Monitoring heart electrical activity is an effective way of detecting existing and developing conditions. This is usually performed as a non-invasive test using a network of up to 12 sensors (electrodes) on the chest and limbs to create an electrocardiogram (ECG). By visually observing these readings, experienced professionals can make accurate diagnoses and, if needed, request further testing. However, the training and experience needed to make accurate diagnoses are significant.
- electrocardiogram
- diagnosis
- particle swarm optimization
- optimization
- recurrent neural networks
1. Introduction
2. AI Approaches in Electrocardiogram Analysis
3. Recurrent Neural Networks
is combined with the current at the time step t. The process is described by Equation (1).
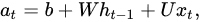
where 𝑎𝑡 is the input activation, b the bias term, and W and U represent the weight matrices of recurrent and input connections, respectively.
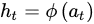
Based on the prediction goal, different functions can be used as 𝜙. The output of the network is derived from the hidden state. The previously described process is mathematically formulated by Equation (3).
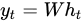
4. Metaheuristics
References
- Mc Namara, K.; Alzubaidi, H.; Jackson, J.K. Cardiovascular disease as a leading cause of death: How are pharmacists getting involved? Integr. Pharm. Res. Pract. 2019, 8, 1–11.
- Ezzati, M.; Obermeyer, Z.; Tzoulaki, I.; Mayosi, B.M.; Elliott, P.; Leon, D.A. Contributions of risk factors and medical care to cardiovascular mortality trends. Nat. Rev. Cardiol. 2015, 12, 508–530.
- Keeney, R.L. Personal decisions are the leading cause of death. Oper. Res. 2008, 56, 1335–1347.
- Berkaya, S.K.; Uysal, A.K.; Gunal, E.S.; Ergin, S.; Gunal, S.; Gulmezoglu, M.B. A survey on ECG analysis. Biomed. Signal Process. Control 2018, 43, 216–235.
- Zhang, Q.; Frick, K. All-ECG: A least-number of leads ECG monitor for standard 12-lead ECG Tracking during Motion. In Proceedings of the 2019 IEEE Healthcare Innovations and Point of Care Technologies, (HI-POCT), Bethesda, MD, USA, 20–22 November 2019; pp. 103–106.
- Antczak, K. Deep recurrent neural networks for ECG signal denoising. arXiv 2018, arXiv:1807.11551.
- Hassaballah, M.; Wazery, Y.M.; Ibrahim, I.E.; Farag, A. Ecg heartbeat classification using machine learning and metaheuristic optimization for smart healthcare systems. Bioengineering 2023, 10, 429.
- Bohr, A.; Memarzadeh, K. The rise of artificial intelligence in healthcare applications. In Artificial Intelligence in Healthcare; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–60.
- Wolpert, D.H.; Macready, W.G. No free lunch theorems for optimization. IEEE Trans. Evol. Comput. 1997, 1, 67–82.
- Velichko, A.; Huyut, M.T.; Belyaev, M.; Izotov, Y.; Korzun, D. Machine learning sensors for diagnosis of COVID-19 disease using routine blood values for internet of things application. Sensors 2022, 22, 7886.
- Akgönüllü, S.; Özgür, E.; Denizli, A. Quartz crystal microbalance-based aptasensors for medical diagnosis. Micromachines 2022, 13, 1441.
- Sadhu, P.K.; Yanambaka, V.P.; Abdelgawad, A.; Yelamarthi, K. Prospect of internet of medical things: A review on security requirements and solutions. Sensors 2022, 22, 5517.
- Al-Kahtani, M.S.; Khan, F.; Taekeun, W. Application of internet of things and sensors in healthcare. Sensors 2022, 22, 5738.
- Phan, D.T.; Nguyen, C.H.; Nguyen, T.D.P.; Tran, L.H.; Park, S.; Choi, J.; Lee, B.i.; Oh, J. A flexible, wearable, and wireless biosensor patch with internet of medical things applications. Biosensors 2022, 12, 139.
- Islam, M.M.; Islam, M.Z.; Asraf, A.; Al-Rakhami, M.S.; Ding, W.; Sodhro, A.H. Diagnosis of COVID-19 from X-rays using combined CNN-RNN architecture with transfer learning. BenchCouncil Trans. Benchmarks Stand. Eval. 2022, 2, 100088.
