Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Theodoros Kelesidis.
Nuclear factor erythroid 2-related factor (NRF2) belongs to the cap “n” collar (CNC) family of transcription factors and is found in the cytoplasm of non-stressed cells in a combined form with KEAP1. Oxidative stress activates the transcription factor NRF2, which plays a key role in alleviating redox-induced cellular injury.
- NRF2 pathway
- respiratory viruses
- viral replication
- inflammation
1. Introduction
Respiratory viruses target the human respiratory system and cause various clinical symptoms in humans, ranging from mild upper respiratory infections to organ failure and life-threatening respiratory diseases [1,2][1][2]. The most common respiratory viruses are rhinoviruses, coronaviruses (CoVs), influenza virus, respiratory syncytial virus (RSV), parainfluenza viruses, enteroviruses, adenoviruses, and human metapneumovirus (hMPV) [3]. Each year, nearly 4 million deaths are attributed to lower respiratory tract infections, with Influenza contributing to approximately half a million of these fatalities [4]. Moreover, morbidity and mortality caused by respiratory viruses increased drastically with the emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the COVID-19 pandemic. Although several host factors have been found to play crucial role in the pathogenesis of respiratory viral infections, the interaction between respiratory viruses and the host cellular response remains poorly understood. Understanding antiviral host pathways and defining their role in the pathogenesis of respiratory viruses may set the foundation for novel antiviral therapies for viral respiratory diseases.
Viral respiratory infections are commonly associated with increased production of reactive oxygen and nitrogen species (ROS and RNS), leading to oxidative stress [5,6][5][6]. Subsequently, increased oxidative stress contributes to reduced host antiviral response, enhanced replication and virus-induced cell and tissue injury apoptosis, ferroptosis, inflammation, causing organ damage [6,7][6][7] and the occurrence of clinical symptoms [5,8,9][5][8][9]. During oxidative stress induced by respiratory viruses, the host deploys antioxidant mechanisms to control signaling pathways and reestablish cellular redox balance. Many respiratory viruses, including influenza [10,11,12,13,14,15,16[10][11][12][13][14][15][16][17],17], CoVs [18[18][19][20][21][22][23][24][25][26],19,20,21,22,23,24,25,26], RSV [27,28,29,30[27][28][29][30][31][32][33][34][35][36][37][38][39][40],31,32,33,34,35,36,37,38,39,40], rhinoviruses [41,42,43,44,45][41][42][43][44][45], enteroviruses such as Coxsackievirus B3 (CVB3) [46], EV71 [47[47][48][49][50],48,49,50], metapneumoviruses [34], and parainfluenza viruses [51[51][52],52], have been demonstrated to disrupt the cell redox homeostasis and induce the production of ROS.
However, in response to virus-induced oxidative stress, host cells deploy a strong antioxidant response characterized by the production of proteins (enzymes) and/or small molecules (vitamins C and E) that are mainly mediated by the nuclear factor erythroid 2-related factor (NRF2) to counteract the redox-induced toxicity and restore cellular redox homeostasis [53,54][53][54].
2. The NRF2 Pathway Regulates Cellular Responses to Stress
The production of ROS and activation of an antioxidant response is known to be controlled by the Kelch-like ECH-associated protein 1 (KEAP1)–NRF2 axis. This regulation occurs through intrinsic mechanisms within different cell types of the airway epithelium (e.g., nasal versus bronchial cells). NRF2 belongs to the cap “n” collar (CNC) family of transcription factors and is found in the cytoplasm of non-stressed cells in a combined form with KEAP1. In quiescent cells, an adapter protein, KEAP1, interacts with NRF2 and recruits cullin-3 (CUL3)-containing E3 ubiquitin ligase to form a complex that regulates the ubiquitination of NRF2. Consequently, polyubiquitination of NRF2 leads to NRF2 degradation via the 26S proteasome machinery, which ensures that the NRF2 level and its activity remain low during redox homeostasis [55]. Contrarily, during a viral respiratory infection or other induced oxidative stress, NRF2 escapes repression by KEAP1. The CUL3/KEAP1 complex that targets NRF2 for ubiquitination undergoes a change to a nonfunctional conformation [56,57,58,59][56][57][58][59]. Thus, upon activation, newly synthesized NRF2 is no longer ubiquitinated/degraded, rapidly accumulates, and translocates to the nucleus where it binds the small maf protein (sMaf) [56,57,58,59][56][57][58][59]. The NRF2–Maf heterodimer binds to the antioxidant response element (ARE) (or multiple Maf recognition elements (MAREs)). This interaction induces the transcription of a wide variety of antioxidant genes, including HO-1 and genes that are involved in the synthesis and recycling of glutathione (Figure 1). A heme sensor known as BTB and CNC homology 1 (BACH-1) can also bind to the ARE in a