related Disease (IgG4-RD)
IgG4-RD is a fibro-inflammatory disease that can involve nearly any organ and tissue. It is a systemic condition characterized by IgG4-positive lymphoplasmacytic tissue that infiltrates with subsequent fibrotic aftereffects. Common manifestations include major salivary and lacrimal gland enlargement, autoimmune pancreatitis, and retroperitoneal fibrosis [24]. Cardiovascular involvement includes coronary stenosis or dilatation surrounded by a tumor-like lesion or periarterial thickening, pericardial thickening and effusion, constrictive pericarditis, cardiac masses (Figure 16), valvular stenosis and regurgitation, periaortitis and aortic aneurysm, pulmonary artery stenosis and pulmonary hypertension, and carotid arteries stenosis. FDG PET/CT can evaluate the activity and severity of the disease and can identify the best biopsy site. Glucocorticoids are considered the first-line agents and azathioprine is frequently used as additional therapy. Moreover, pericardial drainage, pericardiectomy, valve replacement and surgical resection of masses could be performed [25].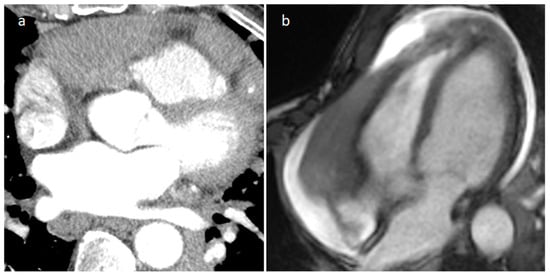
Figure 16. Right atrioventricular groove IgG4-related disease on arterial CT phase (a) and on cine MR sequence (b).
3.8. Sarcoidosis
Sarcoidosis is a multisystem chronic granulomatous disease, and cardiac involvement seems to be responsible for about 10% of sarcoidosis-related deaths. The basal septum is mainly involved, and several reports showed cardiac tumor-like lesions in the ventricular septum, right ventricle, left and right atria [26]. MR imaging can reveal myocardial delayed enhancement, and myocardial oedema can be found in active sarcoidosis (Figure 17). In young patients with heart failure, new atrioventricular (AV) block, and cardiac arrest cardiac sarcoidosis should be excluded. In fact, cardiac MR imaging should be performed as an early screening tool for myocardial involvement in patients presenting with AV block requiring immediate pacing for bradycardia, and FDG PET/CT segmental uptake is the main cardiac sarcoidosis feature related to active disease. Moreover, early initiation of glucocorticoids in patients with second degree AV block could avoid pacemaker implantation [27].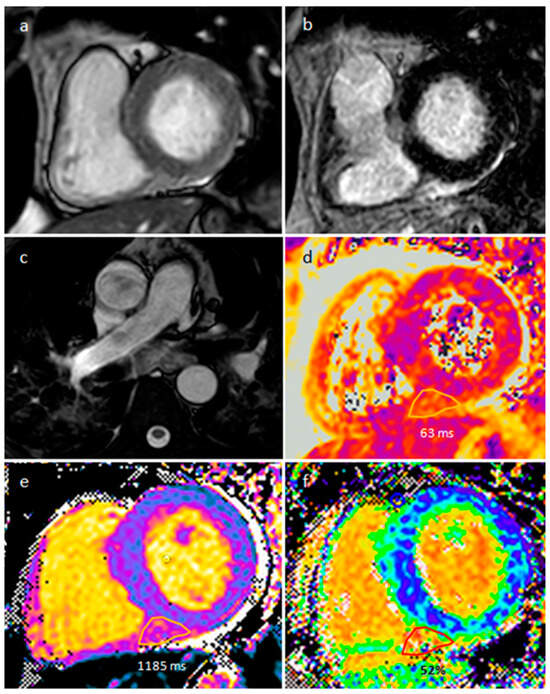
Figure 17. A 57-year-old male patient with sarcoidosis on axial cine sequence (a), short tau inversion recovery sequence (b), hilar and pulmonary involvement on balanced-steady-state free precession images (c), T2 mapping (d), native T1 mapping (e) and post-contrast T1 mapping (f) sequences (normal T2 value < 50 ms and normal native T1 value < 1045 in our site).
