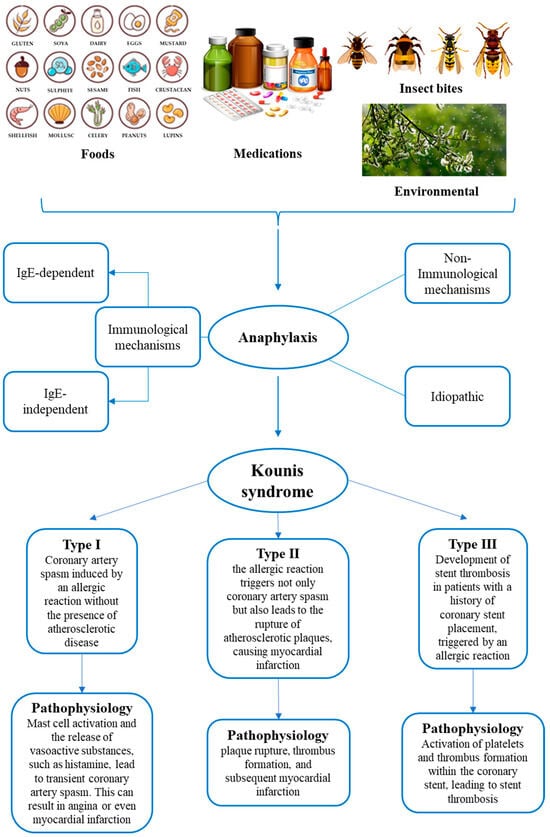
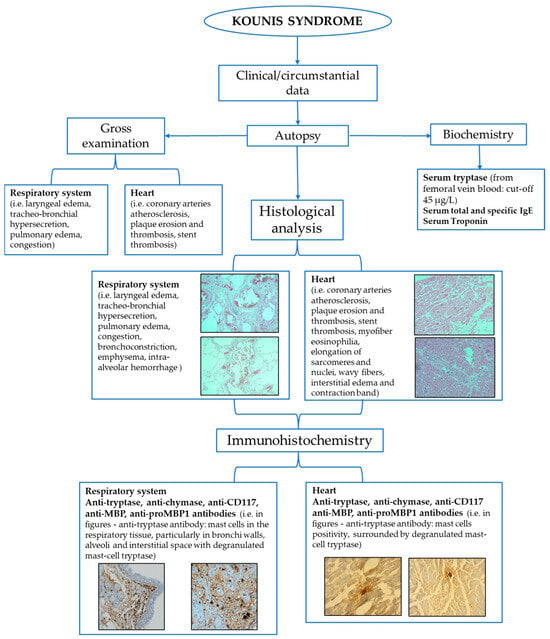
Kounis syndrome (KS) is an acute coronary syndrome triggered by allergic or hypersensitivity reactions. Incidence rates vary, with studies reporting 19.4 per 100.000 among all admissions and 3.4% among allergy patients. The pathophysiology of this syndrome involves a complex interplay between allergic reactions and the cardiovascular system. Mast cell activation, histamine release, leukotrienes, cytokines, and platelet activation can contribute to coronary events. Three types of classification systems (allergic angina, allergic myocardial infarction, allergic stent thrombosis) aid in categorizing presentations. The diagnosis of KS relies on clinical presentation, laboratory findings, and imaging. Postmortem assessment of KS is based on the integration of circumstantial data, autopsy, and histological findings. Biochemical and immunohistochemical analyses also contribute to postmortem diagnosis. In conclusion, a combined, multidisciplinary approach should be used to ease the diagnostic process, which is crucial for forensic practitioners in confirming KS occurrence.
- Kounis syndrome
- anaphylaxis
- postmortem
- immunohistochemistry
- biochemistry
- tryptase
- acute coronary syndrome
1. Epidemiology and Pathophysiology
2. Postmortem Assessment of Kounis Syndrome
References
- Kounis, N.G. Kounis syndrome: An update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin. Chem. Lab. Med. 2016, 54, 1545–1559.
- Akoz, A.; Tanboga, H.I.; Emett, M.; Bayramoglu, A.; Kizrak, Y.; Kantarci, M.; Aslan, S. A prospective study of kounis syndrome: Clinical experience and cardiac magnetic resonance imaging findings for 21 patients. Acta Med. Mediterr. 2013, 29, 811–816.
- Renda, F.; Landoni, G.; Trotta, F.; Piras, D.; Finco, G.; Felicetti, P.; Pimpinella, G.; Pani, L. Kounis Syndrome: An analysis of spontaneous reports from international pharmacovigilance database. Int. J. Cardiol. 2016, 203, 217–220.
- Helbling, A.; Hurni, T.; Mueller, U.R.; Pichler, W.J. Incidence of anaphylaxis with circulatory symptoms: A study over a 3-year period comprising 940000 inhabitants of the Swiss Canton Bern. Clin. Exp. Allergy 2004, 34, 285–290.