KEAP1-independent manner and directly competes with NRF2 for binding to AREs. The interaction of BACH-1 with ARE prevents NRF2 from binding to the ARE, thus repressing HO-1 [60,61,62,63,64,65][60][61][62][63][64][65] (Figure 1). HO-1 catalyzes the degradation of heme into carbon monoxide (CO), Fe2+, and biliverdin, and has antiviral properties through multiple pathways (Figure 2). Importantly, NRF2 activation appears to have an inhibitory effect on the interferon response, which is an important component of the innate immune system’s antiviral defense. The balance between NRF2 activation and the interferon response is regulated by intrinsic cell factors, for which the cell composition varies from nasal to bronchial cells, and potentially influences the susceptibility to viral infections. Notably, activation of the NRF2 pathway appears to mediate protection against viral respiratory infections, including SARS-CoV-2, influenza viruses, and several RNA and DNA viruses that induce oxidative stress (Supplementary Table S1) [5,8,9,10,11,12,13,14,18,19,20,21,22,23,24,27,28,29,30,31,32,33,34,35,36,41,42,43,44,46,47,48,49,51,52,53,54,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93][5][8][9][10][11][12][13][14][18][19][20][21][22][23][24][27][28][29][30][31][32][33][34][35][36][41][42][43][44][46][47][48][49][51][52][53][54][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93]. Overall, the crosstalk between NRF2 and viruses is bidirectional and complex. With regards to the impact of viruses on the NRF2 pathway, respiratory viruses such as influenza [12[12][94],94], SARS-CoV-2 [19[19][20][22][24][95][96],20,22,24,95,96], RSV [27[27][31][32],31,32], rhinovirus [43,44][43][44], and enteroviruses [47] directly alter NRF2 levels and signaling [5,8,9,10,18,33][5][8][9][10][18][33] (Table 1). With regards to the impact of the NRF2 pathway on viruses, host NRF2 pathways also regulate the replication of several respiratory viruses such as influenza [11[11][12][13],12,13], coronaviruses [19[19][20][21][22][23],20,21,22,23], RSV [34[34][35],35], rhinovirus [44], enterovirus [47], metapneumovirus [34], and parainfluenza [51,52][51][52] (Table 2). Notably, the host NRF2 pathways also regulate viral replication, apoptosis, ferroptosis, and inflammation (Table 2, Table 3, Table 4, Table 5 and Table 6). Besides playing an essential role in cell defense against redox stresses by trans-activating cytoprotective genes encoding antioxidant and detoxifying enzymes, NRF2 contributes to the regulation of the anti-inflammatory response and metabolic reprogramming [97].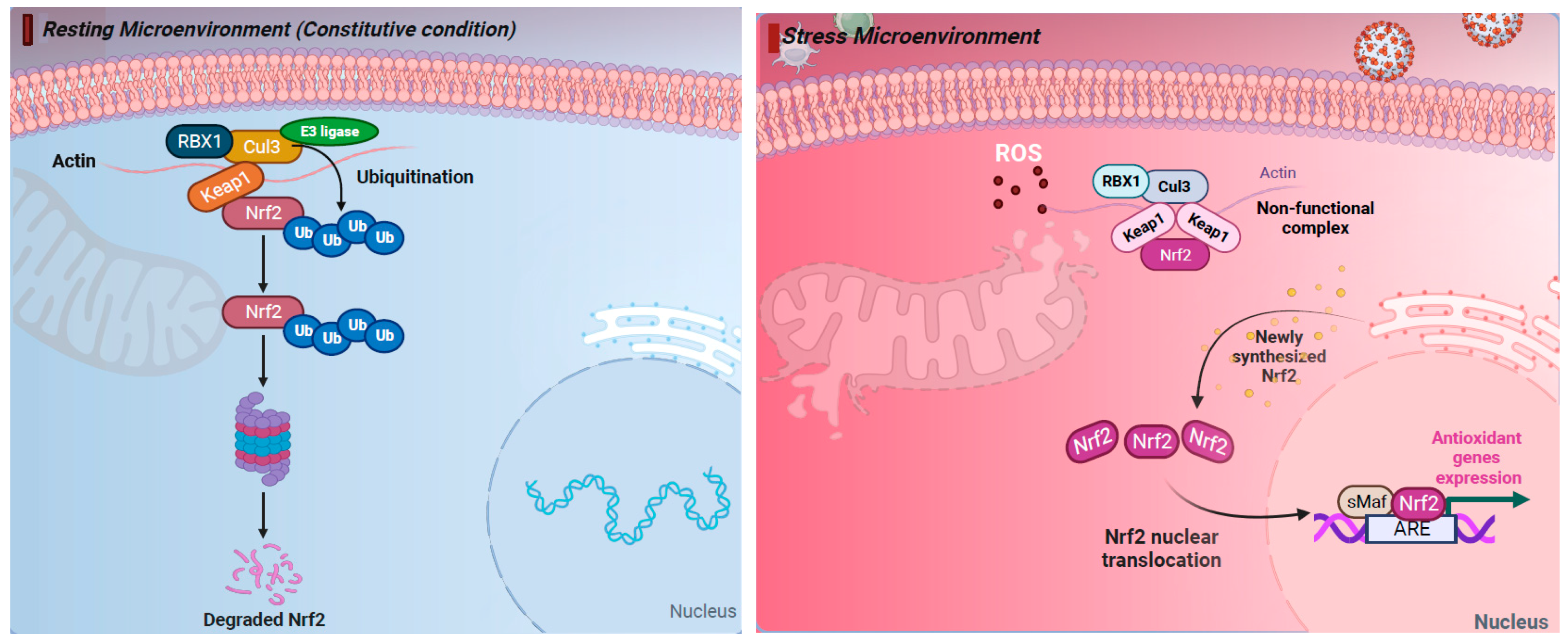
Figure 1. The nuclear factor erythroid 2-related factor 2 (NRF2) pathway regulates cellular responses to stress. (Left) Under resting (constitutive) condition, in the cytoplasm NRF2 is anchored with Kelch-like ECH-associated protein 1 (KEAP1). NRF2 binds KEAP1 and becomes ubiquinated, leading to degradation by the 26S proteasome. (Right) Under oxidative stress response, NRF2 escapes repression by KEAP1. The CUL3/KEAP1 complex that targets NRF2 for ubiquitination undergoes a change to a nonfunctional conformation. Thus, newly synthesized NRF2 is no longer ubiquitinated/degraded, rapidly accumulates, and translocates to the nucleus where it binds the small maf protein (sMaf) and antioxidant response element (ARE). Activation of ARE increases the expression of the antioxidant genes heme oxygenase 1 (HO-1), quinone oxidoreductase (NQO1), and glutathione (GSH), which blocks the progression of oxidative stress (OS). Thus, activation of the NRF2 pathway has cytoprotective effects and plays a key role in maintaining redox balance. Figure generated with Biorender (https://biorender.com/, accessed on 10 November 2023).
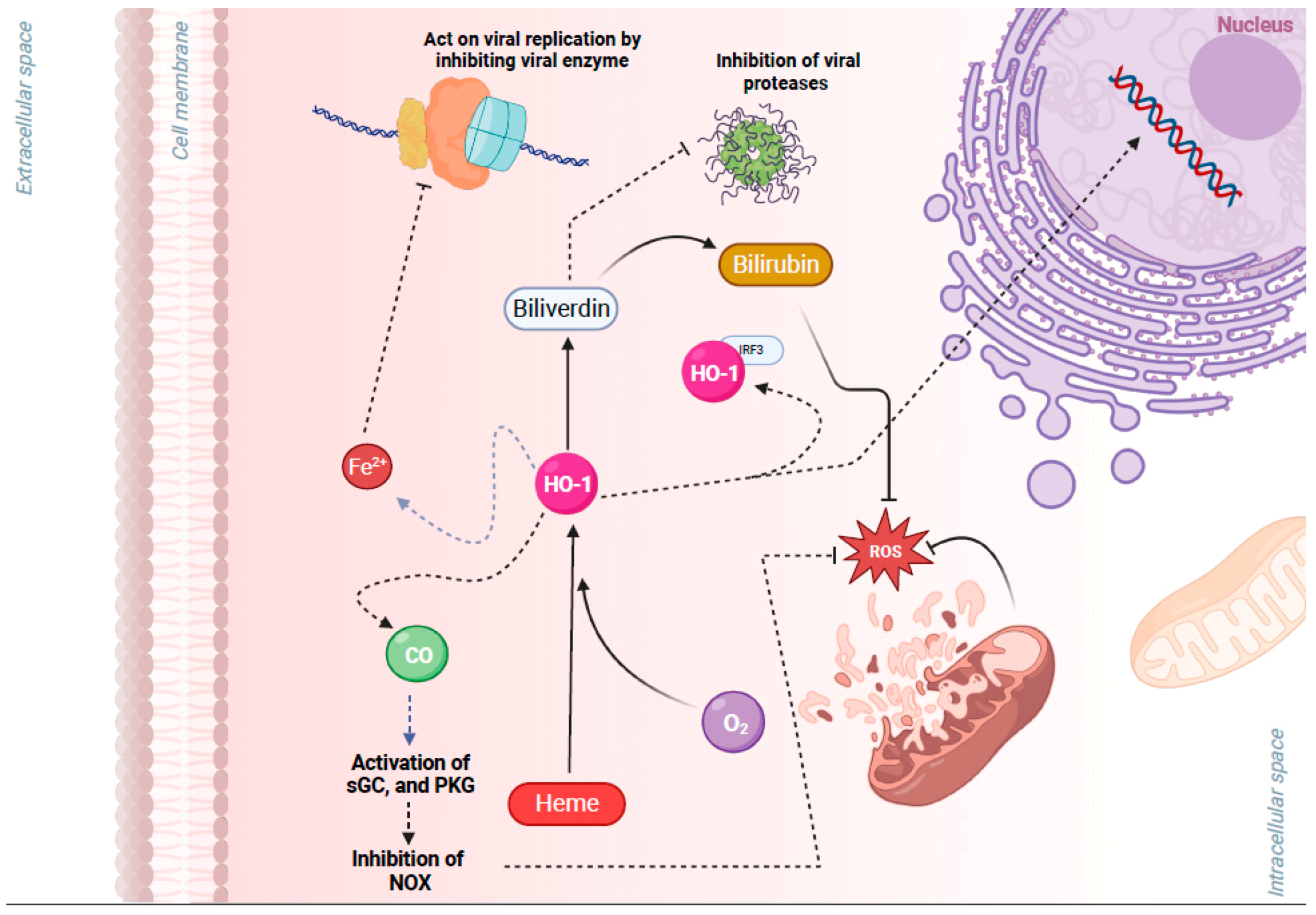
Figure 2. Cytoprotective effects of heme oxygenase 1 (HO-1), a key gene of the nuclear factor erythroid 2-related factor 2 (NRF2) pathway, in viral infection. HO-1 is a metabolic enzyme that utilizes oxygen (O2), heme, and NADPH to catalyze the degradation of heme into carbon monoxide (CO), Fe2+, and biliverdin. HO-1 has antiviral properties through multiple pathways: (1) Free Fe2+ may act on viral replication by binding to the highly conserved divalent metal-binding pocket of the viral RNA and inhibiting enzymes that mediate viral replication. (2) Biliverdin may inhibit viral proteases. (3) Heterodimerization of HO-1 with interferon regulatory factor 3 (IRF3) facilitates the phosphorylation and nuclear translocation of IRF3 and the induction of type I interferon (IFN) gene expression that has antiviral properties. (4) CO activates protein kinase G (PKG), which inhibits NAPDH oxidases (NOX), preventing an increase in reactive oxygen species (ROS) and associated damage. (5) Biliverdin also has antioxidant properties, and it is converted by NRF2/ARE-regulated gene biliverdin reductase to the potent antioxidant bilirubin. Figure generated with Biorender (https://biorender.com/, accessed on 10 November 2023).
Table 1.
