1. Predisposing Factors for C. auris Infection, Clinical Spectrum and Outcomes
The predisposing risk factors for
C. auris infection are similar to other
Candida species, since they are opportunistic pathogens that primarily affect critically ill and immunocompromised patients
[1][63].
Clinical manifestations of
C. auris are diverse and range from colonization and mild, superficial skin infections to invasive disease and deep-seated infections
[2][3][4,5]. Common sites of colonization include the skin, mostly the groin and axilla areas, rectum and mucosal surfaces of the urinary and respiratory tract (e.g., nares, and oropharynx)
[4][5][6][7][8][9][10][11][12][9,15,19,30,41,70,75,76,77]. It is suggested that
C. auris is incapable of colonizing anaerobic environments
[3][5], like the gut, and the salivary antimicrobial peptide histatin 5 exerts a potent candidacidal effect on
C. auris [13][71]. Therefore, unlike
C. albicans, the colonization of the gastrointestinal tract is rare. Infection can occur at multiple body sites and
C. auris has been isolated from both sterile (e.g., blood, cerebrospinal fluid, and bile) and non-sterile samples (e.g., urine, sputum, tissue, wound swabs, and catheter tips)
[2][3][11][14][4,5,36,76]. Progression from colonization to invasive infections is estimated to occur in up to one fourth of affected patients
[7][15][30,72], and candidemia is the predominant type of
C. auris infection, followed by urinary tract, wound and ear infections, and rarely by respiratory tract or intra-abdominal infections, skin abscesses, myocarditis, meningitis and osteomyelitis
[3][5]. It is noteworthy that
C. auris candidemia usually follows colonization and multisite colonization is an independent risk factor for the development of candidemia
[7][30]. Hence, the prompt identification of colonized patients at greater risk for developing candidemia may be beneficial for improving early diagnosis and preventing invasive infection through interventions on modifiable predictors. Lastly, the risk of infection of implantable devices (e.g., defibrillators, pacemakers, prosthetic joints, etc.) when the candidate is already colonized by
C. auris has not yet been addressed in the literature, but according to the authors’ opinion, it is not negligible.
Invasive infections caused by
C. auris are potentially life-threatening and increased mortality rates with significant geographic variation have been reported. In the literature, crude mortality ranges from 27% to 70%
[7][9][16][17][18][19][20][21][11,30,64,65,66,68,70,73], whereas attributable mortality has not been adequately explored. Notably, a recent meta-analysis of 4733
C. auris cases, recorded from 2009 to 2019 in 33 countries worldwide, estimated a crude mortality of 39% and suggested a lower mortality in the European compared to the Asian continent (20% vs. 44%)
[22][78]. Furthermore, as expected, BSIs incur a significant mortality toll, which can be as high as 70%
[9][16][17][21][11,64,70,73], yet a crude mortality of 45% was documented in the aforementioned meta-analysis
[22][78]. Additionally, crude 30-day mortality, reaching almost 60%, was revealed in case of recurrent candidemia in a study of 157 critically ill and
C. auris-colonized patients, of whom 27 patients developed candidemia and 7 had a late recurrent episode
[7][30]. This finding, however, should be interpreted with caution as it may reflect the severity of underlying noninfectious conditions in patients with prolonged ICU stay
[23][79]. In co-infected COVID-19 patients, the estimated mortality is 44.4% and candidemia engenders a mortality of 64.7%
[24][80]. Regarding the specific factors that are associated with unfavorable prognosis, advanced age, and presence of comorbidities,
C. auris infection and particularly candidemia, as well as prolonged hospitalization, were identified in the survival analysis of a study that analyzed outcomes of 108 patients either infected or colonized by
C. auris [19][66]. Similarly, in a retrospective analysis of 92
C. auris-affected patients, only candidemia was causally linked to greater mortality, while both infected and colonized cases shared comparable mortality
[9][70].
