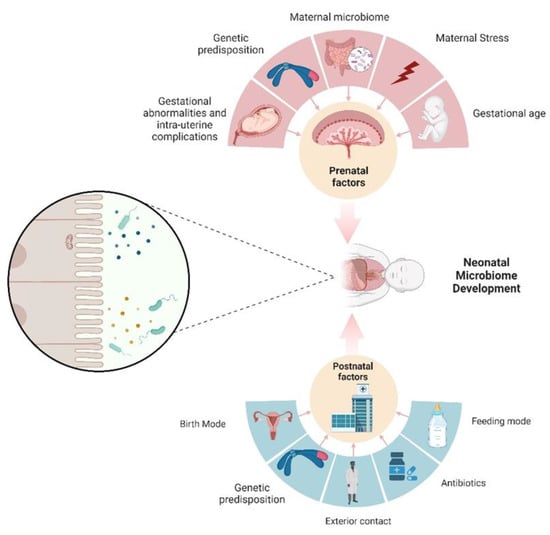
2. The Neonatal Microbiome
The human gut microbiome exhibits remarkable richness and harbors dynamic populations of microorganisms, primarily characterized by the dominance of bacterial communities. This ecosystem comprises approximately 3.8 × 10
13 cells, collectively bearing a genome that surpasses the human genome by approximately 150-fold
[10][16]. The multitude of trillions of cells residing within the gut represents the most abundant microbial population, and their pivotal role in host development and overall health is mediated via direct interactions with the host or through the influence of various metabolites. These interactions occur within the context of a highly homeostatic ecosystem, often referred to as the host–symbiont or holobiont. This conceptual framework acknowledges the integral role of coevolution in shaping the composition of the gut microbiome and its impact on human development. Consequently, any disruption in the long- or short-term selective pressures acting on the microbiome is bound to have significant consequences for neonatal development
[11][12][13][17,18,19].
The objective of this
resear
chticle is to establish a consensus regarding the onset of neonatal microbiome development (
Figure 1). Historically, the prevailing view has held that the intrauterine environment is devoid of microorganisms, with gut colonization commencing only at the moment of birth
[14][20]. Within this paradigm, researchers who have investigated the post-birth neonatal microbiome have portrayed it as an initial set of microbial sets that undergo maturation and progression into a more intricate microbiome, characterized by an enrichment of
Bacteroides and
Firmicutes, which are representative of an adult-like microbiome
[15][21]. Within this perspective, the colonization of the neonatal gut constitutes a de novo construction of a microbial community and is influenced by a multitude of factors. These factors include considerations such as age, dietary regimen, method of delivery, concurrent health conditions, antibiotic usage, and the birthing environment of the infant (NICU)
[16][17][22,23]. The relationship between neonatal age and the mode of birth presents an intriguing connection, given that a significant proportion of premature infants are delivered via a C-section
[18][24]. It is important to note that the microbiota of preterm infants are observed to contribute to the maintenance of an already fragile innate immune system. Consequently, any aberrant colonization of the gut microbiota may lead to unfavorable outcomes
[19][25].
3. Microbial Transfer and Postnatal Influences
The concept of a placental microbiome remains “again” a subject of considerable debate
[20][28]. Aagaard et al. reported the presence of a microbiome in the placenta, resembling that of the oral cavity, under sterile conditions
[21][29]. Subsequent investigators have corroborated Aagaard’s findings and argued that if there is a microbiome in blood and other human niches previously deemed sterile, it logically follows that there should be a distinct microbiome in the placenta as well
[21][22][29,30]. Another intriguing discovery from the same research project involved the investigation of a potential connection between specific placental microbiome types and neonatal outcomes. They identified distinct placental taxa that correlated with preterm birth, shedding light on a potential link between the placental microbiome and adverse pregnancy outcomes
[21][29]. Conversely, other investigators have questioned this entity, and argued that the human placenta does not have a microbiome but it does represent a potential site for microbial acquisition. De Goffau et al. employed distinct methods for DNA extraction and detection, and they based their conclusions on several factors. One key factor was the notably low bacterial sequence biomass in DNA extracted from the placenta, with a significant portion attributed to potential contamination during labor, delivery, or laboratory processes
[23][24][31,32].
The transmission of specific bacterial taxa is heavily dependent on the mode of birth. Infants either inherit a microbial package resembling that found in the mother’s birth canal, or this maternal–fetal microbial overlap is lost when neonates are delivered via a cesarean section
[25][34]. In the case of a cesarean section, other factors appear to have a more significant influence on the postnatal microbiome as compared to the prenatal microbiome. These factors primarily include environmental elements such as delivery and surgical equipment, healthcare workers, and contact with other neonates
[16][22].
Lactobacillus appears to be a prominent feature of vaginal delivery, being highly abundant, particularly in the maternal vagina. Infants born via cesarean section exhibited a consistently low detection rate of
Lactobacilli for up to 6 months after birth. Surprisingly, this disparity in bacterial taxa disappears by the time the infants reach three years of age
[26][35].
Similarly, medication, particularly antibiotics, has been implicated in causing deleterious effects on the human gut microbiome, particularly when administered during the early neonatal phase
[27][28][29][45,46,47]. The microbial alterations induced by antibiotics are deemed to have adverse implications for the well-being and prospective development of newborns. Descriptively, antibiotic-triggered modifications to the microbiome appear to affect distinct facets, specifically, diversity, temporal stability, and quantitative distribution
[30][48].
