1. Introduction
Our earth’s climate is changing, primarily due to anthropogenic global warming due to the burning of fossil fuels. It will continue to change at that pace or even more quickly until we take corrective measures
[1]. Fortunately, we have started acknowledging the negative impacts of climate change on our environment
[2]. As such, the major world powers and the scientific community have focused on renewable energy to curb fossil fuels, a primary culprit in climate change. Since renewable energies are intermittent energy sources, they require energy storage devices to maintain a steady supply. Recently, electric vehicles (EVs) have become increasingly popular, as they are not an active source of carbon emissions and depend on an electric grid or solar array at an invariable or reduced cost. As a result, there has been a great interest in developing efficient electrochemical energy storage (EES) devices.
Among EES technologies, rechargeable batteries (RBs) and supercapacitors (SCs) are the two most desired candidates for powering a range of electrical and electronic devices
[3][4][5][6][7][8][9][10][3,4,5,6,7,8,9,10]. RBs operate on Faradaic processes, whereas the underlying mechanism of SCs varies, as non-Faradaic in electrical double-layer capacitors (EDLCs), Faradaic at the surface of the electrodes in pseudo-capacitors (PCs), and a combination of both non-Faradaic and Faradaic in hybrid SCs (HSCs)
[3][11][3,11]. EDLCs offer high power density but low energy density. HSCs take advantage of the Faradaic process without compromising their capacitive nature. Unlike batteries, supercapacitors provide high power density and numerous charge–discharge cycles; however, they lag batteries in energy density. To take advantage of the merits of both RBs and SCs, researchers have focused on merging the two technologies into a single device known as a “supercapattery” (= supercapacitor + battery)
[12][13][14][15][16][12,13,14,15,16], a generic term used to identify a unique category of energy storage devices that offer high energy density like an RB without compromising the ability to deliver the high power density and large cyclability of EDLCs. Though supercapatteries are relatively new compared to RBs and SCs, supercapatteries have received significant attention, as evidenced by the exponential growth in related publications over the past ten years.
2. Electrochemical Energy Storage (EES) Devices
Batteries and capacitors are two types of energy storage devices relevant to EES devices
[17][29]. Essentially, batteries are non-rechargeable (primary cell) and rechargeable (RBs, secondary cell). On the other hand, capacitors are of three types—non-electrolytic, electrolytic, and electrochemical or supercapacitors (SCs). EES devices comprise RBs, SCs, and their derivatives. EES devices are different in that these devices store energy using different storage mechanisms—non-Faradaic (surface-controlled kinetics) and Faradaic (diffusion-controlled kinetics)—that depend on the materials (electrode and electrolyte) and how those materials are used in the device
[8][18][8,21].
In a non-Faradaic process, charge accumulation occurs electrostatically with opposite charges residing on two interfaces separated by a vacuum (non-electrolytic) or a molecular dielectric (electrolytic). In contrast, a redox reaction achieves the same in a Faradaic process, causing chemical or oxidation state changes in the electroactive materials
[3][17][3,29]. The Faradaic process can be capacitive (pseudocapacitive), as in PCs, or non-capacitive, as in batteries
[19]. Before diving into supercapatteries, understanding three main EES—EDLCs, PCs, and RBs—is essential. The charge storage mechanisms in those devices can be well understood by their electrochemical signature in cyclic voltammetry (CV) and galvanostatic charge/discharge (GCD) profiles. Mathis et al. outlined a set of guidelines for interpreting the performance of EES systems
[18][21]. An EDLC material will show a linear voltage versus time response (a triangular-shaped GCD profile) during constant current charging/discharging and a rectangular CV profile or cyclic voltammogram. In this case, the amount of charge stored depends linearly on potential, and the capacitance of the material can be easily calculated and reported for the EDLC. On the other hand, an RB material will show plateaus in the GCD profile and separated oxidative and reductive peaks in the CV profile. Unlike the case of charge storage at EDLC electrodes, charge storage by RB-type electrodes follows a nonlinear relationship with the applied potential. In the case of pseudocapacitive materials, the GCD profile is symmetric but non-linear, and the CV response does not separate the oxidative and reductive peaks.
2.1. Supercapacitors (SCs)
Depending on the storage mechanism, SCs can be classified mainly into three categories: EDLCs, PCs, and a combination of the two (HSCs
[20][21][22][23][24][18,30,31,32,33] or asymmetric SCs (ASCs)
[19][25][26][19,20,34], where HSCs are a subset of ASCs). Another way of differentiating ASCs from HSCs is that ASCs are configured by combining the electrode materials of EDLCs and PCs. In contrast, HSCs combine the electrode materials of EDLCs and RBs
[27][35].
