Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Bogdan-Marian Tofanica and Version 2 by Camila Xu.
Metallogels can be derived from ready-made cellulose hydrogels using the diffusion–reduction technique. This convenient and cost-effective approach involves the use of chemical or phyto-chemical reducing agents for the nanoparticles’ formation from the precursor salt solutions.
- metallogels
- hydrogels
- cellulose
- metal nanoparticles
- drug delivery
- tissue engineering
1. Introduction
The use of cellulose-based products offers a wide range of environmental and economic benefits. These materials, derived from wood and non-wood sources, show range of properties that make them useful for a variety of applications in the forms of fibers, films, hydrogels, and aerogels, as well as nanoscale materials [1][2][3][4][1,2,3,4]. The structure of cellulose hydrogels provides a superior water-holding capacity and certain properties, namely mechanical strength, porosity, and specific surface area. In addition, an impressive amount of reactive functional groups allow the hydrogel to trap nanoparticles and other additives. As sustainable, biodegradable, non-toxic, biocompatible, and multi-purpose materials, bio-based cellulose materials are in high demand in such fields as medicine, pharmacology, cosmetics, food industry, environmental protection, electronics and energy [5][6][7][8][9][10][11][12][5,6,7,8,9,10,11,12]. For example, in the context of disposable packaging materials, there is a obvious need for biodegradability to alleviate landfill concerns. Alternatively, when used in biomedical applications,such as wound dressings, cellulose hydrogels ensure safe combustion without generating toxic by-products. Moreover, the biocompatibility of these materials is essential for medical devices.
Cost-effectiveness and sustainability of materials are universally paramount considerations [13]. Environmentally friendly production involves the use of various renewable resources as an integral part of the cellulose-based polymer paradigm. Cellulose can be derived from plant-based biomass, such as wood pulp [14], fibers [15], algal biomass [16], cellulose-producing bacteria [17], agricultural [18][19][18,19] and paper wastes [20].
Cellulose hydrogels are typically produced by dissolving cellulose in solvents, such as organic, inorganic, mixed, and ionic liquids, followed by gelation and solvent removal [1][5][1,5]. However, the limited solubility of cellulose is limited, which restricts the choice of suitable solvents for hydrogel production. A comprehensive assessment of cellulose sources and preparation techniques, involving such solvents as N,N-dimethylacetamide (DMAc)/LiCl, dimethyl sulfoxide (DMSO)/LiCl, N-methylmorpholine-N-oxide (NNMO), and NaOH-aqueous solutions, was undertaken by the authors in the first part of the review series “Cellulose-Based Metallogels” [21]. The analysis revealed that DMAc/LiCl and NaOH are the more commonly used solvents, while the other two are less frequently employed by researchers. The NaOH-aqueous system offers cost-effectiveness and environmental advantages, but requires specific pretreatment, conditions, and crosslinking. In contrast, DMAc/LiCl, while less environmentally friendly, provides a simple and attractive process for manufacturing hydrogels.
The addition of metal particles to the gel system provides the resulting metallogels with new capabilities [22][23][24][22,23,24], as summarized in Figure 1. Metal nanoparticles posses advantageous properties, including high specific surface area, diverse catalytic capabilities, unique electromagnetic and optical behavior due to their small size, and enhanced reactivity [25].
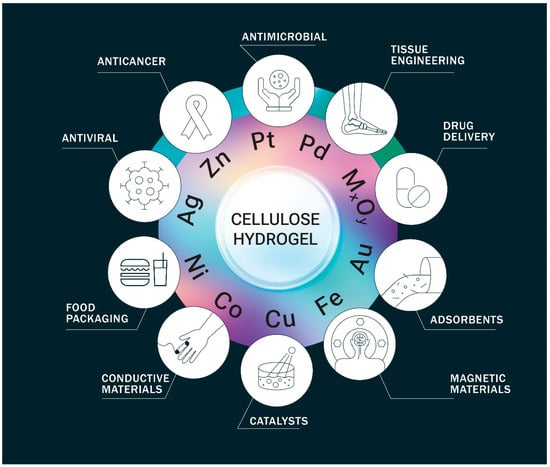
Figure 1.
The main application fields of cellulose-based metallogels.
As discussed in the second part of “Cellulose-Based Metallogels” [12], the incorporation of metal nanoparticles into hydrogels reinforces the mechanical strength of resulting materials. Metal-loaded hydrogels also exhibit improved crystallinity and thermal stability when compared to their pristine counterparts. The addition of metal ions or nanoparticles can provide hydrogel matrices with new and desirable traits, such as optical responsiveness, thermal characteristics, or biological activity. The porous structure of cellulose hydrogels facilitates the exchange of metal ions, and in some cases, enables the formation of metallogels through complexation. This may result in biocidal effects that persists over time [26][27][26,27].