Environmental factors have also been documented to exert an influence on the neonatal microbiome. Studies have revealed disparities, particularly in terms of both the diversity and predominant bacterial types, contingent upon the birthplace. At the compositional level, infants born in hospital settings tend to exhibit lower levels of
Bacteroides,
Bifidobacterium,
Streptococcus, and
Lactobacillus, while displaying higher proportions of the
Clostridium and
Enterobacteriaceae families compared to infants born at home
[31][49]. Notably, these disparities are reflective of a proinflammatory phenotype, marked by an overexpression of various inflammatory markers in infants born in hospital environments
[32][50]. It is essential to emphasize, nevertheless, that some studies have demonstrated that the place of birth plays a role in the divergence of an initially similar microbiome. In other words, the initial bacterial colonization does not differ significantly between the two groups
[33][51].
4. Prenatal Influences on Neonatal Microbiome
The concept of Developmental Origins of Health and Disease (DOHaD) encompasses a field of investigations proposing that detrimental exposures occurring in the early stages of life, while tissues and organs are undergoing development, may elevate the susceptibility to diseases later in life
[34][35][52,53]. The majority of embryonic development and organogenesis happens during the intrauterine period, where the fertilized oocyte develops into a coordinated assembly of interconnected organs. In alignment with this paradigm, compelling epidemiological evidence and experimental data in animal models have substantiated a robust association between the intrauterine environment, often represented by the maternal factors, and the subsequent risk of infants developing diseases in later stages of life
[36][37][54,55].
The concept of allostatic load finds its manifestation in the context of pregnancy, which can be regarded as a unique state of “allostasis” wherein the maternal–fetal dyad faces the intricate challenge of combining the dual objectives of maternal and fetal well-being across the course of development
[38][56]. Maternal adversity experienced during pregnancy can be predominantly associated with environmental factors. The spectrum of maternal stressors is expansive and profoundly impactful, including factors such as partner violence, healthcare accessibility, housing conditions, experiences of humiliation, racial discrimination, and a heightened sense of danger. Many of these stressors can be comprehensively categorized under the broader umbrella of social determinants of health
[39][57]. The mechanisms through which maternal adversity can influence microbiome development are interconnected with dysregulation of the hypothalamo-pituitary axis, pronounced cytokine secretion, direct placental effects, and metabolic alterations
[40][58]. In a rodent model assessing stress during pregnancy, it has been illustrated that maternal stress exerts an influence on the postnatal colonic microbiome, manifesting from postnatal day 2 through postnatal day 28. Notably, a disrupted microbiome structure was detected in male offspring as they displayed characteristics akin to the microbiome patterns typically associated with females, thus suggesting a linkage between stress, hormonal factors, and the gut microbiome
[41][59]. From a descriptive perspective, the stress-induced alterations in the microbiome encompassed a concurrent proliferation of facultative anaerobic microorganisms at the detriment of obligate anaerobes
[42][43][60,61]. This observation is noteworthy, as it implies that specific taxa of facultative anaerobes, such as
Mucispirillum and
Desulfovibrionaceae, collectively possess the capacity for mucin degradation and the production of hydrogen sulfide
[34][52]. These changes are regarded as a plausible foundation for the initiation of intestinal inflammation
[44][45][62,63].
Maternal dietary patterns have the potential to impact the developing fetus through multiple mechanisms
[46][67], and it is widely hypothesized that one of these mechanisms involves influencing the composition of the neonatal microbiome. A study investigating the microbial DNA in the amniotic fluid and placenta from pregnant mothers administered probiotic compounds and revealed a significant alteration in innate immunity gene patterns
[47][68]. In this direction, specific probiotic bacterial-derived metabolites show promise as potential perinatal therapeutic interventions
[48][69]. Numerous medications have been identified as having adverse effects on fetal development
[49][70]. Nonetheless, only a limited number of drugs have been recognized for their ability to mitigate certain effects through the modulation of the maternal and fetal microbiome, with antibiotics being one notable example. In animal models, when administered to pregnant dams, it was noted that there were significant changes in the neonatal gut microbiome, a reduction in intestinal host defense, and an elevated risk of neonatal sepsis. These observations were attributed to a potential decrease in the transmission of bacteria during or shortly after delivery. It is also plausible, however, that maternal antibiotics may limit the colonization of the fetal gut by bacteria even before birth, leading to an atypical immune priming
[50][71].
5. Interpreting Varied Microbiome Profiles
Up to this point,
the reswe
archers have discussed the factors contributing to a variable microbiome in neonates. The continuity involves pinpointing the relationships between specific microbiome taxa and particular health outcomes. It is imperative to acknowledge that an infant’s microbiota are inherently distinguished by lower bacterial abundance and diversity. As the infant matures, the microbiota progressively become more complex. Analogously, the rudimentary, less diverse microbiota in early infancy can be likened to a solid foundation for a building. Should this foundation be flawed, any structure constructed upon it is predisposed to instability and eventual deterioration
[51][52][53][72,73,74]. Considering this perspective, particular microbiome profiles can be directly associated with specific diseases. In-depth research has demonstrated that disruptions in early-life microbiota are conducive to the development of obesity induced by a high-fat diet; further investigation showed that these alterations are primarily instigated by the depletion of
Lactobacillus species within the gut microbiota
[35][53]. In the same experimental model, it elucidated the decrease in Lactobacillus-derived metabolites, particularly phenyllactic acid, known to activate peroxisome proliferator-activated receptor γ (PPAR-γ), a key regulator of lipid metabolism
[54][75]. Another illustration of how the microbiome engages with the immune system involves molecular signaling and the participation of innate immunity, facilitated by various microbial species and microbiome-related molecules. The colonization of the gut by Gram-negative bacteria that secrete lipopolysaccharides (LPSs) has been identified as a pivotal factor in this mechanism. LPS serves as the trigger for initiating inflammatory responses, particularly those mediated through Toll-like receptor 4 (TLR-4) and nuclear factor kappa B (NF-κB)
[55][56][57][76,77,78].