1. Genomic, Mutational and Epigenomic Signatures of HGSTOC Tumor Microenvironment
1.1. Cancer-Associated Fibroblasts (CAFs)
In addition to the identification of HGSTOC epithelial cell subtypes (EC1–EC5—described above), Hao et al.
[1][33] recognized five HGSTOC-isolated fibroblast cell subtypes. The FC1 subtype showed gene enrichment for lipid and steroid metabolism. The FC2 subtype exhibited a preference for genes involved in glycolysis/gluconeogenesis, oxidative phosphorylation, and DNA repair pathways. Moreover, the FC2 population showed expression of tumor epithelial markers and markers of EMT. These cells represent the highly aggressive phenotype of CAFs enhancing tumor chemo-resistance. The FC3 cells were enriched in the genes responsible for the immune response-related pathways. In the FC4 subtype, the most prominent over-expression of angiogenesis-related genes was observed. The next FC5 subtype showed high expression of genes regulating lipid metabolic pathways, extracellular matrix signaling, and cellular stemness
[1][33]. The importance of interactions between cancer cells and CAFs was represented by the expression of relatively high levels of epithelial and fibroblast growth factor receptors on cancer cells and their corresponding ligands on the CAF cells. Some receptor-ligand interactions were expressed at higher levels in metastatic compared to primary tumors, emphasizing their importance in cancer progression.
The study performed by Givel et al.
[2][55] identified four different CAF subsets named CAF-S1 to CAF-S4. It was shown that HGSTOC of the mesenchymal subtype, defined by stromal gene signatures and poor survival, had high numbers of CAF-S1 cells that attract and maintain an immunosuppressive infiltration of Treg CD25+ FoxP3+ T lymphocytes through the expression of CXCL12
β and miR-141/200a dependent-mechanism
[2][55].
The study of the immunological profile of HGSTOC identified a novel immune group of HGSTOC with high infiltration of immune cells, lower chromosomal aberrations, increased neo-antigens, high tumor mutation burden, and microsatellite instability. Tumors belonging to the immune class showed enrichment of immune cell signatures, like T cells, B cells, cytotoxic cells, tertiary lymphoid structures (TLS), macrophages, as well as NK cells and PD-1 signaling. According to the tumor microenvironment, the immune group was divided into two microenvironment-based subsets, namely activated-immune and CAFs-immune subtypes. Both subtypes exhibited high expression of immune molecules; however, the activated-immune subtype showed anti-tumor features exemplified by enrichment of IFN signatures, active immune response, and better prognosis. The CAFs-immune subtype was characterized by tumor-promoting signals like activated stroma, M2 macrophages, WNT/TGF-
β signaling pathway, and a poor prognosis. The activated-immune subtype was more likely than the CAFs-immune subtype to respond to checkpoint blockade immunotherapy
[3][56].
Similar results were obtained in another study. The CAF content was described as the CAF-score. Analysis of immune cell infiltration showed that the infiltration of memory B cells, T-helper cells, T regulatory cells, activated natural killer cells, and dendritic cells was higher, while the infiltration of memory CD4 T cells, M2 macrophages, and neutrophils was significantly lower in tumors with a low CAF-score. A low CAF-score indicated a better prognosis and identified a group of patients with more immunogenic tumors who may be good candidates for immune checkpoint inhibitor therapy
[4][57].
Transcriptome profiles from ovarian cancer CAFs identified two distinct subtypes of CAFs in HGSTOC tumors, including CAF-N and CAF-C. The CAF-C subtype was characterized by more aggressive behavior and a worse prognosis. Therapeutic use of calcitriol in tumor xenograft mice was able to inhibit Smad signaling in CAF-C cells and prolong median mouse survival
[5][58].
1.2. Tumor Microenvironment Different Cell Populations
The study performed by Olbrecht et al.
