Vitamin D was initially known to be essential for bone health and calcium phosphorus homeostasis
[1], but recent research suggests that it may also have important roles in blood pressure regulation, glucose control, wound healing, and immune function, and is even associated with cancer, autoimmune disease, obesity, etc.
[2][3][4][5][2,3,4,5]. Furthermore, vitamin D also may act on the regulation of the microbiome, the modulation of immune and inflammatory processes, and the release of antimicrobial peptides. Vitamin D deficiency has also been associated with many pathologies, including inflammatory bowel disease, colorectal cancer, and sclerosis
[6]. More recently, research on the role of vitamin D in female reproductive health and depression has increased as vitamin D receptors (VDRs) have been detected throughout the female reproductive system and central nervous system (CNS)
[7][8][7,8]. The biological actions of vitamin D are applied through a VDR, which is a ligand-dependent transcription factor located in the nucleus of target cells
[9]. The vitamin D-nuclear receptor complex then acts as a transcription factor and exerts a genomic effect on the ovary or brain
[8][10][8,10]. Although the exact genomic effects on the target cells and the underlying mechanism by which vitamin D may be involved in reproductive and CNS systems are unknown, a direct association between vitamin D and ovarian steroidogenesis or brain-derived neurotrophic factor (BDNF) levels from in vitro and in vivo studies has been suggested
[11][12][13][11,12,13]. In this context, several studies have been published on the relationship between vitamin D and ovarian reserve markers, BDNF, and neurotrophic factors
[14][15][14,15], and there have also been many clinical reports that vitamin D deficiency is associated with reproductive hormonal decline or imbalances such as menopause, polycystic ovarian syndrome (PCOS), primary ovarian insufficiency (POI), and depression in women
[16][17][18][19][16,17,18,19]. Meanwhile, the relationship between female reproductive hormone changes and depression is already well established
[20][21][20,21], and several studies have shown that depression in female reproductive hormone-related diseases is also associated with low vitamin D levels and ovarian reserve markers
[22][23][22,23]. In this regard, vitamin D, which is involved in both female hormones and depression, may be another key to explaining female reproductive depression.
2. The Association of Vitamin D and Ovarian Reserve Markers
Today, the most often used ovarian reserve marker is AMH, a gonadal-specific glycoprotein produced by granulosa cells of small antral or pre-antral follicles. AMH is also known to play a crucial role in folliculogenesis with minimal variations in its levels during the menstrual cycle, making it the most useful predictive marker for assisted reproductive technology
[24]. Antral follicle counts (AFC) and basal follicular stimulation hormone (FSH) levels are also often used to represent ovarian reserve in clinical practice
[25].
2.1. Basic Studies at the Cellular and Genetic Level
There has been increased interest in the potential relationship between vitamin D and ovarian reserve markers, particularly AMH, which is important for folliculogenesis, as reports about the effects of vitamin D on follicular development and steroidogenesis in animal and human cell-line studies have shown. For example, VDR null mutant mice not only have impaired folliculogenesis but also show uterine hypoplasia, decreased aromatase activity, aromatase gene expression, and increased FSH levels
[7][11][7,11]. Also, vitamin D stimulates steroidogenesis in human ovarian cells
[12]. However, there is limited information about how vitamin D affects ovarian reserve markers such as AMH. Malloy et al. demonstrated the presence of functional vitamin D response elements in the human AMH gene promotor region and a direct effect of vitamin D on AMH expression via these response elements
[26]. Wojtusik et al. observed a dose-dependent decrease in AMH mRNA levels in hen granulosa cells after treatment with vitamin D
[27]. In another recent study, treatment of human granulosa cells with vitamin D exhibited altered AMH signaling and an inverse correlation between vitamin D status in follicular fluid and AMH receptor-II (AMHR-II) mRNA gene expression
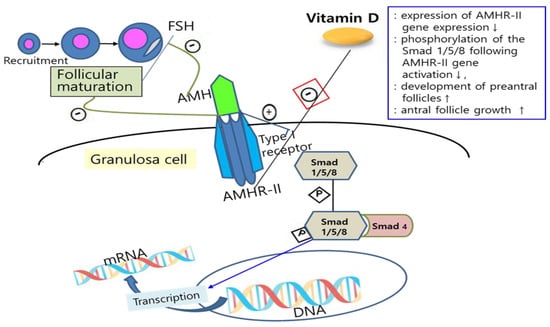
[10]. Binding AMH to AMHR-II activates the type I transmembrane receptor and, subsequently, phosphorylation of Smad 1/5/8, which interacts with Smad 4, followed by this complex regulating transcription of the target gene after moving into the nucleus
[28][29][28,29]. Binding AMH to AMHR-II is also known to suppress follicular maturation by inhibiting primordial follicle recruitment into the growing follicle pool and by decreasing the sensitivity of follicles to FSH
[30]. Since vitamin D plays a role in the downregulation of AMHR-II gene expression, inhibiting the phosphorylation of Smad and its nuclear localization, it is suggested that vitamin D may promote follicle differentiation and development by altering AMH production patterns and FSH sensitivity in ovarian granulosa cells
[31][32][31,32] (
Figure 1). Indeed, Xu et al. showed that vitamin D sustained AMH production and enhanced the antral follicle growth in a rhesus monkey ovarian cell line study
[33]. However, there was a discrepancy between these in vitro studies and clinical studies, which measured plasmatic levels of vitamin D and AMH. This might be due to the possibility that plasmatic levels do not reflect what inherently occurs in tissues. In this regard, there was a study on women with infertility that showed a strong negative correlation of 25-hydroxyvitamin D levels in blood and follicular fluid
[34].