Impact of respiratory viruses on the NRF2 pathway.
| Viruses | Mechanism of NRF2 Activation | Reference |
|---|---|---|
| Several (respiratory) viruses | ∙ ↑ ROS and mito-ROS ↑ ARE elements and phosphorylation of p62. | [5,8,9][5][8][9] |
Abbreviations: ACE2: angiotensin-converting enzyme, ARE: antioxidant response element, COX-2: cyclooxygenase 2, EBV: Epstein Barr Virus, ER: endoplasmic reticulum, EV71: Enterovirus 71, GSK3: glycogen synthase kinase 3, HBV: Hepatitis B virus, HCV: Hepatitis C virus, HO-1: Heme Oxygenase 1, IRG1: immune-responsive gene 1, IV: influenza virus, mito-ROS: mitochondrial ROS, KEAP1: Kelch-like ECH-associated protein 1, KSHV: Kaposi sarcoma-associated herpesvirus, NQO1: NAD(P)H quinone oxidoreductase, NRF2: nuclear factor erythroid 2-related factor, NSP14: nonstructural protein 14, ORF3a: 3a open reading frame 3a, PKC: protein kinase C, PI3K: phosphatidylinositol 3-kinase (PI3K), PGE2: Prostaglandin E2. RNF4: RING finger protein 4, ROS: reactive oxygen species, RSV: respiratory syncytial virus, SIRT1: sirtuin 1, SFTS: severe fever with thrombocytopenia syndrome, sMafs: small Maf proteins, SUMO: small ubiquitin-like modifiers.
Table 2.
Impact of NRF2 on the replication of respiratory viruses.
| Virus | Role of NRF2 in Viral Replication | Reference |
|---|---|---|
| Influenza virus | ∙ Activation of NRF2 leads to ↓ viral replication through interferon host responses. | [11,12,13][11][12][13 |
Abbreviations: ACE2: angiotensin-converting enzyme, ARE: antioxidant response element, BHA: butylated hydroxyanisole, DMF: Dimethyl fumarate, EV-71: Enterovirus 71, hMPV: Human Metapneumovirus, HO-1: Heme Oxygenase 1, HPIV3: Human Parainfluenza virus 3, IBs: inclusion bodies, KEAP1: Kelch-like ECH-associated protein 1, NRF2: nuclear factor erythroid 2-related factor, 4-OI: 4-octyl itaconate, PI4KB: 1-phosphatidylinositol 4-kinase beta, RD: Rhabdomyosarcoma, RSV: respiratory syncytial virus, SARS-CoV-2: severe acute respiratory syndrome-coronavirus 2, siRNA: Small interfering RNA.
Table 3.
Antiviral activity of Heme Oxygenase 1 (HO-1), a key downstream gene of the NRF2 pathway.
| Viruses/NRF2 | HO-1 Antiviral Activity | Viruses/NRF2 | Impact on ApoptosisReference |
|---|---|---|---|
| ] |
Table 4.
Respiratory viruses and the role of NRF2 role in inflammation.
| Virus | Role of NRF2 in Inflammation | References | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Impact on Ferroptosis | Reference | ||||||||||||
| Reference | |||||||||||||
| ∙ Downregulation of NRF2 results in major ↑ of viral entry and, subsequently, viral replication. | |||||||||||||
| Influenza | Influenza virus | ∙ Inhibition of viral replication, growth, and protein expression after activation of NRF2.∙ ↑ expression of HO-1 leads to ↓ viral replication during infection from Influenza A, through the upregulation of IFN- α/β and ISGs. | ∙ Inactivation of NF-κΒ transcription factor. ∙ ↓ of NF-κΒ-mediated inflammation and associated lung permeability damage, mucus hypersecretion, lung permeability damage, as well as mucus hypersecretion, through reduced NF-κΒ-mediated inflammation and associated proinflammatory cytokines [15]. ∙ Induces anti-inflammatory effects in vivo through the HO-1 pathway [100,101][100][101]. ∙ NLRP3 activation form PB1-F2 influenza A protein. ∙ K+ efflux and ROS dependent activation of NLRP3 inflammasome ∙ Impacts function of alveolar macrophages (AMϕ) that are important essential for preventing respiratory failure and mortality after infection from influenza virus in mice | ||||||||||
| Adenoviruses | [17]. ∙ Attenuates virus-induced inflammation through increased GSH levels and IL-8 secretion in ATI-like cells (alveolar epithelial cells) in vitro [12] | [14] | . | ∙ Complex effects. ∙ ↑ apoptosis: ↑ sensitivity to TNFa that induces apoptosis, ↑ PP2A, and ↑ p53. ∙ ↓ apoptosis through several mechanisms: interacts with FADD, ↓ CD95-mediated apoptosis, ↓ phospholipase A2, ↓ Fas, ↓ p53, and ↓ pro-apoptotic proteins of the Bcl-2 family, such as Bax, Bak, BNIP3, and Bnip3L. ∙ ↓ apoptosis of the host cell in order to ↑ efficiently and the capacity of the virus to ‘hijack’ host cell apoptotic machinery. | |||||||||
| NRF2 | ∙ NRF2 ↓ ROS and ↑ antioxidant responses, and ↑ GPX4-induced ↓ of ferroptosis. ∙ NRF2 ↑ Heme Oxygenase 1 (HO-1) that ↓ ferroptosis. ∙ NRF2 ↑ antioxidant enzymes. | [15, | [9]16,17][15][16][17] | ||||||||||
| [ | 103 | ,104,105,106][103][104][105][106] | Influenza | Coronaviruses | ∙ IV strains are thought to activate the NRF2/ARE defense pathway in vitro and in mice by inducing oxidative stress and nuclear translocation and transcriptional activity of NRF2 because transcription of the NRF2 target gene HO-1 was shown to be augmented. | ∙ NRF2 deficiency upregulates ACE2, ↑ viral entry, and, as a result, viral replication. ∙ NRF2 induced production of HO-1 and generated Fe+2, which binds to the RNA- dependent RNA polymerase of SARS-CoV-2, inhibiting its activity and thus viral replication. ∙ NRF2 agonists like 4-OI and DMF inhibit SARS-CoV-2 replication. ∙ Vero cells infected with SARS-CoV-2 and transfected with siRNA to silence KEAP-1, thereby activating NRF2, had a decreased viral load. ∙ Absence of NRF2 in knockout mice ↑ the severity of SARS-CoV-2 infection and viral replication. | [ | [ | |||||
| RSV | ∙ Harmacological activation of HO-1 by CoPP ↓ viral replication of RSV in lung cells of infected mice via induction of IFN-α/β expression. ∙ In vitro data suggest that ↑ of HO-1 can moderate the susceptibility of cells to hRSV infection [36]. | 1219, | Coronavirus | ∙ NRF2 is directly able to inhibit IL6, IL-1B, a key hallmark of the cytokine storm in SARS-CoV-2 infection. ∙ Absence of NRF2 in knockout mice ↑ the severity of SARS-CoV-2 infection, pulmonary inflammation. ∙ In humans, SNPs in the Nrf2 gene promoter region can determine susceptibility to respiratory failure with COPD, indicating the importance of NRF2 in pulmonary inflammation. ∙ Cytokine storm due to T cell depletion and widespread pulmonary inflammation. ∙ Contradictory effect on proinflammatory nature of factors like NF-kB. | ,94][12]20,21,22,23][19][94[20 | [23 | |||||||
| Influenza | , | [25,2326]]][25][21[26] | |||||||||||
| RSV | ] | ||||||||||||
| ∙ ↑ interferons and caspase 1. | ∙ Experimental studies have shown that autophagy plays a very crucial role in RSV replication [107]. | ][22] | [36][23] | ||||||||||
| [ | 105 | ] | ∙ Iron ↓ viral genome amplification and viral replication. ∙ Influenza ↓ cellular GSH and/or affects GPX4 activity. ∙ Neuraminidase of Influenza A binds lysosome-associated membrane proteins and ↑ lysosome rupture. |
[9,108,,112][9109,][108110,][109][110]111[111][112] | SARS-CoV-2 | ∙ SARS-CoV-2 infection ↓ levels of NRF2 in epithelial cells in vitro [19,20,22][19][20][22]. ∙ NRF2 was ↓ in RNA Seq analysis of lung biopsies of COVID-19 patients [22]. ∙ NRF2 deficiency ↑ ACE2, enhancing viral entry and as a result viral replication [19,20][19][20]. ∙ The nonstructural SARS-CoV-2 NSP14 viral protein inhibits NRF2 through ↓ of SIRT1 [95]. ∙ The SARS-CoV-2 ORF3a viral protein recruits KEAP-1 which inhibits NRF2 activity, thereby facilitating ferroptosis through the built-up ROS and the downregulation of genes like HO-1 and NQO1 [96]. ∙ The SARS-CoV-2 ORF3a viral protein binds to human HO-1 protein in vitro [24]. |