[6][59] showed HGSTOC cell clusters originating from ovarian, peritoneal, and omental tissue which were divided into 8 major cell types, including epithelial cancer cells, myeloid cells, dendritic cells (DCs), T cells (TCs), B cells (BCs), fibroblasts (FBs), endothelial cells (ECs) and ovarian stromal cells (OSCs). Based on differential gene expression analysis, cells isolated from omental implants were identified as Langerhans-like dendritic cells and lipid-associated M2 macrophages. In several tumor clusters, the genes of fallopian tube secretory cells were over-expressed, confirming the fimbrial origin of HGSTOC tumors
[6][7][59,60]. The keratin-17 gene-positive cancer cells were represented in both the primary and metastatic localizations indicating that tumor cells at different places show similar transcriptomic profiles, and the observed heterogeneity should be attributed mostly to the differences in stromal cells. Some clusters had prognostic relevance. Mesothelial cells showed active EMT and activation of the IL6/STAT3 signaling pathway, analogically to the fibroblast cluster from the Izar et al. study
[8][61], and were correlated to tumor growth and chemo-resistance. Myofibroblasts were engaged in hypoxia-mediated EMT, active TGF-
β-pathway, and promotion of metastases. Cancer-associated fibroblasts were TGF-
β-dependent and enhanced tumor growth, EMT, metastases, and platinum-resistance. Lymphatic endothelial cells were responsible for the lymphatic spread of metastases. The next prognostic cluster was represented by BMP and activin membrane-bound inhibitor homolog (BAMBI)-expressing tumor cells. Over-expression of BAMBI was observed in recurrent, but not in primary HGSTOC tumors, and was supposed to be one of the markers of platinum-resistance. The cluster represented by plasma cells was a good prognostic marker of an immune-active anti-tumor environment
[6][59].
1.3. Tumor Microenvironment Immune Cell Populations
The study of immune-related gene pairs seems to confirm these observations and enables to differentiation of ovarian cancer patients into high- and low-risk groups according to the clinical outcome. The Toll-like receptor and chemokine signaling pathways were negatively correlated with the risk scores, indicating that the low-risk group tumors had immune infiltration of higher activation status. Contrarily, the p53 signaling and apoptosis pathways had a positive correlation with the risk scores. In addition, the authors also found that the best prognosis was obtained in the immunoreactive, while the worst prognosis was in the mesenchymal ovarian cancer subtype, respectively
[9][62].
Bioinformatics analysis of the expression of the C-X-C motif chemokine receptors (CXCRs) in ovarian cancer indicated higher expression of CXCR3, -4, -7 mRNA and different expression of CXCR1, -2, -3, -4, -7 mRNA in different pathological types of ovarian tumors. High CXCR7 mRNA expression and low CXCR5, -6 expression were associated with unfavorable OS, while high CXCR4, -7 expression and low CXCR5, -6 expression were associated with a decrease of PFS
[10][63]. The role of CXCRs is multifunctional. The CXCR1/CXCR2 are the receptors for IL-8, which is over-expressed in ovarian cancer patients both in serum and ascites
[11][64]. CXCR3 is expressed on activated T, B, and NK cells and mediates ascites-directed tumor cell migration. Is correlated with poor survival in several cancers including ovarian cancer
[12][65]. Over-expressed CXCR4 enhances proliferation and invasion of cancer cells through positive regulation of EMT via CXCR4/CXCL12 signaling, stimulates cellular stemness and chemo-resistance, and in consequence worsens PFS in ovarian cancer patients
[13][14][66,67]. Similarly, CXCR5/CXCL13 signaling regulates EMT and negatively correlates to OS and PFS in ovarian cancer
[10][15][63,68]. The CXCR6/CXCL16 signaling promotes cancer growth by activation of the PI3K/AKT pathway and is associated with TAMs function, expression of TNF-
α, and docetaxel-resistance
[16][69]. The CXCR7 has a high affinity to CXCL12 and transports signal for metalloproteinase-9 over-expression, as well as through CXCR7/CCL19 signaling up-regulates mesenchymal phenotype, thus stimulating metastases
[17][18][70,71]. The CXCR3/4/7 could be potential therapeutic targets in ovarian cancer. The CXCR4 inhibitor AMD3100 was successfully used in in vitro studies and mouse xenograft models of ovarian cancer to restore taxol chemo-sensitivity and prolong survival
[19][20][72,73].