Figure 1. Mechanisms of the effects of vitamin D on AMH signaling and ovarian follicle development. Vitamin D alters the AMH signaling and actions by downregulation of AMHR-II gene expression (red box), inhibiting the phosphorylation of Smad and its nuclear localization, and can also promote follicle differentiation and development by altering AMH production patterns and FSH sensitivity in ovarian granulosa cells. FSH: follicle-stimulating hormone; AMH: anti-Müllerian hormone; AMHR-II: anti-Müllerian hormone receptor-II; mRNA: messenger ribonucleic acid; DNA: deoxyribonucleic acid; P: phosphate; ↑: increase; ↓: decrease.
2.2. Clinical Studies and Meta-Analyses
There have been several clinical observational and interventional studies showing significant relationships between vitamin D and ovarian reserve markers. In observational studies, a positive relationship between serum vitamin D and AMH levels was revealed in a cross-sectional study of 388 premenopausal women
[35], and a negative correlation between serum vitamin D levels and urinary FSH levels was found in another study of 1430 premenopausal women
[36], suggesting that lower vitamin D levels might be associated with lower ovarian reserve in late reproductive-age women (≥40 years) and earlier menopause. Dennis et al. also found that vitamin D might have a positive effect on AMH production in adults, and the seasonal changes in women’s AMH levels also correlates with those in vitamin D levels
[37]. In recent interventional studies, improvements in ovarian reserve markers were reported with vitamin D supplementation, supporting a possible favorable effect of vitamin D on ovarian reserve markers
[38][39][38,39]. However, there were also other studies that revealed no significant association between vitamin D and ovarian reserve markers, such as AMH and AFC
[14][40][41][14,40,41]. Therefore, vitamin D still seems to have inconsistent evidence regarding its relation with ovarian reserves. Nonetheless, it is noteworthy that some recent studies, including meta-analyses, have reported promising results showing that vitamin D supplementation led to improvement in ovarian reserve metrics in a subgroup of normal or diminished ovarian reserves. There were three recent meta-analyses published between 2020 and 2022, two of which provided evidence that vitamin D supplements lead to improved ovarian reserve levels in a subgroup of non-PCOS women with normal ovulation or diminished ovarian reserves
[42][43][42,43], and one found that lower AFC was associated with vitamin D insufficiency/deficiency in a subgroup analysis of Asians
[44]. Moridi et al. highlighted that 18 cross-sectional studies had discrepant findings regarding an association between serum vitamin D and AMH levels, which is likely due to heterogeneity in study subjects and the complex nonlinear relationship between vitamin D and AMH in the systematic review. In contrast, they also demonstrated a cause–effect relationship between vitamin D supplements and AMH in the meta-analysis of six interventional studies, in which, interestingly, serum AMH was significantly increased in ovulatory non-PCOS women and decreased in PCOS patients after vitamin D supplementation
[42]. Similarly, in other meta-analyses, vitamin D supplements led to an increase in AMH levels in non-PCOS women but no increase in AMH levels in PCOS patients
[43].
The role of vitamin D in women with PCOS is even more noteworthy, considering that PCOS can be commonly accompanied by vitamin D deficiency in 67–85% of cases
[45]. Vitamin D supplementation has been reported to reduce serum androgen and AMH levels and endometrial thickness
[46] and to improve fertility indicators by increasing endometrial VDR expression and improving endometrial receptivity
[47][48][47,48]. Zhao et al. showed that both implantation and clinical pregnancy occurrence were significantly higher in patients with normal vitamin D levels compared to patients with decreased vitamin D levels and that the number of high-quality embryos after vitamin D supplements was equivalent to the number of embryos in women with normal vitamin D levels
[49].
3. The Association of Vitamin D and Depression
Observational studies have suggested that women with low vitamin D levels are predisposed to PCOS, infertility, and endometriosis
[50], as well as psychological disorders such as depression
[51]. Women with reproductive diseases that affect fertility are known to have a higher prevalence of depression
[52], so the relationship between vitamin D and depression in relation to fertility is also noteworthy. It is proposed that various mechanisms are involved in the pathogenesis of depression, including those affecting neuroendocrine, immunologic, neurotrophic, and metabolic systems
[53]. Vitamin D is also thought to have a variety of functions, such as neuroimmunomodulation, regulation of neurotrophin, and neuroplasticity, in the brain
[54] and to be involved in serotonin synthesis and maintenance of the circadian rhythm
[55]. Moreover, VDR was found in neurons and glia in many regions of the brain (prefrontal cortex, substantia nigra, cingulate cortex, hippocampus, and hypothalamus) that may play a role in the pathophysiology of depression
[8], and vitamin D is biologically suggested to not only be involved in the synthesis of neurotransmitters such as serotonin, dopamine, adrenalin, and noradrenalin through VDR but also to moderate the hypothalamic–pituitary–adrenal axis (HPA) and γ-aminobutyric acid A (GABA-A) receptor activity
[56].