2.2. Electrical Double-Layer Capacitors (EDLCs)
Like conventional dielectric capacitors, EDLCs store energy electrostatically, forming an electrical double layer (EDL) at the electrode/electrolyte interface, where the applied voltage polarizes the electrolyte, which acts as a dielectric
[20][28][29][30][31][17,18,36,37,38]. The process is purely non-Faradaic and physical in nature. Thus, Equation (1) applies to EDLCs, and the charging–discharging mechanism of EDLCs is very fast and reversible. Additionally, EDLCs primarily utilize carbonaceous materials like activated carbon (AC), graphene, carbon nanotubes (CNTs), carbon aerogel (CA), carbide-derived carbon (CDC), carbon fibers, etc.
[32][39] The capacitance of EDLCs mostly depends on the pore size of the electrode materials. Because of their porous electrodes with large surface areas that allow the formation of compact double layers with atomic range separation between electronic and ionic charges at the electrode surface, EDLCs show greater capacitance than conventional dielectric capacitors
[29][32][36,39].
2.3. Pseudocapacitors (PCs)
2.3. Pseudocapacitors (PCs)
In PCs, energy is stored via a sequence of fast reversible processes, which are Faradaic in nature, at the surface or near-surface of the electrode materials
[8][33][34][35][8,40,41,42]. Conway identified three Faradaic mechanisms—underpotential deposition, electrosorption, and intercalation, that cause pseudocapacitance
[33][36][40,43]. In underpotential deposition, metal ions form an adsorbed monolayer at the surface of a different metal well above their redox potential. An example of such an underpotential deposition is the deposition of lead (Pb) on the surface of a gold (Au) electrode
[37][44]. Redox pseudocapacitance occurs when ions are electrochemically adsorbed onto the surface or near the surface of a material following a Faradaic charge-transfer process. Intercalation pseudocapacitance occurs when ions intercalate into the layers of a redox-active material in a Faradaic charge-transfer process without changing the crystallographic phase
[33][40].
The above three Faradaic processes occur due to different physical processes involving various types of materials that result in similar electrochemical signatures owing to the relationship between potential and the extent of charge developed from adsorption/desorption processes at the electrode/electrolyte interface
[33][40]:
where
E is the potential,
R is the ideal gas constant,
T is the temperature,
n is the number of electrons,
F is the Faraday constant, and
X is the extent of fractional coverage of the surface or inner structure. From Equation (2
5), capacitance (
C) may be defined in regions where the plot of
E vs.
X is linear:
where
m is the molecular weight of the active material. The capacitance,
C, is not always constant, since the plot of
E vs.
X is not entirely linear as in a capacitor, and so is termed pseudocapacitance
[33][40].
There are detailed reviews on PCs and related pseudocapacitive materials
[32][34][35][38][39,41,42,45]. Electrode materials that are used in PCs include transition-metal oxides (TMOs) such as IrO
2, RuO
2, Fe
3O
4, MnO
2, NiO, V
2O
5, Co
3O
4, etc.; transition-metal sulfides (TMSs) such as MoS
2, WS
2, and FeS
2; and conducting polymers (CPs) such as polyaniline (PANI), polythiophene, polypyrrole (PPy), polyvinyl alcohol (PVA), poly (3,4-ethylene dioxythiophene) (PEDOT), polyacetylene, poly (4-styrene sulfonate) (PSS), poly-phenylene-vinylene (PPV), etc.
[32][39][39,46]. Recently, many nanomaterials have been introduced to RBs that have shown fast redox kinetics comparable to pseudocapacitive materials due to the very short ionic diffusion length and high surface area of the nanosized materials. As a result, pseudocapacitive and battery materials are becoming increasingly indistinguishable
[35][42]. According to Brousse et al., some materials are described as “pseudocapacitive” even though their electrochemical signature is analogous to that of a “battery material,” as commonly observed for Ni(OH)
2 in KOH electrolytes. In contrast, true pseudocapacitive electrode materials such as MnO
2 display electrochemical behavior typical of that observed for a capacitive carbon electrode
[40][47]. Faradaic electrodes exhibit electrochemical behavior distinct from that of pseudocapacitive electrodes. Therefore, it is proposed that the term “pseudocapacitive” must be only used to describe electrode materials (e.g., MnO
2) that display electrochemical behavior typical of that observed for a capacitive carbon electrode in a mild aqueous electrolyte to avoid any confusion between battery materials and pseudocapacitive materials.