- Hokimoto, S.; Kaikita, K.; Yasuda, S.; Tsujita, K.; Ishihara, M.; Matoba, T.; Matsuzawa, Y.; Mitsutake, Y.; Mitani, Y.; Murohara, T.; et al. Japanese Circulation Society and Japanese Association of Cardiovascular Intervention and Therapeutics and Japanese College of Cardiology Joint Working Group. JCS/CVIT/JCC 2023 Guideline Focused Update on Diagnosis and Treatment of Vasospastic Angina (Coronary Spastic Angina) and Coronary Microvascular Dysfunction. Circ. J. 2023, 87, 879–936.
- Khan, B.Q.; Kemp, S.F. Pathophysiology of anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 319–325.
- Brown, S.G.A.; Kemp, S.F.; Lieberman, P. Anaphylaxis. In Middleton’s Allergy Principles and Practice, 8th ed.; Adkinson, N.F., Jr., Bochner, B.S., Burks, A.W., Busse, W.W., Holgate, S.T., Lemanske, R.F., O’Hehir, R.E., Eds.; Elsevier Mosby: Philadelphia, PA, USA, 2013; Chapter 77.
- Ginsburg, R.; Bristow, M.R.; Kantrowitz, N.; Baim, D.S.; Harrison, D.C. Histamine provocation of clinical coronary artery spasm: Implications concerning pathogenesis of variant angina pectoris. Am. Heart J. 1981, 102, 819–822.
- Mitsis, A.; Christodoulou, E.; Georgiou, P. Coronary spasm secondary to cefuroxime injection, complicated with cardiogenic shock—A manifestation of Kounis syndrome: Case report and literature review. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 624–630.
- Carl-McGrath, S.; Gràntzdòrffer, I.; Lendeckel, U.; Ebert, M.P.; Ròcken, C. Angiotensin Il-generating enzymes, angiotensin-converting enzyme (ACE) and mast celi chymase (CMA1), in gastric inflammation may be regulated by H. pylori and associated cytokines. Pathology 2009, 41, 419–427.
- Kounis, N.G. Kounis syndrome (allergic angina and allergic myocardial infarction): A natural paradigm? Int. J. Cardiol. 2006, 110, 7–14.
- Biteker, M.; Biteker, F.S.; Özlek, B.; Özlek, E.; Başaran, N. Classification of Kounis syndrome. Int. J. Cardiol. 2017, 247, 13.
- Marchesini, D.; Esperide, A.; Tilli, P.; Santarelli, L.; Covino, M.; Carbone, L.; Franceschi, F. Allergic acute coronary syndrome: A case report with a concise review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11768–11772.
- Mondello, C.; Baldino, G.; Cianci, V.; Forzese, E.; Asmundo, A.; Ieni, A.; Ventura Spagnolo, E. Postmortem Biochemistry and Immunohistochemistry in Anaphylactic Death Due to Hymenoptera Sting: A Forensic Case Report. Int. J. Environ. Res. Public Health 2023, 20, 5640.
- Kitulwatte, I.; Gangahawatte, S.; Perera, U.; Edirisinghe, P. Death following ceftazidime-induced Kounis syndrome. Med. Leg. J. 2017, 85, 215–218.
- Kounis, N.G.; Koniari, I.; Tsigkas, G.; Chourdakis, E.; Velissaris, D.; Soufras, G.D.; Hahalis, G. Death following ceftazidime induced Kounis syndrome: Diagnostic considerations in the realm of forensic pathology. Med. Leg. J. 2020, 88, 48–49.
- Ollo-Morales, P.; Gutierrez-Niso, M.; De-la-Viuda-Camino, E.; Ruiz-de-Galarreta-Beristain, M.; Osaba-Ruiz-de-Alegria, I.; Martel-Martin, C. Drug-Induced Kounis Syndrome: Latest Novelties. Curr. Treat. Options Allergy 2023, 1–18.
- Kounis, N.G.; Soufras, G.D.; Kounis, G.N.; Hahalis, G. Suicidal anaphylactic death: Is Kounis anaphylaxis associated syndrome the cause? Forensic Sci. Int. 2013, 232, e42–e43.
- Kounis, N.G.; Soufras, G.D.; Hahalis, G. Anaphylactic cardiac collapse, sudden death and the Kounis syndrome. J. Postgrad. Med. 2014, 60, 227–229.
- Kounis, N.G.; Soufras, G.D.; Hahalis, G. Accumulation of eosinophils, mast cells, and basophils in the spleen and the coronary arteries in anaphylactic deaths: Is the Kounis hypersensitivity associated syndrome present? Forensic Sci. Med. Pathol. 2014, 10, 150–151.
- Kounis, N.G.; Kounis, G.N.; Soufras, G.D.; Lianas, D.; Patsouras, N. Postmortem diagnosis of drug-induced anaphylactic death: Kounis syndrome and hypersensitivity myocarditis are the likely culprit in death of severe anaphylactic reactions. J. Forensic Leg. Med. 2016, 40, 40–41.