3.9. Foreign Body
Foreign bodies can manifest as cardiac masses. They can be either iatrogenic (gossypiboma, catheter, inferior vena cava filters, guide wires, pacemaker leads) or traumatic (chopstick, bullets). Diagnosis is usually incidental in an asymptomatic patient who previously performed a surgical or intravascular procedure. However, sometimes patients can be highly symptomatic with chest pain and dyspnea caused by heart failure, perforation or obstruction of vessels or cardiac chambers [28,29,30,31][28][29][30][31]. The foreign body is encapsulated in fibroinflammatory tissue, clotted blood and/or thrombus, and the shape and type of the object is sometimes difficult to recognize by imaging. Treatment strategies can vary depending on the patient’s symptoms and the foreign body location and nature. However, surgical removal is often considered in these patients, especially when the patient is symptomatic or there are risks of perforation, embolization, or infection.3.10. Hematoma
Hematoma is usually detected in patients with previous surgery, and some clips can be found around it. Pericardial hematoma is classified as acute in the first week after surgery or trauma, subacute between one and four weeks, and chronic if the hematoma persistent for more than a month. Hematoma can appear mass-like on echocardiography and as mixed density on CT (Figure 18). Density is usually high in the acute phase and then it slowly decreases. On MR it may have different signals, depending on its age and sequence used. It does not show internal enhancement, and chronic hematoma can show internal calcifications. However, sometimes it can show rim-enhancement. A hematoma usually resolves without sequela, however unfrequently it can persist and sometimes become larger over time as blood breakdown products can cause localized inflammatory reactions, which might be at the base of additional bleeding and further inflammation, causing a persisting cycle that evolve into the so called chronic expanding hematomas. On MRI, a chronic expanding hematoma can show a hypointense peripheral capsule and heterogeneous central contents indicating the presence of fresh and old blood. After heart injuries hematomas can cause symptoms within a few days with a potential risk of hemodynamic compromise. In fact, fast or slow-growing space-occupying lesions can cause hemodynamic instability due to compression of great vessels and cardiac chambers. However, patients with post-surgical pericardial hematoma are usually asymptomatic, and serial follow-up examinations are useful to evaluate disease progression, stability or regression [32,33,34][32][33][34].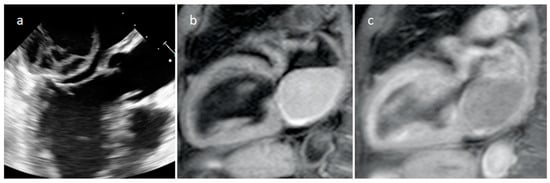
Figure 18. Echocardiography (a), T1-weighted (b) and T1-weighted gadolinium-enhanced MR sequences (c) in a patient with spontaneous acute left atrial hematoma.
3.11. Echinococcus Cyst
The most frequent locations of echinococcosis are the liver and lungs, followed by muscles, bone and kidneys. Cardiac hydatid occurs in about 1% of cases. Usually, the cyst is identified in the LV free wall or RV free wall. Rarely, the pericardium, IVS or atria can be involved [35]. Frequently, cardiac lesions are associated with the liver and/or lung [36]. Diagnosis can frequently be made by echocardiography recognizing a cyst with multiple septa and daughter cysts in a patient with positive laboratory tests. However, CT and MR are useful to define a possible involvement of surrounding structures and for presurgical planning (Figure 19). Cardiac cysts can be asymptomatic or can be associated with dyspnea, chest pain, ventricular arrhythmias, valvular dysfunction, and outflow tract obstruction [37]. Cardiac echinococcosis is usually managed by combining the use of antiparasitic drugs with surgical cyst excision.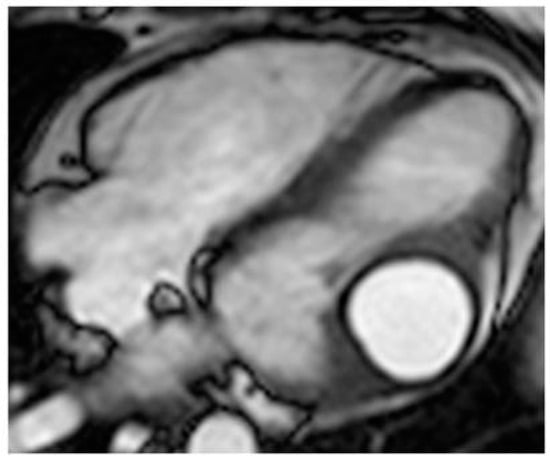
Figure 19. A 26-year-old female patient with left ventricular myocardium echinococcus cyst, which is hyperintense with a dark rim on cine MR sequence.
References
- Maleszewski, J.J.; Basso, C.; Bois, M.C.; Glass, C.; Klarich, K.W.; Leduc, C.; Padera, R.F.; Tavora, F. The 2021 WHO Classification of Tumors of the Heart. J. Thorac. Oncol. 2022, 17, 510–518.
- Wu, C.M.; Bergquist, P.J.; Srichai, M.B. Multimodality Imaging in the Evaluation of Intracardiac Masses. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 55.