2.4. Hybrid Supercapacitors (HSCs)
Hybrid SCs (HSCs) take advantage of the best of EDLCs and the best of PCs or RBs in a single device with different combinations of electrode materials and storage mechanisms (non-Faradaic and Faradaic)
[19][25][41][19,20,48]. Hybridizing different electrode materials into a single electrode or fabricating a hybrid cell configuration consisting of Faradaic and non-Faradaic electrodes has become an obvious strategy for developing high-energy and high-power HSCs
[8]. Thus, asymmetry in HSCs may arise from electrode materials and storage mechanisms. These devices take advantage of the fast kinetics of EDLC materials and the improved energy storage performance of pseudocapacitive or battery electrode materials
[35][42].
2.5. Rechargeable Batteries (RBs)
Like most electrochemical devices, RBs are composed of two electrodes—a cathode and an anode—separated by an electrolyte
[4][42][43][44][4,49,50,51]. In RBs, electrical energy is converted and stored electrochemically within the bulk of the electrodes through reversible chemical reactions at the electrode/electrolyte interface during the charging and discharging processes. There are various kinds of RBs; among them, lithium (Li)-ion batteries (LiBs), a type of metal-ion battery, are the most commercially successful RB technology
[4][42][4,49].
The emergence of LiBs, a Nobel-Prize-winning technology and one of the most popular EES technologies in the nineties, has revolutionized consumer electronics and EVs
[4][7][45][46][4,7,52,53]. Typically, LiBs comprise five key components—the anode, cathode, electrolyte, separator, and current collector
[4]. Generally, copper (Cu) and aluminum (Al) foils are used as current collectors at the anode and the cathode. The negative electrode (anode) is made of carbonaceous materials (e.g., graphite), whereas Li-based metal oxides (e.g., LiCoO
2) are used in the positive electrode (cathode). Other materials used in the anode are germanium-based materials, transition metal chalcogenides, silicon, and metallic oxides
[47][54]. The two electrodes are separated by a separator, typically a porous polyolefin film soaked in a non-aqueous solution of a lithium salt (e.g., LiPF
6 in ethylene carbonate, ethyl methyl carbonate, or diethyl carbonate)
[4][42][4,49]. In LiBs, the reversible chemical reaction occurs in two ways: displacement and insertion into the electrodes
[4]. During the charging cycle, the positive electrolyte ions (Li
+) are deintercalated (displaced) from the cathode and intercalated (inserted) into the anode. The reverse process occurs during the discharging cycle, where the positive ions transport from the anode to the cathode and electrons travel from the anode to the cathode via an external load and thereby complete the circuit. This displacement/insertion process involves reversible Faradaic processes that can be identified as well-separated oxidative and reductive peaks in the CV profile and asymmetric curves in the GCD profile. Emerging RBs based on abundant alkali, alkaline earth, and transition metals—sodium (Na), potassium (K), magnesium (Mg), calcium (Ca), zinc (Zn), and aluminum (Al)—are promising alternatives to LiBs
[6][44][6,51].
2.6. Supercapatteries
Supercapatteries are hybrid EES devices that combine the advantages of SCs and RBs, such as high energy density, high power density, and a long cycle life. This EES hybrid design involves a combination of an SC electrode with an RB electrode, such as in the so-called Li-ion capacitor
[48][49][50][55,56,57], Na-ion capacitors
[49][51][24,56], and other hybrid EES devices
[15]. Supercapatteries can exhibit capacitive performances like conventional capacitors, including CV and linear GCD profiles. Thus, the fundamentals of conventional capacitors can also be applied to supercapatteries, in which capacitance (
𝐶) is the ratio of the change in stored charge (
Δ𝑄) to the variation in applied voltage (
Δ𝑉) as the voltage of a capacitor is swept at a constant voltage scan rate (
𝜈=𝑑𝑉/𝑑𝑡) in CV. Because the current (
𝑖) flowing through a capacitor is proportional to
𝜈, this proportionality is also equal to
C, as described in Equation (
427)
[15]:
2.7. Electrode and Electrolyte Materials in Supercapatteries
Different supercapatteries can be fabricated utilizing electrodes with capacitive, pseudocapacitive, or battery-type materials. Balasubramaniam et al. provided a comprehensive account of mechanisms, materials selection, and performance evaluation for supercapatteries
[52][63]. Electrode materials with high surface area, electrical conductivity, porous structure, and short ion/electron diffusion lengths have essential characteristics for the performance improvement of supercapatteries. To comprehensively discuss these hybrid devices, Liu and Chen constructed several hypothetical supercapatteries to illustrate their performance using corresponding GCD plots, one by one, and these hypothetical devices were confirmed using relevant experimental data from the literature
[15]. At the negatrode, mostly activated carbon of different sources was used in asymmetric battery/EDLC and pseudocapacitive/EDLC type supercapatteries. On the other hand, different metal iodides (BiOI-Bi
9I
2)
[53][64], metal oxides (β-NiMoO
4 [54][65], phosphate ion-functionalized NiO (P-NiO)
[55][66], Zn
0.5CoO
0.5Mn(PO
4)
2 [56][67], Co
3(PO
4)
2 [57][68], Co
0.5Ni
0.5WO
4 [58][69], etc.), metal hydroxides (Co–Ni LDH
[59][70]), metal sulfides (FeCoCuS
2 [60][71], Co
0.125Cu
0.375Mn
0.500S
[61][72], etc.), composites (Co-MOF-PANI
[62][73], Sr
3P
2-PANI
[63][74], MWCNT-NiMnPO
4 [64][75], graphitic carbon nitride (g-C
3N
4)-BiVO
4 [65][76], Zn-Carbon cloths,
[66][77], etc.), etc. were used for the positrode.