RSV | ∙ NRF2 knockout mice showed significantly ↑ viral titers in the lungs. ∙ Treatment of the NRF2 agonist sulforaphane on NRF2−/− and NRF2+/+ mice before RSV infection ↓ virus replication, but this significant effect was not observed in NRF2−/− mice [37]. ∙ Compared to wild-type mice, RSV-infected NRF2 KO had ↓ antioxidant enzymes and enzymes in the airway, which modulated the endogenous hydrogen sulfide (H2S) pathway that has a significant antiviral function [34]. ∙ Inducers of the NRF2-ARE pathway, such as BHA treatment, ↑ the viral clearance in murine lungs [35]. |
|||||
| HCV | ∙ Type 1 IFN-dependent anti-HCV activity due to ↑ levels of HO-1 resulting from the usage of HO-1 agonists/inducers. | RSV | ∙ Iron stopped viral replication of HCV by direct binding to the Mg+2 binding pocket of the RNA polymerase of the virus. |
∙ Severe inflammation in NRF2−/− mice compared to NRF2+/+ mice. ∙ RSV-infected NRF2 KO mice are reported to have a significant ↑ in airway neutrophilia and inflammatory cytokines. | |||||||||
| SARS-CoV-2, SARS-CoV, other coronaviruses | ∙ ↓ lung inflammation when pretreated with sulforaphane. ∙ ↓ ROS- and K+ efflux-dependent activation of NLRP3 inflammasome. ∙ SH viroporin activates NLRP3 inflammasome. |
∙ SARS-CoV-2 Potentially causes cellular iron overload and iron scavenging. ∙ SARS-CoV-2 ↑serum ferritin concentration. ∙ CoVs↓ cellular GSH and/or affect GPX4 activity. ∙ SARS-CoV ORF-3a viral protein ↑ lysosomal damage and dysfunction. | [19,20,22,24,95,96][19][[3420],35,37][34][35][22][37[24][[96] | ||||||||||
| Influenza also regulates autophagy, which interacts with NRF2 and is involved in influenza replication | ] | ||||||||||||
| [ | 88,89 | ||||||||||||
| Influenza | ∙ Impacts function of alveolar macrophages (AMϕ), which are important to attenuate virus-induced inflammation. | , | ∙ ↑ Fas expression. ∙ ↓ PKR and apoptosis. ∙ Apoptosis plays a role in viral release. | 90,91,92][88][[3789],38,39,40,[38 | [113][39102][37] | [105,106][105][106][90],114,115,116,117][113][114]][115][91][40][116]95][92] | [102] | [117] | RSV | ∙ RSV deregulates the NRF2 expression and its activity along with the upregulation of downstream ARE-responsive genes [98]. ∙ RSV ↓ mRNA levels of NRF2 in airway epithelial cells [27]. ∙ RSV ↑ NRF2 deacetylation, ubiquitination, and degradation through a proteasome-dependent pathway in a SUMO-specific E3 ubiquitin ligase RNF4-dependent manner. ∙ Another possible mechanism of RSV-associated NRF2 activation is the activation of [31], which activates the NRF2 pathway through direct alkylation of the NRF2 partner—KEAP1 [32] |
Rhinovirus | . | ∙ Silence of NRF2 in cells led to a ↓ in the secretion of antiviral interferons and higher viral titers. |
| Coronaviruses | ∙ Fe+2 binds to the RNA-dependent RNA polymerase of SARS-CoV-2 inhibiting its activity and ↓ viral replication. ORF3a protein binds to human HMOX1 protein in vitro [24 | [27,[44] | |||||||||||
| ] | . | 31,32,98][27] | [19][31][32][98] | ||||||||||
| Rhinovirus | ∙ Rhinovirus RNA stimulates the innate immune sensor RIG-I within airway epithelial cells and activates the antiviral interferon response (greater activation in nasal cells than in bronchial cells) and the NRF2-mediated response to oxidative stress (greater activation in bronchial cells compared to nasal cells) [43]. | ||||||||||||
| Metapneumovirus | Enterovirus 71 (EV71) | ∙ However, the inhibitory effects were reversed in cells pretreated with the antioxidant, N-acetyl cysteine. Moreover, the secretion of anti-viral interferons ↑ in cells treated with the NRF2 agonist sulforaphane but ↓ in cells where NRF2 was silenced [44]. |
[43 | ∙ Silencing of NRF2 is beneficial for viral replication. | ,44][43] | ||||||||
| [ | 99] | ||||||||||||
| EV71 | ∙ Activating NRF2 through downregulation of KEAP1 led to ↓ viral replication in RD cells. | ||||||||||||
| Rhinovirus, enteroviruses | ∙ NRF2 KO mice infected with hMPV had ↓ expression of antioxidant enzymes (AOE) and ↑ viral-mediated oxidative stress and airway damage compared to NRF2+/+ mice. | ||||||||||||
| RSV | ∙ ↑ apoptosis through unknown mechanism. | [34] | |||||||||||
| [ | 105 | ] | ∙ ↑ the expression of 12/15-LOX and mitochondrial iron content. | [118] | ∙ The overexpression of HO-1 ↓ NADPH oxidase/ROS production that is induced by enterovirus 71 and hence ↓ viral replication. This effect was abolished if cells were pretreated with zinc–protoporphyrin IX, an HO-1 activity inhibitor. | ][47] | |||||||
| ∙ Bilirubin has also been found to exert antiviral activity against EV71 reducing its replication and as a result infectivity in vitro. | [ | 48,49][ | Enterovirus 7148 | ∙ By silencing KEAP1, the induced ROS, apoptosis, and inflammation was ↓ in the EV71 infected cells. However, when both KEAP1 and NRF2 were silenced in Vero and RD cells, these effects were restored. ∙ Inflammation-promoting cytokines and chemokines influence the severity of the EV71 infection. | ][44 | [47,50][47]50] | |||||||
| Coronaviruses | ∙ ↑ apoptosis through ORF proteins and unknown mechanisms. | [105[49] | ][ | ||||||||||
| EV-71, CB3 | ∙ Iron ↓ viral genome amplification and viral replication of EV-71. ∙ CB3 ↑the expression NRAMP (DMT) and ↑cellular iron uptake. |
[108 | Enterovirus 71 (EV71) | ∙ EV71 ↑ KEAP1 and ↓ NRF2 | Metapneumovirus | ∙ ↓ of NRF2-dependent genes ↑ viral replication and clinical disease upon hMPV infection. | [34] | ||||||
| , | 119 | ,120][108][119][120] | [47]. | HIV[47 | ∙ BV and BR have been identified to act as inhibitors for the protease of HIV, interfering with the life cycle of the virus. | [ | Rhinovirus] | ||||||
| 93 | ∙ 2B viroporin activates NLRP3 inflammasome | ] | |||||||||||
| Non-respiratory viruses: HBV, HCV, WNV, Dengue virus, HSV, KSHV |
∙ HBV, HCV: ↑ serum and cellular iron uptake and ↓ hepcidin expression, ↑ serum ferritin concentration, and uses TfR1 as a cellular receptor. ∙ HIV ↓ serum iron, ↑ the expression of hepcidin, ↑cellular iron via hepcidin mediated degradation of ferroportin, ↓ cellular GSH and/or affects GPX4 activity, and upregulates the expression of system xc-. ∙ WNV ↑ the expression NRAMP (DMT) and ↑cellular iron uptake. ∙ Dengue virus ↓ cellular GSH and/or affects GPX4 activity. ∙ HSV ↓ cellular GSH and/or affects GPX4 activity. ∙ JEV ↓ cellular GSH and/or affects GPX4 activity, produces lipid peroxide free radicals, and ↑ the expression of system xc-. ∙ KSHV ↓ cellular GSH and/or affects GPX4 activity. ∙ Zika virus ↓ cellular GSH and/or affects GPX4 activity. | [16,45][16][45] | RSV, influenza, coronaviruses, HCV | ∙ ↑ phosphorylation of the redox-sensitive PKC ↑ NRF2 dissociation from KEAP1. | Parainfluenza viruses | ∙ Cotreatment or post infection treatment with curcumin, ↓ the expression of HN viral protein, indicating that curcumin may ↓ viral entry affecting viral replication and subsequently different steps in viral replication ∙ Curcumin, an NRF2 activator, led to ↓ of F-actin, ↓ the formation of viral IBs, and ↓ viral replication. ∙ Curcumin ↓ HPIV3 replication by ↓ the endogenous PI4KB level in the cells, and ↓ the colocalization of PI4KB and IBs, affecting IB formation. | [10, | [5118,,52][51][52]33 |
Abbreviations: CoPP: cobalt protoporphyrin, EV-71: Enterovirus 71, HCV: Hepatitis C virus, HO-1: Heme Oxygenase 1, ISGs: interferon stimulated genes. NRF2: nuclear factor erythroid 2-related factor, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, RSV: respiratory syncytial virus.
| ] | ||
| [ | ||
| 10 | ||
| ] | ||
| [ | 18 | ][33] |
Abbreviations: EV-71: Enterovirus 71, KEAP1: Kelch-like ECH-associated protein 1, hMPV: human metapneumovirus, NRF2: nuclear factor erythroid 2-related factor, NRF2-KO: NRF2 knocked out; NF-κB: nuclear factor kappa B, ROS: reactive oxygen species, RSV: respiratory syncytial virus.