- Bernheim, A.; Gore, A.; Goyal, N. Evaluation of Incidental Cardiac Masses on Computed Tomography Imaging: An Algorithmic Approach. J. Thorac. Imaging. 2019, 34, W1–W9.
- Aghayev, A.; Cheezum, M.K.; Steigner, M.L.; Mousavi, N.; Padera, R.; Barac, A.; Kwong, R.Y.; Di Carli, M.F.; Blankstein, R. Multimodality imaging to distinguish between benign and malignant cardiac masses. J. Nucl. Cardiol. 2022, 29, 1504–1517.
- Gonzalez-Casal, D.; Datino, T.; Soto, N.; González-Panizo, J.; Sánchez-Quintana, D.; Macias, Y.; Cabrera, J.Á. Anatomy of the left atrial appendage for the interventional cardiologist. Herzschrittmacherther. Elektrophysiol. 2022, 33, 195–202.
- Lak, H.M.; Kerndt, C.C.; Unai, S.; Maroo, A. Cardiac papillary fibroelastoma originating from the coumadin ridge and review of literature. BMJ Case Rep. 2020, 13, e235361.
- Moustafa, S.; Patton, D.J.; Connelly, M.S.; Alvarez, N.; Prieur, T.; Mookadam, F. An atypical case of left atrial myxoma. Rev. Port. Cardiol. 2015, 34, 75–77.
- Lakhani, D.A.; Balar, A.B.; Kim, C. Prominent crista terminalis mimicking a right atrial mass: A case report and brief review of the literature. Radiol. Case Rep. 2021, 17, 434–438.
- Kucybała, I.; Ciuk, K.; Klimek-Piotrowska, W. Clinical anatomy of human heart atria and interatrial septum—Anatomical basis for interventional cardiologists and electrocardiologists. Part 1: Right atrium and interatrial septum. Kardiol. Pol. 2018, 76, 499–509.
- Islam, A.K.; Sayami, L.A.; Zaman, S. Chiari network: A case report and brief overview. J. Saudi Heart Assoc. 2013, 25, 225–229.
- Meetham, K.; Taerujjirakul, T.; Garitjirapath, N.; Navic, P.; Shinlapawittayatorn, K.; Mahakkanukrauh, P. The morphometric study of the moderator band in Thais. Anat. Sci. Int. 2022, 97, 188–196.
- Rajiah, P.; MacNamara, J.; Chaturvedi, A.; Ashwath, R.; Fulton, N.L.; Goerne, H. Bands in the Heart: Multimodality Imaging Review. Radiographics 2019, 39, 1238–1263.
- Hamer, O.W. Lipomatous Hypertrophy of the Atrial Septum. Dtsch. Arztebl. Int. 2022, 119, 187.
- Yavar, Z.; Gilge, J.L.; Patel, P.J.; Patel, A.C.; Garcia-Cortes, R.S.; Fetters, J.K.; Fouts, A.M.; Salerno, C.T. Lipomatous Hypertrophy of the Interatrial Septum Manifesting as Third Degree Atrioventricular Block. JACC Case Rep. 2020, 2, 2235–2239.
- Siminiak, N.; Rajewska-Tabor, J.; Pyda, M.; Czepczyński, R.; Ruchała, M. Changing appearance of lipomatous hypertrophy of the interatrial septum on positron emission tomography scan. Kardiol. Pol. 2021, 79, 1032–1033.
- Adeniyi, A.; Abadir, S.; Parikh, K.; Khanna, R.; Yusuf, S.; Anais Hichard, M. Atypical Intracavitary Cardiac Mass: Tumor or Thrombus? Cureus 2022, 14, e21937.
- Lee, J.W.; Park, C.H.; Im, D.J.; Lee, K.H.; Kim, T.H.; Han, K.; Hur, J. CT-based radiomics signature for differentiation between cardiac tumors and a thrombi: A retrospective, multicenter study. Sci. Rep. 2022, 12, 8173.
- Johnson, E.M.; Gage, K.L.; Feuerlein, S.; Jeong, D. Cardiac Magnetic Resonance for the Evaluation of Suspected Cardiac Thrombus: Conventional and Emerging Techniques. J. Vis. Exp. 2019, 148, e58808.
- Gatti, M.; D’Angelo, T.; Muscogiuri, G.; Dell’aversana, S.; Andreis, A.; Carisio, A.; Darvizeh, F.; Tore, D.; Pontone, G.; Faletti, R. Cardiovascular magnetic resonance of cardiac tumors and masses. World J. Cardiol. 2021, 13, 628–649.