- Palmiere, C. Postmortem diagnosis of drug-induced anaphylactic death. J. Forensic Leg. Med. 2016, 41, 28–29.
- Mondello, C.; Cardia, L.; Ventura-Spagnolo, E. Immunohistochemical detection of early myocardial infarction: A systematic review. Int. J. Leg. Med. 2017, 131, 411–421.
- Mondello, C.; Cardia, L.; Bartoloni, G.; Asmundo, A.; Ventura Spagnolo, E. Immunohistochemical study on dystrophin expression in CAD-related sudden cardiac death: A marker of early myocardial ischaemia. Int. J. Leg. Med. 2018, 132, 1333–1339.
- Tse, R.; Wong, C.X.; Kesha, K.; Garland, J.; Tran, Y.; Anne, S.; Elstub, H.; Cala, A.D.; Palmiere, C.; Patchett, K.L. Post mortem tryptase cut-off level for anaphylactic death. Forensic Sci. Int. 2018, 284, 5–8.
- Edston, E.; Eriksson, O.; van Hage, M. Mast cell tryptase in postmortem serum-reference values and confounders. Int. J. Leg. Med. 2007, 121, 275–280.
- Cecchi, R. Diagnosis of anaphylactic death in forensics: Review and future perspectives. Leg. Med. 2016, 22, 75–81.
- Xiao, N.; Li, D.R.; Wang, Q.; Zhang, F.; Yu, Y.G.; Wang, H.J. Postmortem Serum Tryptase Levels with Special Regard to Acute Cardiac Deaths. J. Forensic Sci. 2017, 62, 1336–1338.
- McLean-Tooke, A.; Goulding, M.; Bundell, C.; White, J.; Hollingsworth, P. Postmortem serum tryptase levels in anaphylactic and non-anaphylactic deaths. J. Clin. Pathol. 2014, 67, 134–138.
- Garland, J.; Philcox, W.; McCarthy, S.; Hensby-Bennet, S.; Ondruschka, B.; Woydt, L.; Da Broi, U.; Palmiere, C.; Lam, L.; Ahn, Y.; et al. The effects of different sampling techniques on peripheral postmortem tryptase levels: A recommended sampling method. Int. J. Leg. Med. 2019, 133, 1477–1483.
- Wang, X.; Yin, C.; Su, X.; Su, M. Reliable Postmortem Molecular Diagnosis of Anaphylaxis: Co-localization of Mast Cell Degranulation and Immunoglobulin E in Allergic Throat Tissues. Am. J. Forensic Med. Pathol. 2020, 41, 249–258.
- Bañón, R.; Hernández-Romero, D.; Navarro, E.; Pérez-Cárceles, M.D.; Noguera-Velasco, J.A.; Osuna, E. Combined determination of B-type natriuretic peptide and high-sensitivity troponin I in the postmortem diagnosis of cardiac disease. Forensic Sci. Med. Pathol. 2019, 15, 528–535.
- Edston, E. Accumulation of eosinophils, mast cells, and basophils in the spleen in anaphylactic deaths. Forensic Sci. Med. Pathol. 2013, 9, 496–500.
- Kounis, N.G.; Cervellin, G.; Koniari, I.; Bonfanti, L.; Dousdampanis, P.; Charokopos, N.; Assimakopoulos, S.F.; Kakkos, S.K.; Ntouvas, I.G.; Soufras, G.D.; et al. Anaphylactic cardiovascular collapse and Kounis syndrome: Systemic vasodilation or coronary vasoconstriction? Ann. Transl. Med. 2018, 6, 332.
- Costantino, A.; Mezzetti, E.; De Matteis, A.; Volonnino, G.; De Simone, S.; Fazio, V. Comparative study between conventional and new methods in defining the cause of death from anaphylactic shock. Clin. Ter. 2021, 172, 369–371.
- Luongo, S.; Frontalini, C.; Pesaresi, M.; Valsecchi, M.; Tagliabracci, A. Histopathological markers for the diagnosis of anaphylactic death. Med. Sci. Law 2011, 51 (Suppl. 1), S30–S36.
- Unkrig, S.; Hagemeier, L.; Madea, B. Postmortem diagnostics of assumed food anaphylaxis in an unexpected death. Forensic Sci. Int. 2010, 198, e1–e4.
- Osawa, M.; Satoh, F.; Horiuchi, H.; Tian, W.; Kugota, N.; Hasegawa, I. Postmortem diagnosis of fatal anaphylaxis during intravenous administration of therapeutic and diagnostic agents: Evaluation of clinical laboratory parameters and immunohistochemistry in three cases. Leg. Med. 2008, 10, 143–147.
- Del Duca, F.; Manetti, A.C.; Maiese, A.; Napoletano, G.; Ghamlouch, A.; Pascale, N.; Giorgio, B.; Paola, F.; Russa, R. Death Due to Anaphylactic Reaction: The Role of the Forensic Pathologist in an Accurate Postmortem Diagnosis. Medicina 2023, 59, 2184.