2. Infection Prevention and Control Strategies
Prompt and accurate microbiological identification, as well as robust implementation of evidence-based IPC strategies are crucial for controlling and preventing C. auris outbreaks in healthcare settings. It is worth mentioning that C. auris is transmissible whether a patient is colonized or infected; thus, IPC measures are the same for both patient groups.
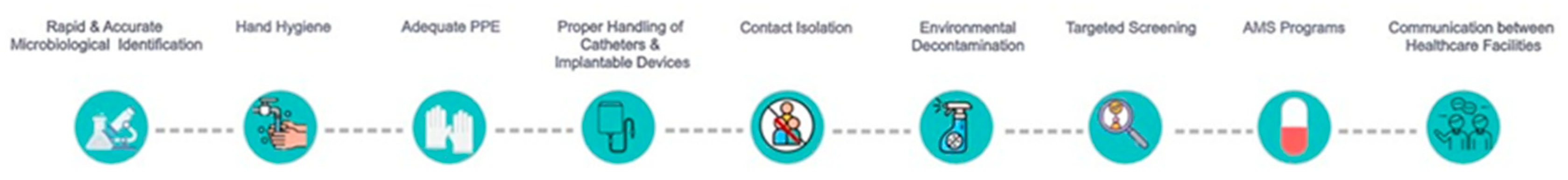
The IPC strategies which have, so far, been successfully implemented in diverse healthcare settings worldwide
[6][25][26][27][28][29][30][31][32][33][34][19,20,81,82,83,84,85,86,87,88,89] are illustrated in
Figure 1 and they are discussed in the following paragraphs.
Figure 1.
Optimal strategies for controlling and minimizing intra-hospital transmission of
C. auris.
AMS: antimicrobial stewardship; PPE: personal protective equipment.
2.1. Rapid and Accurate Identification
C. auris has overlapping phenotypic characteristics with other closely related species, such as
C. haemulonii,
C. sake and
R. glutinis, which compromise its rapid and accurate identification
[35][90]. Due to misidentification issues, it is essential that microbiology laboratories update their commercial identification software to enable them to easily and efficiently identify
C. auris cases
[36][67], while the need for full identification in patients at greater risk for
C. auris colonization or infection should be communicated.
A substantial progress has been made to improve
C. auris identification methods. The first step included the development of a high-salt, high-temperature enrichment culture-based method that enables the accurate isolation of
C. auris [37]. Once an isolate is obtained, matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry can be successfully applied for yeast identification, provided that the reference database contains the necessary information
[38][39][6,91]. In case that MALDI-TOF is not available, sequence analysis of the internal transcribed spacer and D1/D2 region of the 28 s ribosomal deoxyribonucleic acid (DNA) can be performed
[38][40][6,10]. Nevertheless, DNA sequencing is a time-consuming, expensive and is not available in all diagnostic lab methods, and its applicability may be limited, at least in developing countries
[39][91]. For this reason, various sequencing-independent DNA-based methods, including end-point or multiplex polymerase chain reaction (PCR) assays, have been designed; they are highly sensitive and some of them were successfully validated for the direct detection of
C. auris in clinical and environmental samples
[39][91].
Lately, other culture-independent methods, such as PCR-restriction fragment length polymorphism (RFLP)
[41][92], Taqman quantitative PCR (qPCR)
[42][43][93,94], SYBR green qPCR
[44][95], GPS™ MONODOSE CanAur dtec-qPCR (Genetic PCR Solutions™, Elche, Alicante, Spain)
[45][96] and T2 Magnetic Resonance assay
[46][97], have emerged as an attractive alternative approach for rapid detection, mostly in surveillance samples, as they are accompanied by accurate and reproducible identification of
C. auris with a significantly reduced turnaround time compared to culture/MALDI-TOF-based methods.
2.2. Transmission
An alarming characteristic of
C. auris is its inter-patient transmissibility and the fact that even colonized patients can serve as a reservoir for nosocomial spread. Specifically, it is efficiently transmitted from patient to patient, either directly or indirectly by sharing the same room or contaminated items, and by the colonized hands of healthcare workers (HCWs)
[10][75]. Notably, contact with contaminated items is by far the most common method of colonization
[17][25][37][47][48][20,37,43,44,64], and close contact of cases (e.g., current or past room contacts within a prior month) has a documented colonization rate of 12–21%
[17][49][22,64]. The minimum contact period for the acquisition of
C. auris from an infected person or surface is estimated to be 4 h
[50][21], and invasive infections have occurred in patients within 48 h of ICU admission
[51][98].