Table 5.
Impact of viruses and NRF2 on the apoptosis pathway.
Abbreviations: FADD: Fas-associated death domain protein, GPX4: Glutathione Peroxidase 4, HO-1: Heme Oxygenase 1, NRF2: nuclear factor erythroid 2-related factor, ORF: Open Reading Frame, PKR: protein kinase R, p53: tumor protein 53, PP2A: Protein Phosphatase 2A, ROS: reactive oxygen species, RSV: respiratory syncytial virus, TNFa: tumor necrosis factor a.
Table 6.
Impact of viruses and NRF2 on the ferroptosis pathway.
| Viruses/NRF2 |
|---|
| ∙ Other viruses (e.g., hemorrhagic viruses) use NRAMP or TfR1 as a cellular receptor. |
| [ |
| 121 |
| ] |
Abbreviations: CB3: Coxsackievirus B3, EV-71: Enterovirus 71, GSH: Glutathione, GPX4: Glutathione Peroxidase 4, HBV: Hepatitis B virus, HCV: Hepatitis C virus, HIV: human immunodeficiency virus, HSV: Herpes simplex virus, JEV: Japanese encephalitis virus, KSHV: Kaposi sarcoma-associated herpesvirus, 12/15-LOX:12/15-lipoxygenase, NRAMP: Natural Resistance-Associated Macrophage Proteins, ORF-3a: Open Reading Frame-3a, RSV: respiratory syncytial virus, SARS-CoV-2: severe acute respiratory syndrome-coronavirus-2, SARS-CoV: severe acute respiratory syndrome coronavirus, TfR1: transferrin receptor 1 protein, WNV: West Nile virus.
References
- Clementi, N.; Ghosh, S.; De Santis, M.; Castelli, M.; Criscuolo, E.; Zanoni, I.; Clementi, M.; Mancini, N. Viral Respiratory Pathogens and Lung Injury. Clin. Microbiol. Rev. 2021, 34, e00103-20.
- Newton, A.H.; Cardani, A.; Braciale, T.J. The host immune response in respiratory virus infection: Balancing virus clearance and immunopathology. Semin. Immunopathol. 2016, 38, 471–482.
- Shi, T.; Arnott, A.; Semogas, I.; Falsey, A.R.; Openshaw, P.; Wedzicha, J.A.; Campbell, H.; Nair, H.; Investigators, R. The Etiological Role of Common Respiratory Viruses in Acute Respiratory Infections in Older Adults: A Systematic Review and Meta-analysis. J. Infect. Dis. 2020, 222, S563–S569.
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Prim. 2018, 4, 3.
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392.
- Gain, C.; Song, S.; Angtuaco, T.; Satta, S.; Kelesidis, T. The role of oxidative stress in the pathogenesis of infections with coronaviruses. Front. Microbiol. 2022, 13, 1111930.
- Moreno-Solis, G.; Dela Torre-Aguilar, M.J.; Torres-Borrego, J.; Llorente-Cantarero, F.J.; Fernandez-Gutierrez, F.; Gil-Campos, M.; Tunez-Finana, I.; Perez-Navero, J.L. Oxidative stress and inflamatory plasma biomarkers in respiratory syncytial virus bronchiolitis. Clin. Respir. J. 2017, 11, 839–846.
- Jiang, T.; Harder, B.; Rojo de la Vega, M.; Wong, P.K.; Chapman, E.; Zhang, D.D. p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 2015, 88, 199–204.
- Lane, D.J.R.; Metselaar, B.; Greenough, M.; Bush, A.I.; Ayton, S.J. Ferroptosis and NRF2: An emerging battlefield in the neurodegeneration of Alzheimer’s disease. Essays Biochem. 2021, 65, 925–940.
- Mondal, A.; Dawson, A.R.; Potts, G.K.; Freiberger, E.C.; Baker, S.F.; Moser, L.A.; Bernard, K.A.; Coon, J.J.; Mehle, A. Influenza virus recruits host protein kinase C to control assembly and activity of its replication machinery. eLife 2017, 6, e26910.
- Kesic, M.J.; Simmons, S.O.; Bauer, R.; Jaspers, I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic. Biol. Med. 2011, 51, 444–453.
- Kosmider, B.; Messier, E.M.; Janssen, W.J.; Nahreini, P.; Wang, J.; Hartshorn, K.L.; Mason, R.J. Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir. Res. 2012, 13, 43.
- Shoji, M.; Arakaki, Y.; Esumi, T.; Kohnomi, S.; Yamamoto, C.; Suzuki, Y.; Takahashi, E.; Konishi, S.; Kido, H.; Kuzuhara, T. Bakuchiol Is a Phenolic Isoprenoid with Novel Enantiomer-selective Anti-influenza A Virus Activity Involving Nrf2 Activation. J. Biol. Chem. 2015, 290, 28001–28017.
- Ma, L.L.; Wang, H.Q.; Wu, P.; Hu, J.; Yin, J.Q.; Wu, S.; Ge, M.; Sun, W.F.; Zhao, J.Y.; Aisa, H.A.; et al. Rupestonic acid derivative YZH-106 suppresses influenza virus replication by activation of heme oxygenase-1-mediated interferon response. Free Radic. Biol. Med. 2016, 96, 347–361.
- Yageta, Y.; Ishii, Y.; Morishima, Y.; Masuko, H.; Ano, S.; Yamadori, T.; Itoh, K.; Takeuchi, K.; Yamamoto, M.; Hizawa, N. Role of Nrf2 in host defense against influenza virus in cigarette smoke-exposed mice. J. Virol. 2011, 85, 4679–4690.
- McAuley, J.L.; Tate, M.D.; MacKenzie-Kludas, C.J.; Pinar, A.; Zeng, W.; Stutz, A.; Latz, E.; Brown, L.E.; Mansell, A. Activation of the NLRP3 inflammasome by IAV virulence protein PB1-F2 contributes to severe pathophysiology and disease. PLoS Pathog. 2013, 9, e1003392.
- Schneider, C.; Nobs, S.P.; Heer, A.K.; Kurrer, M.; Klinke, G.; van Rooijen, N.; Vogel, J.; Kopf, M. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014, 10, e1004053.
- Huang, C.; Feng, F.; Shi, Y.; Li, W.; Wang, Z.; Zhu, Y.; Yuan, S.; Hu, D.; Dai, J.; Jiang, Q.; et al. Protein Kinase C Inhibitors Reduce SARS-CoV-2 Replication in Cultured Cells. Microbiol. Spectr. 2022, 10, e0105622.
- Cuadrado, A.; Pajares, M.; Benito, C.; Jimenez-Villegas, J.; Escoll, M.; Fernandez-Gines, R.; Garcia Yague, A.J.; Lastra, D.; Manda, G.; Rojo, A.I.; et al. Can Activation of NRF2 Be a Strategy against COVID-19? Trends Pharmacol. Sci. 2020, 41, 598–610.
- Zhao, S.; Ghosh, A.; Lo, C.S.; Chenier, I.; Scholey, J.W.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.L.; Chan, J.S.D. Nrf2 Deficiency Upregulates Intrarenal Angiotensin-Converting Enzyme-2 and Angiotensin 1-7 Receptor Expression and Attenuates Hypertension and Nephropathy in Diabetic Mice. Endocrinology 2018, 159, 836–852.
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020, 368, 779–782.
- Olagnier, D.; Farahani, E.; Thyrsted, J.; Blay-Cadanet, J.; Herengt, A.; Idorn, M.; Hait, A.; Hernaez, B.; Knudsen, A.; Iversen, M.B.; et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020, 11, 4938.
- Qu, Y.; Haas de Mello, A.; Morris, D.R.; Jones-Hall, Y.L.; Ivanciuc, T.; Sattler, R.A.; Paessler, S.; Menachery, V.D.; Garofalo, R.P.; Casola, A. SARS-CoV-2 Inhibits NRF2-Mediated Antioxidant Responses in Airway Epithelial Cells and in the Lung of a Murine Model of Infection. Microbiol. Spectr. 2023, 11, e0037823.