An immune-related lncRNA pairing model for predicting tumor immune infiltration and cancer prognosis was constructed based on the expression of seven lncRNA pairs. The high-risk lncRNA signatures correlated with tumor infiltration with macrophages, neutrophils, T and mast cells, and CAFs. Moreover, the tumors with high-risk signatures showed low expression of immune checkpoint-related genes
[21][51].
1.4. Tumor Microenvironment Cell Populations in Ascites
Izar et al.
[8][61] analyzed cellular populations of HGSTOC ascites using single-cell RNA-sequencing and demonstrated significant variability in cellular states among malignant and non-malignant cells. The identified populations were composed of epithelial cells, cancer-associated fibroblasts, dendritic cells, B cells, T cells, and erythrocytes. Among CAFs, there were identified distinct cell states, including sub-populations with expression of immune-related genes, such as complement factors and cytokines. Among macrophages generally, two groups were identified. Group 1 cells co-expressed several genes identified as markers of M1-type macrophages and suppressors of M2 differentiation, whereas Group 2 cells expressed genes regulating M2 differentiation. Authors concluded that the previously described “immunoreactive” and “mesenchymal” subtypes of HGSTOC, reflected the abundance of immune infiltrates and fibroblasts rather than distinct subsets of malignant cells
[8][61].
The cellular components of ascites include either single cells or cell aggregates called spheroids. The single-cell population was composed mostly of immune cells, some tumor cells, and CD90+ mesenchymal-like cells. The spheroid population was composed almost exclusively of EpCAM+ and CD24+ tumor cells. Ascitic spheroids showed, compared to the primary or metastatic ovarian cancer cells, significantly up-regulated genes related to the oxidative phosphorylation pathways, chemo-resistance, encoding glycosylation enzymes, and transcription factors. Over-expressed protein-glutamine gamma-glutamyltransferase K gene (
TGM1) and up-regulated heat shock proteins in the spheroid cells promoted their stemness and chemo-resistance, whereas proteins with mechanical barrier function, supporting cellular aggregation (plakophillin, periplakin, claudin, filaggrin) could protect cancer cells from immune recognition and destruction
[22][74]. Spheroids contain M2-type tumor-associated macrophages (TAMs) and also CAFs which together enhance the aggregation and adhesion of these tumor cell aggregates
[23][75]. The spheroids indicated down-regulation of angiogenesis and extra-cellular structure organization pathways and significant up-regulation of the mitochondrial oxidative phosphorylation (OXPHOS) pathway. This is typical for quiescent cells with low proliferation profile and low chemo-sensitivity, as are spheroids
[24][25][76,77]. Drugs inhibiting the OXPHOS pathway could be considered in ovarian cancer therapy. Potential OXPHOS inhibitors include metformin, which was tested in pre-clinical and clinical studies with conflicting results, showing either its anti-tumor effects or denying its clinical efficacy
[26][78]. The reason for these observations might lie in the relative, but not complete, sensitivity to OXPHOS inhibitors
[22][74].
The immune TME in malignant ascites was also characterized by transcriptomic analysis and used to construct a tumor-associated macrophage-related gene (TAMRG) prognostic signature. As expected, M1-type TAMs correlated positively with the patient’s OS, whereas M2-type TAMs, memory T CD4+ lymphocytes, neutrophils, and mast cells exhibited negative correlation, respectively. The TAMRG-based gene signature was associated with poor or favorable prognosis, depending on the gene expression profile
[27][79].
1.5. Lipid Metabolism in Tumor Microenvironment
Lipid metabolism is extremely important for the growth and nutrition of peritoneal and omental implants and ovarian cancer stem cells, therefore a prognostic model based on eleven lipid metabolism gene signatures was proposed. The expression levels of several genes regulating drug-mediated and p53-mediated apoptosis, the proliferative and migratory properties of tumor cells, and the recruitment of immune cells to the tumor allowed the differentiation of patients into high- and low-risk groups
[28][29][30][31][32][80,81,82,83,84].