Metallogels can be produced from pre-existing cellulose hydrogels using the diffusion–reduction technique [21][24][28][21,24,28]. This approach is convenient and cost-effective, involving the use of chemical or phyto-chemical reducing agents to form the nanoparticles from precursor salt solutions. Alternatively, another chemical route to obtain cellulose metallogels relies on certain metal ions that initiate gelation and act as cross-linking agents for cellulose [29]. While this method is appealing, it is limited to cellulose solvents that are aqueous in nature [21].
2. Metallogel Science, Approaches, and Applications
2.1. Medical Application
The development of biomaterials is embracing metallogels, with such metals as Ag, Au, Zn, and others, as well as their oxides, taking the forefront. These materials are gaining traction due to their capacity to react to diverse physical and chemical cues (for instance, electrical, pH, magnetic, and light stimuli) while also offering supplementary attributes [30][34].2.1.1. Antimicrobial, Antifungal, and Antiviral Properties
Due to the widespread and often uncontrolled usage of antibiotics in recent decades, many microorganisms have become resistant to the effect of antibiotics, which makes it difficult to cure even the simplest infections. Metal and metal oxide nanoparticles (Ag, Cu, CuO, Cu2O, Au, ZnO, Al2O3, TiO2, Co3O4, In2O3, MgO, ZrO2, Cr2O3, NiO, Ni2O3, Mn2O3, and CoO) have demonstrated selective effectiveness against priority pathogens. The non-specific bacterial toxicity mechanisms of metal nanoparticles, devoid of binding to specific bacterial cell receptors, hinder resistance development and expand the range of antibacterial effects [31][32][35,36]. A number of metal and metal oxide nanoparticles (such as silver, gold, copper, titanium dioxide, and zinc oxide) have been recently introduced as antiviral agents [33][34][35][36][37,38,39,40]. As was revealed in the review by Sánchez-López et al., nanoparticles employ distinctive mechanisms of action that differ from conventional treatments. This grants them the ability to combat antibiotic-resistant bacteria and target various biomolecules, hindering the emergence of resistant strains. Concerns about potential human toxicological effects related to metal nanoparticles stem from their physico-chemical traits, dosage, and administration method, which dictate their behavior within the body [31][35]. Addressing this challenge, a polymer matrix can play a crucial role in managing the targeting and gradual release of nanoparticles. Hydrogels exhibit porosity, remarkable flexibility, and a capacity to retain water. These attributes create a moist, tissue-like environment, rendering it a fitting biomaterial for diverse biomedical uses [36][40]. These requisites are realized in cellulose hydrogels, making them an ideal carrier for metal nanoparticles. As was shown in numerous studies, introducing metals such as silver, gold, copper, and zinc, as well as metal oxides, in nanosized or colloid states into cellulose matrices confers antimicrobial and antifungal properties to the composite material [24][28][37][38][39][40][41][24,28,41,42,43,44,45]. An effective method for producing antimicrobial cellulose/metal composite hydrogels involves the chemical or biological reduction of the metal ions within the ready-made cellulose hydrogel [20]. For instance, hydrogels prepared from solutions of hardwood and flax powder celluloses in DMAc/LiCl demonstrated high porosity and specific surface areas. These attributes are crucial for anchoring the reduced metal nanoparticles within the cellulose matrix. The synthesis of silver and gold nanoparticles was carried out via the Turkevich method of reducing gold ions from a sodium tetrachloroaurate (III) Na[AuCl4] solution and silver ions from a silver nitrate AgNO3 solution with trisodium 2-hydroxypropane-1,2,3-tricarboxylate (trisodium citrate) as a reducing agent. The digital pictures of the resulting Au- and Ag-containing metallogels are presented in Figure 2 (a and b, respectively). The nanoparticles intercalated into cellulose hydrogels exhibited various shapes (spherical or rectangular) and sizes. Gold nanoparticles predominantly ranged from 40 to 120 nm, sometimes forming larger agglomerates of several micrometers (Figure 2d). A higher concentration of trisodium citrate facilitated the rapid stabilization of smaller-sized Au nanoparticles, while a lower concentration led to larger Au nanoparticles due to aggregation. Silver nanoparticle sizes ranged from 20 to 260 nm (Figure 2e), showing a more uniform size distribution for gold nanoparticles compared to silver nanoparticles [42][46]. It was demonstrated that cellulose hydrogels that contained from 0.30 to 5.66 wt.% of Ag or Au nanoparticles revealed antimicrobial activity against Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria (Figure 2c) [24][42][43][24,46,47]. The biocidal activity was not notably influenced by the metal content in the given range. This suggests that there is no necessity to elevate it beyond 1 wt.%, avoiding the possible toxic effect of metal nanoparticles on humans. All examples are briefly summarized in Table 1.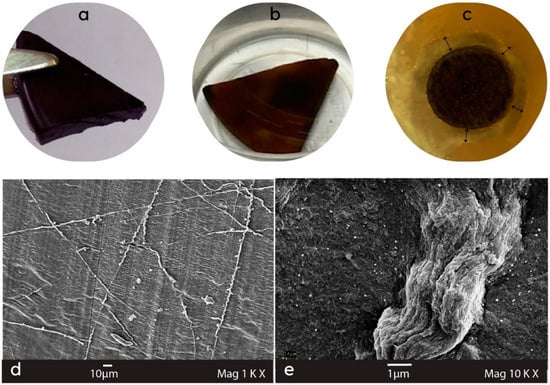
Figure 2. Digital photographs and SEM micrographs, respectively, of (a,d) Au/cellulose metallogel (2.9 wt.% Au), (b,e) Ag/cellulose metallogel (2.1 wt.% Ag), and (c) no-growth zone on agar inoculated with E. coli bacteria in the experiment with Ag/cellulose metallogel after 24 h of incubation.