- Batra, N.; De Souza, C.; Batra, J.; Raetz, A.G.; Yu, A.M. The HMOX1 Pathway as a Promising Target for the Treatment and Prevention of SARS-CoV-2 of 2019 (COVID-19). Int. J. Mol. Sci. 2020, 21, 6412.
- Luo, X.H.; Zhu, Y.; Mao, J.; Du, R.C. T cell immunobiology and cytokine storm of COVID-19. Scand. J. Immunol. 2021, 93, e12989.
- Hua, C.C.; Chang, L.C.; Tseng, J.C.; Chu, C.M.; Liu, Y.C.; Shieh, W.B. Functional haplotypes in the promoter region of transcription factor Nrf2 in chronic obstructive pulmonary disease. Dis. Markers 2010, 28, 185–193.
- Hosakote, Y.M.; Liu, T.; Castro, S.M.; Garofalo, R.P.; Casola, A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am. J. Respir. Cell Mol. Biol. 2009, 41, 348–357.
- Casola, A.; Burger, N.; Liu, T.; Jamaluddin, M.; Brasier, A.R.; Garofalo, R.P. Oxidant tone regulates RANTES gene expression in airway epithelial cells infected with respiratory syncytial virus. Role in viral-induced interferon regulatory factor activation. J. Biol. Chem. 2001, 276, 19715–19722.
- Liu, T.; Castro, S.; Brasier, A.R.; Jamaluddin, M.; Garofalo, R.P.; Casola, A. Reactive oxygen species mediate virus-induced STAT activation: Role of tyrosine phosphatases. J. Biol. Chem. 2004, 279, 2461–2469.
- Komaravelli, N.; Ansar, M.; Garofalo, R.P.; Casola, A. Respiratory syncytial virus induces NRF2 degradation through a promyelocytic leukemia protein—Ring finger protein 4 dependent pathway. Free Radic. Biol. Med. 2017, 113, 494–504.
- Ren, K.; Lv, Y.; Zhuo, Y.; Chen, C.; Shi, H.; Guo, L.; Yang, G.; Hou, Y.; Tan, R.X.; Li, E. Suppression of IRG-1 Reduces Inflammatory Cell Infiltration and Lung Injury in Respiratory Syncytial Virus Infection by Reducing Production of Reactive Oxygen Species. J. Virol. 2016, 90, 7313–7322.
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117.
- San-Juan-Vergara, H.; Peeples, M.E.; Lockey, R.F.; Mohapatra, S.S. Protein kinase C-alpha activity is required for respiratory syncytial virus fusion to human bronchial epithelial cells. J. Virol. 2004, 78, 13717–13726.
- Ivanciuc, T.; Sbrana, E.; Casola, A.; Garofalo, R.P. Protective Role of Nuclear Factor Erythroid 2-Related Factor 2 Against Respiratory Syncytial Virus and Human Metapneumovirus Infections. Front. Immunol. 2018, 9, 854.
- Castro, S.M.; Guerrero-Plata, A.; Suarez-Real, G.; Adegboyega, P.A.; Colasurdo, G.N.; Khan, A.M.; Garofalo, R.P.; Casola, A. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am. J. Respir. Crit. Care Med. 2006, 174, 1361–1369.
- Espinoza, J.A.; Leon, M.A.; Cespedes, P.F.; Gomez, R.S.; Canedo-Marroquin, G.; Riquelme, S.A.; Salazar-Echegarai, F.J.; Blancou, P.; Simon, T.; Anegon, I.; et al. Heme Oxygenase-1 Modulates Human Respiratory Syncytial Virus Replication and Lung Pathogenesis during Infection. J. Immunol. 2017, 199, 212–223.
- Cho, H.Y.; Imani, F.; Miller-DeGraff, L.; Walters, D.; Melendi, G.A.; Yamamoto, M.; Polack, F.P.; Kleeberger, S.R. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am. J. Respir. Crit. Care Med. 2009, 179, 138–150.
- Segovia, J.; Sabbah, A.; Mgbemena, V.; Tsai, S.Y.; Chang, T.H.; Berton, M.T.; Morris, I.R.; Allen, I.C.; Ting, J.P.; Bose, S. TLR2/MyD88/NF-kappaB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS ONE 2012, 7, e29695.
- Triantafilou, K.; Kar, S.; Vakakis, E.; Kotecha, S.; Triantafilou, M. Human respiratory syncytial virus viroporin SH: A viral recognition pathway used by the host to signal inflammasome activation. Thorax 2013, 68, 66–75.
- Reed, J.L.; Brewah, Y.A.; Delaney, T.; Welliver, T.; Burwell, T.; Benjamin, E.; Kuta, E.; Kozhich, A.; McKinney, L.; Suzich, J.; et al. Macrophage impairment underlies airway occlusion in primary respiratory syncytial virus bronchiolitis. J. Infect. Dis. 2008, 198, 1783–1793.
- Biagioli, M.C.; Kaul, P.; Singh, I.; Turner, R.B. The role of oxidative stress in rhinovirus induced elaboration of IL-8 by respiratory epithelial cells. Free Radic. Biol. Med. 1999, 26, 454–462.
- Wu, Q.; van Dyk, L.F.; Jiang, D.; Dakhama, A.; Li, L.; White, S.R.; Gross, A.; Chu, H.W. Interleukin-1 receptor-associated kinase M (IRAK-M) promotes human rhinovirus infection in lung epithelial cells via the autophagic pathway. Virology 2013, 446, 199–206.
- Mihaylova, V.T.; Kong, Y.; Fedorova, O.; Sharma, L.; Dela Cruz, C.S.; Pyle, A.M.; Iwasaki, A.; Foxman, E.F. Regional Differences in Airway Epithelial Cells Reveal Tradeoff between Defense against Oxidative Stress and Defense against Rhinovirus. Cell Rep. 2018, 24, 3000–3007.e3.
- Lee, S.H.; Han, M.S.; Lee, T.H.; Lee, D.B.; Park, J.H.; Lee, S.H.; Kim, T.H. Hydrogen peroxide attenuates rhinovirus-induced anti-viral interferon secretion in sinonasal epithelial cells. Front. Immunol. 2023, 14, 1086381.
- Triantafilou, K.; Kar, S.; van Kuppeveld, F.J.; Triantafilou, M. Rhinovirus-induced calcium flux triggers NLRP3 and NLRC5 activation in bronchial cells. Am. J. Respir. Cell Mol. Biol. 2013, 49, 923–934.
- Ai, F.; Zheng, J.; Zhang, Y.; Fan, T. Inhibition of 12/15-LO ameliorates CVB3-induced myocarditis by activating Nrf2. Chem. Biol. Interact. 2017, 272, 65–71.
- Bai, Z.; Zhao, X.; Li, C.; Sheng, C.; Li, H. EV71 virus reduces Nrf2 activation to promote production of reactive oxygen species in infected cells. Gut Pathog. 2020, 12, 22.
- Tung, W.H.; Hsieh, H.L.; Lee, I.T.; Yang, C.M. Enterovirus 71 induces integrin beta1/EGFR-Rac1-dependent oxidative stress in SK-N-SH cells: Role of HO-1/CO in viral replication. J. Cell. Physiol. 2011, 226, 3316–3329.
- Santangelo, R.; Mancuso, C.; Marchetti, S.; Di Stasio, E.; Pani, G.; Fadda, G. Bilirubin: An Endogenous Molecule with Antiviral Activity in vitro. Front. Pharmacol. 2012, 3, 36.
- Lin, T.Y.; Hsia, S.H.; Huang, Y.C.; Wu, C.T.; Chang, L.Y. Proinflammatory cytokine reactions in enterovirus 71 infections of the central nervous system. Clin. Infect. Dis. 2003, 36, 269–274.
- Zhang, C.; Zhang, K.; Zang, G.; Chen, T.; Lu, N.; Wang, S.; Zhang, G. Curcumin Inhibits Replication of Human Parainfluenza Virus Type 3 by Affecting Viral Inclusion Body Formation. Biomed. Res. Int. 2021, 2021, 1807293.
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133.
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777.
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and Its Activators in Respiratory Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 7090534.
- Ramezani, A.; Nahad, M.P.; Faghihloo, E. The role of Nrf2 transcription factor in viral infection. J. Cell. Biochem. 2018, 119, 6366–6382.
- Baird, L.; Dinkova-Kostova, A.T. Diffusion dynamics of the Keap1-Cullin3 interaction in single live cells. Biochem. Biophys. Res. Commun. 2013, 433, 58–65.