1.6. Mechanisms of the Regulated Cell Death (RCD)
1.6.1. Autophagy
Autophagy is a lysosomal-driven form of genetically determined regulated cell death (RCD). In response to multiple stressors and malnutrition, cells can control self-digestion enable survival, and maintains homeostasis. Autophagy functions as a double-edged sword, as in early cancer stages prevents tumor progression, however, in advanced tumors augments cancer stem cells and helps to survive hypoxia, drug-derived stress, and starvation. Therefore, inhibiting autophagy could be beneficial for the elimination of the tumor
[33][34][85,86]. Seven autophagy-related genes have been identified as regulators of tumor immune infiltration and predictors of prognosis
[35][87]. All the marker genes had associations with
BRCA1 and the immune pathway in ovarian cancer. Consequently, the numbers of tumor-infiltrating T, B, NK, Treg cells, neutrophils, and macrophages differed between high- and low-risk tumors. The marker genes also affect the signaling pathways, including P53-related, PI3K/AKT/mTOR, and IL-6/JAK/STAT3 pathways
[35][87].
The study on lncRNA expression in ovarian cancer CAFs allowed the identification of several lncRNAs involved in metabolic processes and regulation of autophagy, the increase of which was correlated with a worse prognosis of patients. Another
MIR155HG lncRNA was responsible for the regulation of genes associated with the function of the immune system, especially T cell activation, antigen presentation, cytokine signaling, and ECM interactions. Tumors with high
MIR155HG expression had significantly higher numbers of activated T cells, M1-type macrophages, cytotoxic T CD8+ cells, and T CD4+ helper cells, and were correlated with more favorable outcomes
[36][88].
1.6.2. Ferroptosis
Except for apoptosis or autophagy, the two recently discovered forms of RCD are ferroptosis and necroptosis
[37][89]. Ferroptosis is an iron-dependent mode of cell death, which is characterized by cytological changes in mitochondrial structure caused by the imbalance between the production and degradation of intracellular lipid reactive oxygen species. Ferroptosis eliminates malignant cells damaged by nutritional deficiency or stress
[38][90]. In the ferroptosis-related prognostic model, the expression of genes regulating cell degradation and its recycling, defense reactions against oxidative agents, integrin-dependent signaling related to normal cell growth and tumorigenesis, the reaction of lysosomal exocytosis, prostaglandin synthesis, iron-sulfur regulation of cellular enzymes, transmembrane energy transporting, and fatty acids metabolism were correlated with patient’s OS
[39][91]. The next study showed similarly that expression of ferroptosis-related genes including lncRNAs were correlated with prognosis in ovarian cancer patients. The prognostic model based on the 8 lncRNAs enabled the division of patients into high- and low-risk groups presented with differential clinical outcomes, mutation burden, and immune cell infiltration, and to predict the response to immunotherapy. Moreover, the expression of lncRNA RP11 correlated with the platinum sensitivity
[40][92].
Another lncRNA signature was based on a comprehensive analysis of ferroptosis and iron metabolism-related lncRNAs (FIRLs). Patients with different FIRL signatures would be expected to respond disparately to immunotherapy. The high-risk FIRL signature differed according to the extent of infiltration by B cells, T CD4+ memory cells, NK cells, and macrophages, and high-risk FIRL scores were correlated to higher immunosuppression in the tumor environment. In this group of patients, one could anticipate a better response to immune checkpoint inhibitors-based therapy. Moreover, the low-risk FIRL signature patients were more sensitive to docetaxel, doxorubicin, etoposide, paclitaxel, cisplatin, and gemcitabine
[41][93].