- Kamble, D.D.; Kale, P.H.; Nitture, S.P.; Waghmare, K.V.; Aher, C.N. Heart disease detection through deep learning model RNN. In Proceedings of the Smart Intelligent Computing and Applications, Volume 2: Proceedings of Fifth International Conference on Smart Computing and Informatics (SCI 2021); Springer: Berlin/Heidelberg, Germany, 2022; pp. 469–480.
- Djemili, R.; Djemili, I. Nonlinear and chaos features over EMD/VMD decomposition methods for ictal EEG signals detection. Comput. Methods Biomech. Biomed. Eng. 2023, 1–20.
- Pandey, P.; Seeja, K. Subject independent emotion recognition from EEG using VMD and deep learning. J. King Saud Univ.-Comput. Inf. Sci. 2022, 34, 1730–1738.
- Saini, S.K.; Gupta, R. Artificial intelligence methods for analysis of electrocardiogram signals for cardiac abnormalities: State-of-the-art and future challenges. Artif. Intell. Rev. 2022, 55, 1519–1565.
- Mincholé, A.; Camps, J.; Lyon, A.; Rodríguez, B. Machine learning in the electrocardiogram. J. Electrocardiol. 2019, 57, S61–S64.
- Fiorina, L.; Maupain, C.; Gardella, C.; Manenti, V.; Salerno, F.; Socie, P.; Li, J.; Henry, C.; Plesse, A.; Narayanan, K.; et al. Evaluation of an ambulatory ECG analysis platform using deep neural networks in routine clinical practice. J. Am. Heart Assoc. 2022, 11, e026196.
- Jin, Y.; Li, Z.; Qin, C.; Liu, J.; Liu, Y.; Zhao, L.; Liu, C. A novel attentional deep neural network-based assessment method for ECG quality. Biomed. Signal Process. Control 2023, 79, 104064.
- Li, W.; Tang, Y.M.; Yu, K.M.; To, S. SLC-GAN: An automated myocardial infarction detection model based on generative adversarial networks and convolutional neural networks with single-lead electrocardiogram synthesis. Inf. Sci. 2022, 589, 738–750.
- Boda, S.; Mahadevappa, M.; Dutta, P.K. An automated patient-specific ECG beat classification using LSTM-based recurrent neural networks. Biomed. Signal Process. Control 2023, 84, 104756.
- Lee, J.A.; Kwak, K.C. Personal identification using an ensemble approach of 1D-LSTM and 2D-CNN with electrocardiogram signals. Appl. Sci. 2022, 12, 2692.
- Rai, H.M.; Chatterjee, K. Hybrid CNN-LSTM deep learning model and ensemble technique for automatic detection of myocardial infarction using big ECG data. Appl. Intell. 2022, 52, 5366–5384.
- Wasimuddin, M.; Elleithy, K.; Abuzneid, A.S.; Faezipour, M.; Abuzaghleh, O. Stages-based ECG signal analysis from traditional signal processing to machine learning approaches: A survey. IEEE Access 2020, 8, 177782–177803.
- Celin, S.; Vasanth, K. ECG signal classification using various machine learning techniques. J. Med. Syst. 2018, 42, 241.
- Maturo, F.; Verde, R. Pooling random forest and functional data analysis for biomedical signals supervised classification: Theory and application to electrocardiogram data. Stat. Med. 2022, 41, 2247–2275.
- Myrovali, E.; Hristu-Varsakelis, D.; Tachmatzidis, D.; Antoniadis, A.; Vassilikos, V. Identifying patients with paroxysmal atrial fibrillation from sinus rhythm ECG using random forests. Expert Syst. Appl. 2023, 213, 118948.
- Ma, C.; Wang, Z.; Yang, M.; Li, J.; Liu, C. Decision Tree-based Model for Signal Quality Scanning in Wearable ECG. In Proceedings of the 2022 Computing in Cardiology (CinC), Tampere, Finland, 4–7 September 2022; Volume 498, pp. 1–4.