- Baird, L.; Lleres, D.; Swift, S.; Dinkova-Kostova, A.T. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc. Natl. Acad. Sci. USA 2013, 110, 15259–15264.
- Eggler, A.L.; Liu, G.; Pezzuto, J.M.; van Breemen, R.B.; Mesecar, A.D. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc. Natl. Acad. Sci. USA 2005, 102, 10070–10075.
- Iso, T.; Suzuki, T.; Baird, L.; Yamamoto, M. Absolute Amounts and Status of the Nrf2-Keap1-Cul3 Complex within Cells. Mol. Cell. Biol. 2016, 36, 3100–3112.
- Sun, J.; Hoshino, H.; Takaku, K.; Nakajima, O.; Muto, A.; Suzuki, H.; Tashiro, S.; Takahashi, S.; Shibahara, S.; Alam, J.; et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002, 21, 5216–5224.
- Kurokawa, H.; Motohashi, H.; Sueno, S.; Kimura, M.; Takagawa, H.; Kanno, Y.; Yamamoto, M.; Tanaka, T. Structural basis of alternative DNA recognition by Maf transcription factors. Mol. Cell. Biol. 2009, 29, 6232–6244.
- MacLeod, A.K.; McMahon, M.; Plummer, S.M.; Higgins, L.G.; Penning, T.M.; Igarashi, K.; Hayes, J.D. Characterization of the cancer chemopreventive NRF2-dependent gene battery in human keratinocytes: Demonstration that the KEAP1-NRF2 pathway, and not the BACH1-NRF2 pathway, controls cytoprotection against electrophiles as well as redox-cycling compounds. Carcinogenesis 2009, 30, 1571–1580.
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47.
- Jancova, P.; Anzenbacher, P.; Anzenbacherova, E. Phase II drug metabolizing enzymes. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2010, 154, 103–116.
- Lee, J.; Jang, J.; Park, S.M.; Yang, S.R. An Update on the Role of Nrf2 in Respiratory Disease: Molecular Mechanisms and Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 8406.
- Herengt, A.; Thyrsted, J.; Holm, C.K. NRF2 in Viral Infection. Antioxidants 2021, 10, 1491.
- Han, D.; Lu, X.; Yin, W.; Fu, H.; Zhang, X.; Cheng, L.; Liu, F.; Jin, C.; Tian, X.; Xie, Y.; et al. Activation of NRF2 blocks HIV replication and apoptosis in macrophages. Heliyon 2023, 9, e12575.
- Staitieh, B.S.; Ding, L.; Neveu, W.A.; Spearman, P.; Guidot, D.M.; Fan, X. HIV-1 decreases Nrf2/ARE activity and phagocytic function in alveolar macrophages. J. Leukoc. Biol. 2017, 102, 517–525.
- Mastrantonio, R.; Cervelli, M.; Pietropaoli, S.; Mariottini, P.; Colasanti, M.; Persichini, T. HIV-Tat Induces the Nrf2/ARE Pathway through NMDA Receptor-Elicited Spermine Oxidase Activation in Human Neuroblastoma Cells. PLoS ONE 2016, 11, e0149802.
- Carvajal-Yepes, M.; Himmelsbach, K.; Schaedler, S.; Ploen, D.; Krause, J.; Ludwig, L.; Weiss, T.; Klingel, K.; Hildt, E. Hepatitis C virus impairs the induction of cytoprotective Nrf2 target genes by delocalization of small Maf proteins. J. Biol. Chem. 2011, 286, 8941–8951.
- Vomund, S.; Schafer, A.; Parnham, M.J.; Brune, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772.
- Ivanov, A.V.; Smirnova, O.A.; Ivanova, O.N.; Masalova, O.V.; Kochetkov, S.N.; Isaguliants, M.G. Hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. PLoS ONE 2011, 6, e24957.
- Edwards, M.R.; Johnson, B.; Mire, C.E.; Xu, W.; Shabman, R.S.; Speller, L.N.; Leung, D.W.; Geisbert, T.W.; Amarasinghe, G.K.; Basler, C.F. The Marburg virus VP24 protein interacts with Keap1 to activate the cytoprotective antioxidant response pathway. Cell Rep. 2014, 6, 1017–1025.
- Page, A.; Volchkova, V.A.; Reid, S.P.; Mateo, M.; Bagnaud-Baule, A.; Nemirov, K.; Shurtleff, A.C.; Lawrence, P.; Reynard, O.; Ottmann, M.; et al. Marburgvirus hijacks nrf2-dependent pathway by targeting nrf2-negative regulator keap1. Cell Rep. 2014, 6, 1026–1036.
- Ma, J.Q.; Tuersun, H.; Jiao, S.J.; Zheng, J.H.; Xiao, J.B.; Hasim, A. Functional Role of NRF2 in Cervical Carcinogenesis. PLoS ONE 2015, 10, e0133876.
- Yin, Q.; McBride, J.; Fewell, C.; Lacey, M.; Wang, X.; Lin, Z.; Cameron, J.; Flemington, E.K. MicroRNA-155 is an Epstein-Barr virus-induced gene that modulates Epstein-Barr virus-regulated gene expression pathways. J. Virol. 2008, 82, 5295–5306.
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223.
- Gjyshi, O.; Flaherty, S.; Veettil, M.V.; Johnson, K.E.; Chandran, B.; Bottero, V. Kaposi’s sarcoma-associated herpesvirus induces Nrf2 activation in latently infected endothelial cells through SQSTM1 phosphorylation and interaction with polyubiquitinated Keap1. J. Virol. 2015, 89, 2268–2286.
- Gjyshi, O.; Roy, A.; Dutta, S.; Veettil, M.V.; Dutta, D.; Chandran, B. Activated Nrf2 Interacts with Kaposi’s Sarcoma-Associated Herpesvirus Latency Protein LANA-1 and Host Protein KAP1 To Mediate Global Lytic Gene Repression. J. Virol. 2015, 89, 7874–7892.
- Liu, B.; Fang, M.; He, Z.; Cui, D.; Jia, S.; Lin, X.; Xu, X.; Zhou, T.; Liu, W. Hepatitis B virus stimulates G6PD expression through HBx-mediated Nrf2 activation. Cell Death Dis. 2015, 6, e1980.
- Choi, Y.; Jiang, Z.; Shin, W.J.; Jung, J.U. Severe Fever with Thrombocytopenia Syndrome Virus NSs Interacts with TRIM21 To Activate the p62-Keap1-Nrf2 Pathway. J. Virol. 2020, 94, e01684-19.
- Smirnova, O.A.; Ivanova, O.N.; Mukhtarov, F.S.; Tunitskaya, V.L.; Jansons, J.; Isaguliants, M.G.; Kochetkov, S.N.; Ivanov, A.V. Analysis of the Domains of Hepatitis C Virus Core and NS5A Proteins that Activate the Nrf2/ARE Cascade. Acta Nat. 2016, 8, 123–127.
- Guo, H.; Zhou, T.; Jiang, D.; Cuconati, A.; Xiao, G.H.; Block, T.M.; Guo, J.T. Regulation of hepatitis B virus replication by the phosphatidylinositol 3-kinase-akt signal transduction pathway. J. Virol. 2007, 81, 10072–10080.
- Rada, P.; Rojo, A.I.; Chowdhry, S.; McMahon, M.; Hayes, J.D.; Cuadrado, A. SCF/beta-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell. Biol. 2011, 31, 1121–1133.
- Cheng, Y.L.; Lin, Y.S.; Chen, C.L.; Tsai, T.T.; Tsai, C.C.; Wu, Y.W.; Ou, Y.D.; Chu, Y.Y.; Wang, J.M.; Yu, C.Y.; et al. Activation of Nrf2 by the dengue virus causes an increase in CLEC5A, which enhances TNF-alpha production by mononuclear phagocytes. Sci. Rep. 2016, 6, 32000.
- Lee, J.; Koh, K.; Kim, Y.E.; Ahn, J.H.; Kim, S. Upregulation of Nrf2 expression by human cytomegalovirus infection protects host cells from oxidative stress. J. Gen. Virol. 2013, 94, 1658–1668.
- Gjyshi, O.; Bottero, V.; Veettil, M.V.; Dutta, S.; Singh, V.V.; Chikoti, L.; Chandran, B. Kaposi’s sarcoma-associated herpesvirus induces Nrf2 during de novo infection of endothelial cells to create a microenvironment conducive to infection. PLoS Pathog. 2014, 10, e1004460.
- Yu, J.S.; Chen, W.C.; Tseng, C.K.; Lin, C.K.; Hsu, Y.C.; Chen, Y.H.; Lee, J.C. Sulforaphane Suppresses Hepatitis C Virus Replication by Up-Regulating Heme Oxygenase-1 Expression through PI3K/Nrf2 Pathway. PLoS ONE 2016, 11, e0152236.