1.6.3. Necroptosis
Necroptosis is regulated programmed cell necrosis, morphologically exhibiting the same features as necrosis, and mediated by pro-inflammatory cytokines (TNF-
α, IFN-
α, and IFN-γ), Toll-like receptors (TLR3, TLR4, and TLR9), and nucleic acid (DNA and RNA) receptors. The receptor-interacting serine/threonine kinase1 and 3 (RIPK1/3) and mixed lineage kinase domain-like pseudokinase (MLKL) are important proteins involved in the development of necroptosis
[42][94]. Necroptosis could either indirectly enhance (via stimulation of inflammation) or directly inhibit tumorigenesis
[43][95]. In the necroptosis-related model, the expression of genes regulating transcription activators, calcium-mediated signaling, nucleosome structure of the chromosomal fibers, activation of Janus protein kinases and death-inducing signaling pathway, initiation of an inflammatory response, and metabolism of glucose in stress conditions were identified as factors determining patient’s OS
[39][91]. Another study devoted to necroptosis-related gene signatures identified 33 differentially expressed genes (DEGs) engaged in the regulation of necroptosis. Among genes playing a central role in the tumorigenesis and development of ovarian cancer, were those involved in TNF-
α and NF-
ĸB signaling pathways
[44][96]. Then authors constructed a risk model based on the regression analysis of a 5-gene signature which successfully differentiated patients into high or low-risk groups, with high-risk patients indicating significantly shorter survival, a decrease in expression of immune checkpoint proteins, and chemo-resistance
[44][96].
1.7. Summary
In summary, the commonly known dualistic model dividing ovarian tumors into two distinct populations (type I and type II) characterized by different genetic landscapes, biology, and prognosis, has been recently found to be more complex. At least four different tumor types have been identified, namely subtypes with high stromal response, immune signature, low stromal response, and mesenchymal subtype, with low immune signature. Several studies have confirmed such division showing the presence of similar subtypes defined as proliferative, immunoreactive, differentiated, and mesenchymal tumors
[45][46][47][48][49][17,24,25,26,27]. All other classifications more or less correspond to these four basic subtypes
[1][50][51][52][32,33,37,38]. All these studies indicate, that HGSTOC cancer has different biological and clinical behaviors depending on the genetic profile and mutational burden in cancer cells, but the cellular composition of the tumor, mainly concerning the cells of both stromal and immune lineage seems to be another important determinant of tumor growth. Similarly, to cancer subtypes, the cells of the tumor microenvironment have different functional signatures which have prognostic meaning for the patients. Altogether this means, that different ovarian cancer subtypes have diverse immunological host responses, and could diversely respond to targeted therapy, therefore demanding different therapeutic approaches
[51][37]. Moreover, diversified expression of several genes both in cancer cells themselves and in tumor microenvironment cells, could discriminate between chemo-resistant and chemo-sensitive ovarian tumors
[53][36]. Molecular profiling indicates also that in primary HGSTOC tumors, there is a significant heterogeneity with the co-existence of several gene signatures determining its resultant phenotype and behavior, however, peritoneal cancer metastases show a reduction of heterogeneity and expansion of selected tumor cell population
[52][38]. They also differ according to stromal and immune cell composition. That points out a potential necessity to use a different therapeutic approach to primary tumors, peritoneal implants, and recurrences.