3.1. Basic Studies in Cellular and Genetic Level
Although the pathophysiology of how vitamin D affects depression has not yet been fully elucidated, several main mechanisms have been reported. First, VDR and 1-α-hydroxylase (vitamin D activating enzyme) are widely distributed in the brain, especially in the hippocampus, which plays a key role in the mechanisms of depression
[57]. Numerous studies conducted on in vitro hippocampal cells and in vivo adult rodents have shown that vitamin D deficiency alters the structure or function of the hippocampus during development
[58]. Additionally, it has been demonstrated that vitamin D can regulate neurotrophins such as nerve growth factor (NGF), BDNF, and neurotrophin (NT)-3, which are essential for the survival and differentiation of neurons during development
[59]. It has been reported that vitamin D may affect depression by increasing BDNF, which plays an important role in the survival, differentiation, and function of newborn neurons in the adult hippocampus
[13][60][13,60]. Second, vitamin D could influence serotonin synthesis by alleviating tryptophan hydroxylase 2 (TPH2) and repressing tryptophan hydroxylase 1 (TPH1). It thus has an antidepressant effect by modulating the serotonergic system
[61][62][61,62]. A strong body of evidence of the role of serotonin in the pathophysiology of depression has been built, and serotonin also acts on the hippocampus
[63]. Vitamin D is known to be involved in depression by also affecting the levels of dopamine and noradrenalin
[64]. Third, vitamin D is thought to control inflammation by reducing the expression of inflammatory cytokines, exhibiting a neuroprotective effect
[65]. Grudet et al. have also shown that vitamin D insufficiency increased inflammatory markers such as IL-1β and IL-6 in depressive rats, although the underlying mechanisms are not clear yet
[66]. In addition, vitamin D has been reported to be related to depression through mechanisms such as antioxidant effects in the CNS, regulation of expression of calcium homeostasis genes, regulation of mitochondrial protein expression, and regulation of demethylation, but additional studies and evidence seem to be necessary for further understanding
[60].
3.2. Clinical Studies and Meta-Analyses on Cohort and Interventional Studies
There have been numerous observational studies on the relationship between serum vitamin D and depression and interventional studies on the effect of vitamin D supplements on depression. Several meta-analyses have also been conducted, which reflects the strong interest in the role of vitamin D. Observational studies have generally suggested an association between low serum vitamin D levels and depression. Vitamin D deficiency was related to depression-like symptoms, and subjects with anxiety or depression showed lower serum vitamin D levels
[51][67][51,67]. Seasonal affective disorder was also suggested to be related to low vitamin D levels in northern latitudes with less sunlight exposure and in winter
[68]. However, recent studies have failed to prove this relationship in female or elderly populations
[69][70][69,70]. These discrepancies may result from not considering possible modulating factors such as subject characteristics and sociodemographic factors (sex, body mass index, diet, underlying diseases, drinking and smoking, etc.) and limitations of cross-sectional studies (biases caused by reverse causality: low vitamin D due to less outdoor activity/reduced nutrient intake, self-scored depression, unadjusted data, etc.). Interventional studies on the effect of vitamin D supplements in reducing depression have also provided inconsistent results. In this regard, several meta-analyses of randomized controlled trials (RCTs) of depressive patients were conducted, and some found a positive effect of vitamin D on depression severity
[71][72][71,72], while others showed insignificant vitamin D efficacy for depressive symptoms
[73][74][73,74]. However, interpretation of these results should also take into account that they also did not examine some factors that may modulate the efficacy of vitamin D in different settings. Also, considering there is reciprocity between vitamin D and epigenetic mechanisms
[75], there may be a complex nonlinear relationship between vitamin D and reproduction or depression due to epigenetic changes caused by vitamin D supplementation. Recently, Musazadeh et al. conducted an umbrella meta-analysis on ten meta-analyses of interventional RCTs and four meta-analyses of cohort observational studies
[76]. This umbrella meta-analysis demonstrated a significant reduction in depressive symptoms in patients with vitamin D supplements and increased odds of depression in patients with low serum vitamin D levels. In another recent meta-analysis of 18 RCTs, vitamin D supplements were effective in depressed patients in heterogeneous data
[77]. In these two recent meta-analyses, there were subgroup analyses of several factors that might modulate vitamin D efficacy, and no differences in the efficacy of vitamin D supplementation according to gender were observed, suggesting that vitamin D supplementation is beneficial to both men and women
[76][77][76,77]. To date, there is growing promise that low vitamin D levels are related to the risk of depression and that vitamin D supplementation may be effective in treating depression.