- Botros, J.; Mourad-Chehade, F.; Laplanche, D. CNN and SVM-Based Models for the Detection of Heart Failure Using Electrocardiogram Signals. Sensors 2022, 22, 9190.
- Mattheakis, M.; Protopapas, P. Recurrent neural networks: Exploding vanishing gradients & reservoir computing. In Advanced Topics in Data Science; Harvard Press: Cambridge, MA, USA, 2019.
- Beni, G. Swarm intelligence. In Complex Social and Behavioral Systems: Game Theory and Agent-Based Models; Springer: New York, NY, USA, 2020; pp. 791–818.
- Eberhart, R.; Kennedy, J. Particle swarm optimization. In Proceedings of the IEEE International Conference on Neural Networks, Perth, WA, Australia, 27 November–1 December 1995; Volume 4, pp. 1942–1948.
- Mirjalili, S.; Mirjalili, S. Genetic algorithm. In Evolutionary Algorithms and Neural Networks: Theory and Applications; Springer: Cham, Switzerland, 2019; pp. 43–55.
- Mirjalili, S. SCA: A sine cosine algorithm for solving optimization problems. Knowl.-Based Syst. 2016, 96, 120–133.
- Yang, X.S.; Slowik, A. Firefly algorithm. In Swarm Intelligence Algorithms; CRC Press: Boca Raton, FL, USA, 2020; pp. 163–174.
- Faris, H.; Aljarah, I.; Al-Betar, M.A.; Mirjalili, S. Grey wolf optimizer: A review of recent variants and applications. Neural Comput. Appl. 2018, 30, 413–435.
- Abualigah, L.; Abd Elaziz, M.; Sumari, P.; Geem, Z.W.; Gandomi, A.H. Reptile Search Algorithm (RSA): A nature-inspired meta-heuristic optimizer. Expert Syst. Appl. 2022, 191, 116158.
- Gurrola-Ramos, J.; Hernàndez-Aguirre, A.; Dalmau-Cedeño, O. COLSHADE for real-world single-objective constrained optimization problems. In Proceedings of the 2020 IEEE Congress on Evolutionary Computation (CEC), Glasgow, UK, 19–24 July 2020; pp. 1–8.
- Bezdan, T.; Zivkovic, M.; Tuba, E.; Strumberger, I.; Bacanin, N.; Tuba, M. Glioma brain tumor grade classification from mri using convolutional neural networks designed by modified fa. In Proceedings of the International Conference on Intelligent and Fuzzy Systems, Istanbul, Turkey, 21–23 July 2020; Springer: Cham, Switzerland, 2020; pp. 955–963.
- Jovanovic, D.; Antonijevic, M.; Stankovic, M.; Zivkovic, M.; Tanaskovic, M.; Bacanin, N. Tuning machine learning models using a group search firefly algorithm for credit card fraud detection. Mathematics 2022, 10, 2272.
- Petrovic, A.; Bacanin, N.; Zivkovic, M.; Marjanovic, M.; Antonijevic, M.; Strumberger, I. The adaboost approach tuned by firefly metaheuristics for fraud detection. In Proceedings of the 2022 IEEE World Conference on Applied Intelligence and Computing (AIC), Sonbhadra, India, 17–19 June 2022; pp. 834–839.
- Strumberger, I.; Tuba, E.; Zivkovic, M.; Bacanin, N.; Beko, M.; Tuba, M. Dynamic search tree growth algorithm for global optimization. In Technological Innovation for Industry and Service Systems: Proceedings of the 10th IFIP WG 5.5/SOCOLNET Advanced Doctoral Conference on Computing, Electrical and Industrial Systems, DoCEIS 2019, Costa de Caparica, Portugal, May 8–10, 2019, Proceedings 10; Springer: Cham, Switzerland, 2019; pp. 143–153.
- Zamani, H.; Nadimi-Shahraki, M.H.; Gandomi, A.H. Starling murmuration optimizer: A novel bio-inspired algorithm for global and engineering optimization. Comput. Methods Appl. Mech. Eng. 2022, 392, 114616.
- Nadimi-Shahraki, M.H.; Zamani, H. DMDE: Diversity-maintained multi-trial vector differential evolution algorithm for non-decomposition large-scale global optimization. Expert Syst. Appl. 2022, 198, 116895.