2. Personalization of Treatment—State of Art and Future Directions
The attempts to get the heterogeneity of HGSTOC right have driven to the diversification of tumors into different genotypes and functional phenotypes with distinct clinical behaviors and sensitivity to drugs. Moreover, the environments of primary tumors, peritoneal implants, and ascites have been characterized by different gene signatures giving the possibility to modify the therapy according to the tumor localization and temporal changes in the course of the disease. Identification of the hub genes and their downstream pathways and targets could enable the selection of the candidate drugs for ovarian cancer treatment and improve the personalized approach to the therapy. This could change the philosophy of therapy not only in ovarian cancer but in cancer generally. An example of such an approach is an ongoing nonrandomized, multicenter phase II TAPUR clinical trial (NCT02693535) testing the use of drugs already approved by the FDA that target a specific tumor mutation in individuals with advanced cancer according to their molecular profile regardless of the tissue origin or cancer type
[54][99]. Two study cohorts have been closed due to a lack of anti-tumor activity, but 12 cohorts have expanded to the second stage of enrollment due to promising preliminary activity. The next phase II clinical trial called NCI-MATCH (NCT02465060) attempted to answer the question of how effective treatment was based on genetic profiling in patients with solid tumors or lymphomas that had progressed despite at least one line of standard treatment
[55][100]. The study demonstrated that next-generation sequencing in biopsy specimens from patients with relapsed-refractory cancer enables the qualification of nearly 20% of patients to evidence-based investigational therapy. However, co-occurring resistance mutations were common and that fact needs investigation of drug combination therapy regimens
[56][101]. Another example of individualized therapy was a prospective MOSCATO 01 clinical trial. Material extracted from fresh-frozen tumor biopsies was analyzed by array comparative genomic hybridization, next-generation sequencing, and RNA sequencing. Patients were treated based on their genomic signature, and the PFS2 was compared to the PFS 1 from the recently performed therapy. An actionable molecular alteration was identified in 49% of patients and 24% of patients were treated with a targeted therapy matched to a genomic alteration. The PFS2/PFS1 ratio was >1.3 in 33% of the treated patients
[57][102]. In the next similar trial, patients with metastatic solid tumors who had progressed on at least one line of standard-of-care therapy were referred to the Indiana University Health Precision Genomics Program. Tumor samples were studied by DNA & RNA next-generation sequencing, fluorescence in situ hybridization, and immunohistochemistry to find actionable targets. Altogether, 43% of patients treated with genomically guided therapy had a PFS ratio ≥1.3, whereas only 5% of patients treated with standard therapy had reached that result. Further, patients treated with genomically guided therapy had a superior median PFS (86 days vs. 49 days). The main limitation of the study was decision-making by a multi-disciplinary tumor board instead of an objective algorithm
[58][103]. Another study compared the PFS in the cohort of patients with metastatic cancer of diverse subtypes who received genomic testing and targeted therapy (precision medicine), and control patients who received standard chemotherapy or best supportive care. The PFS was 23 weeks for the precision medicine group and 12 weeks for the control group, and although the inclusion of patients with supportive care could bias the results, it seemed that precision medicine may improve survival for patients with refractory cancer
[59][104]. All these examples speak for the personalization of the treatment and implementation of drug combination therapy. Taking into consideration the aggressive course of HGSTOC personalization of the treatment is not just an option, but rather the only reasonable way and necessity.
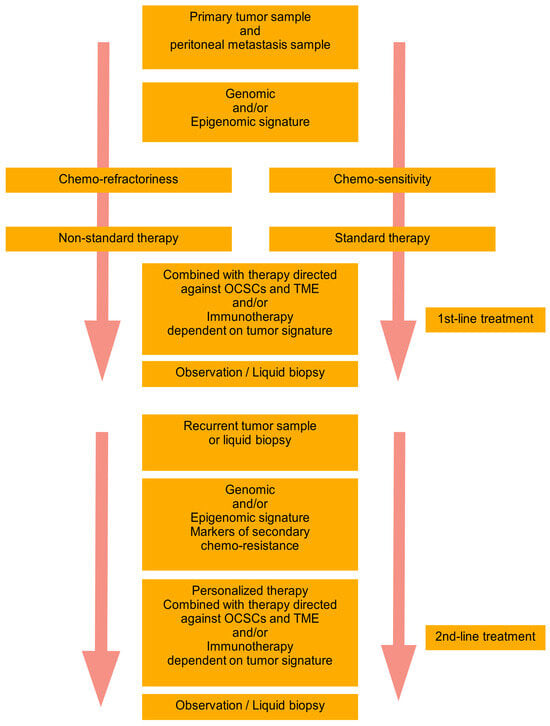
Based on the recent trends and achievements in the field of HGSTOC diversity, there is a need to create a novel complex personalized model of therapy. The researchers have postulated the basic premises of such a model called “DEPHENCE” system