Sources of contamination have been found within the patient’s room, including bedding materials (e.g., bed rails and pans, mattress, linen, and pillows), furniture, door handles, flooring, walls, radiators, window sills, faucets and sinks
[3][14][17][38][51][52][53][5,6,36,42,64,98,99]. It has also been isolated from high-touch surfaces and medical equipment, such as oxygen masks, axillary temperature probes, sphygmomanometer cuffs, pulse oxygen meters, electrocardiograph leads, catheter tips, infusion pumps and ventilators, particularly in outbreak settings
[14][17][25][38][40][48][49][50][51][53][54][55][6,10,17,20,21,22,36,44,64,98,99,100]. For instance, following the identification of a cluster of
C. auris infections in the neurosciences ICU, Eyre and colleagues concluded that patients exposed to reusable skin-surface axillary temperature probes had a sevenfold risk of infection or colonization
[48][44].
Transmission-Based Precautions
In a systematic review of 17 studies reporting multidrug-resistant (MDR) outbreaks in ICUs, mainly caused by
C. auris (n = 6), during the COVID-19 pandemic, the most commonly identified factors contributing to the outbreaks were inadequate PPE or a shortage of PPE, hand hygiene non-adherence, and high antibiotic use, followed by environmental contamination, prolonged critical illness and lack of trained HCWs
[56][101]. Therefore, all HCWs attending
C. auris-infected or -colonized patients should apply standard hand hygiene practices and perform adequate hand hygiene with soap and water, alcohol-based hand sanitizers, or chlorhexidine hand rubs
[25][35][38][40][49][57][58][59][6,10,20,22,90,102,103,104]. Sharing of medical supplies and equipment is prohibited and use of disposable PPE (e.g., gloves, aprons, and gowns) is recommended
[35][38][40][57][59][6,10,90,102,104]. Hospital infection control teams should raise awareness about
C. auris, ensure that enough quantities of hand hygiene materials are available and monitor HCW adherence with recommended hand hygiene practices and PPE use, as well as train the personnel and retrain them at regular intervals. Additionally, as a low HCW/patient ratio is a well-established risk factor for MDR-organism (MDRO) transmission
[60][105], a minimum number of HCWs should be designated for
C. auris cases.
Strict isolation of patients harboring
C. auris is recommended by the CDC and ECDC in order to prevent horizontal transfer to other patients
[38][40][6,10]. Ideally, they should be placed in single-occupancy rooms with designated medical equipment and attached toilet facilities and be restricted there, except for medically necessary procedures
[38][59][6,104]. Their rooms should be clearly marked and limited contact with visitors should be allowed. In case the number of single rooms is limited, they should be reserved for patients at the highest risk for transmission, such as those with uncontained secretions or diarrhea
[35][90].
C. auris patients can also be cohorted
[35][59][90,104], taking into account that these patients are usually co-infected with other MDROs. Strict isolation measures should not be an excuse for suboptimal patient care or result in the subject’s stigmatization
[61][106]. Safety indicators and tools should be developed to avoid rupture in the flow of care as well as isolated patients’ emotional stress.