- Chen, M.H.; Lee, M.Y.; Chuang, J.J.; Li, Y.Z.; Ning, S.T.; Chen, J.C.; Liu, Y.W. Curcumin inhibits HCV replication by induction of heme oxygenase-1 and suppression of AKT. Int. J. Mol. Med. 2012, 30, 1021–1028.
- Tseng, C.K.; Hsu, S.P.; Lin, C.K.; Wu, Y.H.; Lee, J.C.; Young, K.C. Celastrol inhibits hepatitis C virus replication by upregulating heme oxygenase-1 via the JNK MAPK/Nrf2 pathway in human hepatoma cells. Antivir. Res. 2017, 146, 191–200.
- Zhu, Z.; Mathahs, M.M.; Schmidt, W.N. Restoration of type I interferon expression by heme and related tetrapyrroles through inhibition of NS3/4A protease. J. Infect. Dis. 2013, 208, 1653–1663.
- Fillebeen, C.; Rivas-Estilla, A.M.; Bisaillon, M.; Ponka, P.; Muckenthaler, M.; Hentze, M.W.; Koromilas, A.E.; Pantopoulos, K. Iron inactivates the RNA polymerase NS5B and suppresses subgenomic replication of hepatitis C Virus. J. Biol. Chem. 2005, 280, 9049–9057.
- McPhee, F.; Caldera, P.S.; Bemis, G.W.; McDonagh, A.F.; Kuntz, I.D.; Craik, C.S. Bile pigments as HIV-1 protease inhibitors and their effects on HIV-1 viral maturation and infectivity in vitro. Biochem. J. 1996, 320 Pt 2, 681–686.
- Yamada, Y.; Limmon, G.V.; Zheng, D.; Li, N.; Li, L.; Yin, L.; Chow, V.T.; Chen, J.; Engelward, B.P. Major shifts in the spatio-temporal distribution of lung antioxidant enzymes during influenza pneumonia. PLoS ONE 2012, 7, e31494.
- Zhang, S.; Wang, J.; Wang, L.; Aliyari, S.; Cheng, G. SARS-CoV-2 virus NSP14 Impairs NRF2/HMOX1 activation by targeting Sirtuin 1. Cell Mol. Immunol. 2022, 19, 872–882.
- Liu, L.; Du, J.; Yang, S.; Zheng, B.; Shen, J.; Huang, J.; Cao, L.; Huang, S.; Liu, X.; Guo, L.; et al. SARS-CoV-2 ORF3a sensitizes cells to ferroptosis via Keap1-NRF2 axis. Redox Biol. 2023, 63, 102752.
- Emanuele, S.; Celesia, A.; D’Anneo, A.; Lauricella, M.; Carlisi, D.; De Blasio, A.; Giuliano, M. The Good and Bad of Nrf2: An Update in Cancer and New Perspectives in COVID-19. Int. J. Mol. Sci. 2021, 22, 7963.
- Koyuncu, E.; Budayeva, H.G.; Miteva, Y.V.; Ricci, D.P.; Silhavy, T.J.; Shenk, T.; Cristea, I.M. Sirtuins are evolutionarily conserved viral restriction factors. mBio 2014, 5, e02249-14.
- Zhou, Z.; Jiang, X.; Liu, D.; Fan, Z.; Hu, X.; Yan, J.; Wang, M.; Gao, G.F. Autophagy is involved in influenza A virus replication. Autophagy 2009, 5, 321–328.
- Cummins, N.W.; Weaver, E.A.; May, S.M.; Croatt, A.J.; Foreman, O.; Kennedy, R.B.; Poland, G.A.; Barry, M.A.; Nath, K.A.; Badley, A.D. Heme oxygenase-1 regulates the immune response to influenza virus infection and vaccination in aged mice. FASEB J. 2012, 26, 2911–2918.
- Wang, C.; Zhang, Y.; Han, L.; Guo, L.; Zhong, H.; Wang, J. Hemin ameliorates influenza pneumonia by attenuating lung injury and regulating the immune response. Int. J. Antimicrob. Agents 2017, 49, 45–52.
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624.
- Thomson, B.J. Viruses and apoptosis. Int. J. Exp. Pathol. 2001, 82, 65–76.
- Shen, Y.; Shenk, T.E. Viruses and apoptosis. Curr. Opin. Genet. Dev. 1995, 5, 105–111.
- Roulston, A.; Marcellus, R.C.; Branton, P.E. Viruses and apoptosis. Annu. Rev. Microbiol. 1999, 53, 577–628.
- Everett, H.; McFadden, G. Apoptosis: An innate immune response to virus infection. Trends Microbiol. 1999, 7, 160–165.
- Li, M.; Li, J.; Zeng, R.; Yang, J.; Liu, J.; Zhang, Z.; Song, X.; Yao, Z.; Ma, C.; Li, W.; et al. Respiratory Syncytial Virus Replication Is Promoted by Autophagy-Mediated Inhibition of Apoptosis. J. Virol. 2018, 92, e02193-17.
- Wang, H.; Li, Z.; Niu, J.; Xu, Y.; Ma, L.; Lu, A.; Wang, X.; Qian, Z.; Huang, Z.; Jin, X.; et al. Antiviral effects of ferric ammonium citrate. Cell Discov. 2018, 4, 14.
- Kumar, R.; Nayak, M.; Sahoo, G.C.; Pandey, K.; Sarkar, M.C.; Ansari, Y.; Das, V.N.R.; Topno, R.K.; Bhawna; Madhukar, M.; et al. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019, 25, 325–329.
- Nencioni, L.; Iuvara, A.; Aquilano, K.; Ciriolo, M.R.; Cozzolino, F.; Rotilio, G.; Garaci, E.; Palamara, A.T. Influenza A virus replication is dependent on an antioxidant pathway that involves GSH and Bcl-2. FASEB J. 2003, 17, 758–760.
- Alsuwaidi, A.R.; Almarzooqi, S.; Albawardi, A.; Benedict, S.; Kochiyil, J.; Mustafa, F.; Hartwig, S.M.; Varga, S.M.; Souid, A.K. Cellular bioenergetics, caspase activity and glutathione in murine lungs infected with influenza A virus. Virology 2013, 446, 180–188.
- Ju, X.; Yan, Y.; Liu, Q.; Li, N.; Sheng, M.; Zhang, L.; Li, X.; Liang, Z.; Huang, F.; Liu, K.; et al. Neuraminidase of Influenza A Virus Binds Lysosome-Associated Membrane Proteins Directly and Induces Lysosome Rupture. J. Virol. 2015, 89, 10347–10358.
- Edeas, M.; Saleh, J.; Peyssonnaux, C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int. J. Infect. Dis. 2020, 97, 303–305.
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin. Pract. 2020, 10, 1271.
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062.
- Polonikov, A. Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in COVID-19 Patients. ACS Infect. Dis. 2020, 6, 1558–1562.
- Yue, Y.; Nabar, N.R.; Shi, C.S.; Kamenyeva, O.; Xiao, X.; Hwang, I.Y.; Wang, M.; Kehrl, J.H. SARS-Coronavirus Open Reading Frame-3a drives multimodal necrotic cell death. Cell Death Dis. 2018, 9, 904.
- Salimi, V.; Ramezani, A.; Mirzaei, H.; Tahamtan, A.; Faghihloo, E.; Rezaei, F.; Naseri, M.; Bont, L.; Mokhtari-Azad, T.; Tavakoli-Yaraki, M. Evaluation of the expression level of 12/15 lipoxygenase and the related inflammatory factors (CCL5, CCL3) in respiratory syncytial virus infection in mice model. Microb. Pathog. 2017, 109, 209–213.
- Lin, T.Y.; Chu, C.; Chiu, C.H. Lactoferrin inhibits enterovirus 71 infection of human embryonal rhabdomyosarcoma cells in vitro. J. Infect. Dis. 2002, 186, 1161–1164.
- Ilback, N.G.; Frisk, P.; Mohamed, N.; Gadhasson, I.L.; Blomberg, J.; Friman, G. Virus induces metal-binding proteins and changed trace element balance in the brain during the course of a common human infection (coxsackievirus B3) in mice. Sci. Total Env. 2007, 381, 88–98.
- Wang, M.P.; Joshua, B.; Jin, N.Y.; Du, S.W.; Li, C. Ferroptosis in viral infection: The unexplored possibility. Acta Pharmacol. Sin. 2022, 43, 1905–1915.
More