[60][10]. During primary cytoreductive surgery, the samples of the primary tumor and peritoneal metastases are collected for the identification of genomic and/or epigenomic signatures. A tumor could be also sampled during exploratory laparoscopy before neoadjuvant chemotherapy. The identification of tumor signature and prognosis of chemo-sensitivity is the base for planning the following therapy. In the case of anticipated chemo-refractoriness, the non-standard therapy is started, whereas in the case of expected chemo-sensitivity, the standard chemotherapy is started. However, independently of the basic therapy, multimodal supplementary treatment is always introduced, including anti-ovarian cancer stem cell- and tumor environment components-related therapy, combined with immunotherapy or immune-potentization of the patient’s immune system. After the first line of treatment observation of the patient with a classical approach supplemented with repetitive liquid biopsies to monitor for response to therapy and eventually the recurrence are performed. In the case of recurrence, the tumor is again biopsied, or liquid biopsy is performed (it is a better option since ctDNA is freed from all tumor localizations, while harvest of the tissue during ordinary biopsy gives material from only one localization). Again, the genomic/epigenomic signature of the tumor is tested together with markers of chemo-resistance and appropriate personalized therapy is implemented. Similarly, to the first-line treatment, the second-line of therapy is also supplemented with anti-stem cell and anti-tumor environment drugs and/or immunotherapy. Due to the ovarian cancer genomic heterogeneity and temporal heterogeneity, the therapy should be tailored to the tumor genotype/phenotype and may be completely different in consecutive lines of treatment. After the second-line therapy patient is again subjected to observation and liquid biopsy, until another recurrence (
Figure 1). The researchers have met with the opinion that such a model of therapy is non-realistic. It is true for now, but searching for the hidden order in the heterogenous HGSTOC tumors will sooner or later bring therapeutically important conclusions. Novel genetic tests and techniques are increasingly more available and cheaper. Bioinformatics enables the designing of new drugs instead of a blind search. So let us be a little more optimistic, as the history of science has taught us, that progress has many times come faster than it was expected. There is a reasonable assumption in the HGSTOC therapy to adjust the lines of treatment to the unique subtype of tumor, as well as its spatial (primary/metastatic tumor) and temporal (primary/recurrent tumor) characteristics. Without the multidirectional approach, this extremely lethal cancer will still be a step forward.
Figure 1. “DEPHENCE” system in ovarian cancer treatment. During primary cytoreductive surgery, the samples of the primary tumor and peritoneal metastases are collected for identification of genomic and/or epigenomic signatures. A tumor could be also sampled during exploratory laparoscopy before neoadjuvant chemotherapy. The prognosis of chemo-sensitivity or chemo-refractoriness is a base for planning the therapy. In the case of anticipated chemo-refractoriness, the non-standard therapy is started, whereas in the case of expected chemo-sensitivity, the standard chemotherapy is started. However, independently of the basic therapy, multimodal supplementary treatment is always introduced, including anti-OCSCs and TME therapy combined with immunotherapy or immune-potentization of the patient’s immune system. After the first line of treatment observation of the patient with a classical approach supplemented with repetitive liquid biopsies to monitor for response to therapy and eventually the recurrence are performed. In the case of recurrence, the tumor is again biopsied, or liquid biopsy is performed (it is a better option since ctDNA is freed from all tumor localizations, while harvest of the tissue during ordinary biopsy gives material from only one localization). Again, the genomic/epigenomic signature of the tumor is tested together with markers of chemo-resistance and appropriate personalized therapy is implemented. Similarly, to the first-line treatment, the second-line of therapy is also supplemented with anti-OCSCs and anti-TME drugs and/or immunotherapy. Due to the ovarian cancer genomic heterogeneity and temporal heterogeneity, the therapy should be tailored to the tumor genotype/phenotype and may be completely different in consecutive lines of treatment. After the second-line therapy patient is again subjected to observation and liquid biopsy, until another recurrence. OCSCs—ovarian cancer stem cells; TME—tumor microenvironment; The arrow represents Time.