In the electrolyte, mostly, aqueous KOH at different concentrations has been used in supercapatteries
[53][67][68][69][70][64,78,79,80,81]. Other electrolytes used include LiPF
6 [66][77], K
2SO
4 [71][82], H
2SO
4 [72][83], KBr
[71][82], NaClO
4 [66][77], PVA-KOH
[57][68], KI/VOSO
4 [72][83], etc. Electrolytes with additional redox species have been investigated in supercapatteries with significantly enhanced energy capacity
[22][73][74][31,58,84]. Also, EDLC materials with redox electrolytes showed enhanced performance
[75][85].
2.8. Performance and Experimental Evaluation of Supercapatteries
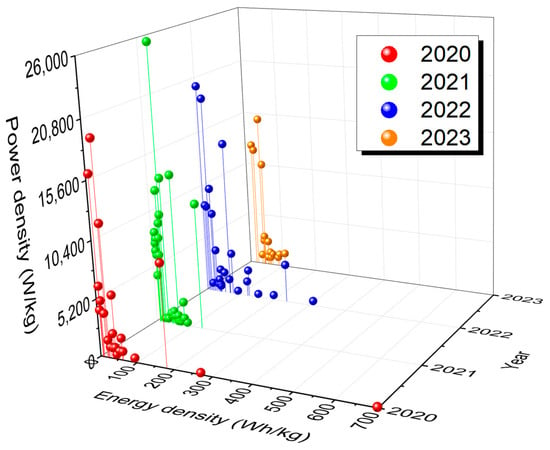
Figure 1 shows Ragone plots of supercapatteries reported in published articles during the last four years, from 2020 to 2023. Primarily, the reported supercapatteries performed either like an EDLC or an RB. However, Devi et al. reported a Na-ion supercapattery with outstanding specific energy of 236 W h kg
−1 at a higher specific power of 3630 W kg
−1 with appreciable retention of over 95% even at the 10,000th cycle
[66][77]. It is important to note that capacitance can be used only when there is a linear relationship between charge and voltage, and the capacitance value should be a single constant value in the chosen potential window; any deviation from this behavior requires that integration be used to calculate the charge being stored or delivered. Also, capacity instead of capacitance should be measured for RBs. As Chen has pointed out, many authors have ignored the critical differences between SCs and RBs as they have applied the concept of pseudocapacitance to some new battery-type materials
[20][18]. As a result, deceptively high specific capacitance values have been claimed, and high specific capacitance has been also used in calculating specific energy.
Figure 1. Ragone plots for different supercapatteries reported over the last four years (2020–2023) showing relative energy and power density.
2.9. Classification of EES Devices
From the above discussion, it is evident that RBs and SCs belong to EES, where EDLCs and PCs belong to SCs. However, placing HSCs and supercapatteries in the classification tree is inconsistent throughout the literature
[8][12][20][21][22][23][25][8,12,18,20,30,31,32]. Even ASCs have been placed parallel to HSCs or in place of HSCs, making HSCs a subclass of ASCs
[25][20]. Initially, HSCs appeared to be defined as hybrids of EDLCs and PCs, whereas supercapatteries were considered hybrids of EDLCs and RBs. Guan et al. proposed defining and differentiating storage mechanisms in EES devices without explicitly differentiating ASCs and HSCs
[19]. Considering all possible combinations of electrode materials and storage mechanisms, EES devices were classified vividly in tabulated form
[20][18], where the combination results in nine different devices depending on electrode materials and storage mechanisms.