- Bacanin, N.; Bezdan, T.; Tuba, E.; Strumberger, I.; Tuba, M.; Zivkovic, M. Task scheduling in cloud computing environment by grey wolf optimizer. In Proceedings of the 2019 27th Telecommunications Forum (TELFOR), Belgrade, Serbia, 26–27 November 2019; pp. 1–4.
- Zivkovic, M.; Venkatachalam, K.; Bacanin, N.; Djordjevic, A.; Antonijevic, M.; Strumberger, I.; Rashid, T.A. Hybrid genetic algorithm and machine learning method for COVID-19 cases prediction. In Proceedings of the International Conference on Sustainable Expert Systems: ICSES 2020; Springer: Singapore, 2021; pp. 169–184.
- Bezdan, T.; Cvetnic, D.; Gajic, L.; Zivkovic, M.; Strumberger, I.; Bacanin, N. Feature selection by firefly algorithm with improved initialization strategy. In Proceedings of the 7th Conference on the Engineering of Computer Based Systems, Novi Sad, Serbia, 26–27 May 2021; pp. 1–8.
- Zivkovic, M.; Bacanin, N.; Tuba, E.; Strumberger, I.; Bezdan, T.; Tuba, M. Wireless sensor networks life time optimization based on the improved firefly algorithm. In Proceedings of the 2020 International Wireless Communications and Mobile Computing (IWCMC), Limassol, Cyprus, 15–19 June 2020; pp. 1176–1181.
- Zivkovic, M.; Bacanin, N.; Zivkovic, T.; Strumberger, I.; Tuba, E.; Tuba, M. Enhanced grey wolf algorithm for energy efficient wireless sensor networks. In Proceedings of the 2020 Zooming Innovation in Consumer Technologies Conference (ZINC), Novi Sad, Serbia, 26–27 May 2020; pp. 87–92.
- Ahmadpour, M.R.; Ghadiri, H.; Hajian, S.R. Model predictive control optimisation using the metaheuristic optimisation for blood pressure control. IET Syst. Biol. 2021, 15, 41–52.
- Khan, M.A.; Algarni, F. A healthcare monitoring system for the diagnosis of heart disease in the IoMT cloud environment using MSSO-ANFIS. IEEE Access 2020, 8, 122259–122269.
- Gupta, A.; Chhikara, R. Diabetic retinopathy: Present and past. Procedia Comput. Sci. 2018, 132, 1432–1440.
- Mahbod, A.; Schaefer, G.; Wang, C.; Ecker, R.; Ellinge, I. Skin lesion classification using hybrid deep neural networks. In Proceedings of the ICASSP 2019-2019 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Brighton, UK, 12–17 May 2019; pp. 1229–1233.
- Ren, Z.; Zhang, Y.; Wang, S. A hybrid framework for lung cancer classification. Electronics 2022, 11, 1614.
- Zhang, Y.; Zhao, Z.; Deng, Y.; Zhang, X.; Zhang, Y. ECGID: A human identification method based on adaptive particle swarm optimization and the bidirectional LSTM model. Front. Inf. Technol. Electron. Eng. 2021, 22, 1641–1654.
- Baños, F.S.; Romero, N.H.; Mora, J.C.S.T.; Marín, J.M.; Vite, I.B.; Fuentes, G.E.A. A Novel Hybrid Model Based on Convolutional Neural Network with Particle Swarm Optimization Algorithm for Classification of Cardiac Arrhytmias. IEEE Access 2023, 11, 55515–55532.
- Karthiga, M.; Santhi, V.; Sountharrajan, S. Hybrid optimized convolutional neural network for efficient classification of ECG signals in healthcare monitoring. Biomed. Signal Process. Control 2022, 76, 103731.
- Predić, B.; Jovanovic, L.; Simic, V.; Bacanin, N.; Zivkovic, M.; Spalevic, P.; Budimirovic, N.; Dobrojevic, M. Cloud-load forecasting via decomposition-aided attention recurrent neural network tuned by modified particle swarm optimization. Complex Intell. Syst. 2023, 1–21.