- Shah, B.N.; Babu-Narayan, S.; Li, W.; Rubens, M.; Wong, T. Severe mitral annular calcification: Insights from multimodality imaging. Tex. Heart Inst. J. 2014, 41, 245–247.
- Motwani, M.; Kidambi, A.; Herzog, B.A.; Uddin, A.; Greenwood, J.P.; Plein, S. MR imaging of cardiac tumors and masses: A review of methods and clinical applications. Radiology 2013, 268, 26–43.
- Tower-Rader, A.; Kwon, D. Pericardial Masses, Cysts and Diverticula: A Comprehensive Review Using Multimodality Imaging. Prog. Cardiovasc. Dis. 2017, 59, 389–397.
- Glockner, J.F. Magnetic Resonance Imaging and Computed Tomography of Cardiac Masses and Pseudomasses in the Atrioventricular Groove. Can. Assoc. Radiol. J. 2018, 69, 78–91.
- Chen, L.Y.C.; Mattman, A.; Seidman, M.A.; Carruthers, M.N. IgG4-related disease: What a hematologist needs to know. Haematologica 2019, 104, 444–455.
- Fathala, A. Multimodalities Imaging of Immunoglobulin 4-related Cardiovascular Disorders. Curr. Cardiol. Rev. 2019, 15, 224–229.
- Fujimoto, R.; Asano, T.; Maezawa, H.; Shimojima, H.; Tsujiuchi, M.; Hori, Y.; Ebato, M.; Suzuki, H. Cardiac Sarcoidosis Mimicking Septal Tumor with Intermittent Complete Atrioventricular Block. Int. Heart J. 2018, 59, 1473–1479.
- Yodogawa, K.; Fujimoto, Y.; Hagiwara, K.; Oka, E.; Hayashi, H.; Murata, H.; Yamamoto, T.; Iwasaki, Y.K.; Shimizu, W. Possibility of steroid therapy without pacemaker implantation in patients with sarcoidosis presenting atrioventricular block. Heart Vessel. 2022, 37, 1892–1898.
- Pyo, W.K.; Kim, W.K.; Kim, J.B. A Huge Pericardial Gossypiboma Causing Severe Cardiac Dysfunction. Ann. Thorac. Surg. 2020, 109, e167–e169.
- Caushi, F.; Çoku, L.; Skenduli, I.; Xhemalaj, D.; Mezini, A.; Hysa, E.; Rulli, F. Intrapericardial gossypiboma found 14 years after coronary artery bypass grafting. J. Cardiothorac. Surg. 2019, 14, 69.
- Stewart, J.; Baltabaeva, A.; Beeton, I.; Wignall, O. Case report of a mysterious myocardial mass: An aetiological conundrum. Eur. Heart J. Case Rep. 2018, 2, yty018.
- Choi, C.H.; Elahi, M.M.; Konda, S. Iatrogenic retained foreign body in the right atrium. Lessons to Learn. Int. J. Surg. Case Rep. 2013, 4, 985–987.
- Takahashi, H.; Chomei, S.; Gan, K. A rare case of idiopathic chronic expanding pericardial hematoma presenting as pericardial tamponade: A case report and literature review. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 996–999.
- Hirai, S.; Hamanaka, Y.; Mitsui, N.; Isaka, M.; Kobayashi, T. Chronic expanding hematoma in the pericardial cavity after cardiac surgery. Ann. Thorac. Surg. 2003, 75, 1629–1631.
- Yamaguchi, T.; Terashima, M.; Takamura, C.; Sakurai, H.; Ooishi, K.; Yoshizaki, T.; Yamaguchi, J.; Hijikata, S.; Iwai, T.; Sagawa, Y.; et al. Cardiac Magnetic Resonance Imaging of Very Late Intrapericardial Hematoma 8 Years after Coronary Artery Bypass Grafting. Intern. Med. 2018, 57, 975–978.
- Prasad, K.; Kumar, R.; Halder, V.; Raju, M.; Negi, S.L.; Naganur, S. Multimodality imaging of an interventricular septum hydatid cyst. Egypt. Heart J. 2021, 73, 23.
- Khalilian, M.R.; Norouzi, A.R.; Zamani, H.; Nia, S.K.F.; Teymoordash, S.N. A Rare Case of Cardiac Hydatid Disease without Liver and Lungs Involvement. Iran. J. Public Health. 2021, 50, 2332–2336.
- Ameen, A.; Hilal, K.; Shaikh, A.; Khan, F.; Fatimi, S. Cardiac hydatid cyst presenting as ventricular arrhythmia: A case report. Egypt. Heart J. 2021, 73, 105.
More