2.3. Decontamination and Disinfection Procedures
Extensive contamination of the healthcare environment has been described in facilities with
C. auris outbreaks, highlighting the crucial role of enhanced daily and terminal disinfection in spread prevention
[38][40][6,10]. Nevertheless, there are currently no standardized cleaning or disinfection procedures. Prior to decontamination, visible organic materials (e.g., body fluids) from the patient care area should be removed and cleaned
[35][90], and the frequency of cleaning and disinfection is recommended to be at least twice daily, up to three times during outbreaks, and at least on all high-touch surfaces, such as bedrails and bedside tables
[38][6]. Moreover, in case of patient discharge or transfer, terminal cleaning and disinfection should be carried out with great diligence and environmental sampling for
C. auris culture should be performed in an outbreak setting
[35][38][40][59][6,10,90,104]. To date, only sodium hypochlorite of 100 ppm concentration and topical hydrogen peroxide-based disinfectants are widely recommended for use
[25][38][62][63][6,20,107,108], since commercially available products have been proven ineffective in eradicating
C. auris [64][109]. For this reason, the US Environmental Protection Agency (EPA) has registered a list of qualified products for use and released a standardized quantitative disk carrier method, with the acronym SOP-MB-35-00, for evaluating the efficacy of antimicrobials against
C. auris on hard, non-porous surfaces
[65][110].
It is worth mentioning that disinfectant selection should be made weighting toxicity. For instance, exceptionally toxic disinfectants, like high-strength sodium hypochlorite agents of 5000 ppm concentration, should be reserved for terminal cleaning and not used on a regular basis. In addition to routine cleaning with disinfectants, peracetic acid
[66][111], hydrogen peroxide < 1%
[67][112], vaporized hydrogen peroxide
[68][113], and ultraviolet subtype-C (UV-C) are other measures that can be used for optimal decontamination
[69][70][114,115]. For example, UV-C is sufficient to prevent biofilm formation
[70][115], and repeated flushing of colonized sinks in the patient’s room with ozonated water (2.5 ppm) (cycles of 30 s every 4 h) resulted in yeast elimination within 2 days
[71][116]. Recently, silver nanoparticles are recognized as promising antifungal agents, as they exhibited both inhibitory effects on the growth of
C. auris and antibiofilm formation activity
[72][117]. Finally, as already mentioned, dedicated and single-use items (e.g., pillows, and bedding material) and equipment (e.g., thermometers, and blood pressure cuffs) should be used and, for equipment that cannot be dedicated to patients harboring
C. auris, it is mandatory for it to be thoroughly disinfected after use
[35][59][90,104].
2.4. Decolonization Protocols
The efficacy of decolonization protocols is still under investigation and not supported by regulatory bodies. Schelenz and colleagues suggest oral nystatin use, bathing with single-use wipes of 2% chlorhexidine gluconate twice daily and mouth washing with chlorhexidine 0.2% or chlorhexidine 1% dental gel in oropharyngeal-colonized, skin-colonized and ventilated patients, respectively
[50][21]. However, if transient decolonization is achieved, the occurrence of recolonization is a potent scenario and high-touch areas may be the source of contamination where
C. auris persists for long periods of time
[25][50][20,21]. For this reason, patients with a history of colonization/infection by
C. auris in the past should be considered as potentially colonized for at least one year in case of readmission, until surveillance cultures prove negative.
2.5. Targeted Screening and Labelling of the Patients
Targeted screening serves as a useful tool to prevent hospital transmission by rapidly implementing IPC practices. Once a
C. auris-positive case has been identified, the infection control team should be immediately informed in order to trace the contact of origin and identify other potential patients who may have been exposed to the fungi. Moreover,
C. auris cases should be followed until discharge and flagged for at least one year after the first negative screening culture
[57][102], whereas HCWs and persons in close contact with them should be placed under strict contact precautions
[50][21].
The CDC recommends that screening should be considered for close healthcare contacts with newly identified
C. auris cases (colonized and/or infected) and patients reporting an overnight healthcare facility stay in a country outside the US in the previous year, especially if the country has documented
C. auris cases
[38][6]; pre-emptive screening of patients with international exposure is based on the finding that patients with a history of abroad hospitalization are at higher risk of MDRO carriage during ICU admission
[73][118], and approximately 1 in 2 are estimated to be positive
[74][119]. Notably, similar recommendations are supported by the ECDC
[40][10].