2.1.2. Anticancer Properties
Nanoparticles of certain metals are recognized as promising anticancer agents due to their effectiveness against drug-resistant tumor cells through distinct mechanisms [61][65]. Au, Ag, and Zn nanoparticles [62][63][64][66,67,68], as well as bimetallic colloidal particles [65][66][69,70], exhibit effects both in cancer diagnosis and treatment, offering alternatives to current toxic anticancer drugs. Existing treatments produce harmful side effects and drug resistance along with rapid metabolism and clearance, limiting efficacy. Metal nanoparticles can be combined with drugs or polymer-coated for targeting cancer cells [61][65]. The major mechanisms by which metal nanoparticles exert their anticancer properties involve cellular uptake via endocytosis. Following this, vesicles are dispersed throughout the cytoplasm and nucleus, causing toxic effects that ultimately result in apoptosis (programmed cell death) [67][68][71,72]. For instance, silver and gold nanoparticles can induce inflammation in treated cells by activating macrophages [69][73]. They also possess antiangiogenic properties by blocking the completion of signaling pathways [70][71][74,75], along with antiproliferative effects attributed to the induction of genomic and cytoskeletal instability [72][73][76,77]. Size-dependent effects indicate smaller nanoparticles are more toxic, generating ROS more efficiently [74][78]. Gold nanoparticles can serve as delivery systems in cancer therapy [75][79] or are combined with therapeutic molecules [76][80], including genes, enhancing their efficacy against cancer cells. Their distinct photo-optical attributes enable successful utilization in both photothermal (inducing hyperthermia and eventual cell necrosis) and photodynamic (creating ROS) therapies [77][78][81,82]. Thus, metal and metal oxide nanoparticles exhibit promising potential in cancer treatment; however, their direct skin or oral application is not feasible. A carrier is essential to control the release of active nanoparticles at precise concentrations and timings. As one viable approach, nanoparticles can be incorporated within polymer hydrogels or chemically cross-linked into their networks. Cellulose’s capacity for liquid absorption and loading active substances, such as drugs and metal nanoparticles, makes it a promising material for diverse cancer therapies [79][83]. Hydrogels composed of hydrophilic cellulose have been extensively utilized for skin cancer treatment, providing a water-rich environment [55][59]. Nanocomposite hydrogels can induce controlled high temperatures via near-infrared irradiation, effectively eradicating tumor cells and inhibiting their growth [80][84]. Owing to their favorable mechanical, biological, and physico-chemical properties [12], they mitigate the risk of post-surgical tumor recurrence [81][85]. Skin cancer simultaneously couples with skin wound infection. Nanocomposite hydrogels with simultaneous photothermal antitumor and antibacterial efficacy can meet the dual demand of cutaneous melanoma treatment and skin wound healing [55][59]. For instance, cellulose-based metallogels with ZnO nanoparticles, enriched by quercetin and curcumin, exhibited both anticancer and antimicrobial capabilities [51][52][55,56]. Similarly, cellulose hydrogels incorporating coordination complexes of zinc, palladium, platinum, cobalt, and silver with 1,10-phenanthrocyanates demonstrated promising potential in both anticancer and antimicrobial applications [48][52].2.1.3. Drug Delivery
The domain of drug delivery has made remarkable strides in recent times, particularly with the rapid advancement of nanomedicine, coupled with enhanced insights into infectious and cancerous conditions [82][86]. One of the crucial pharmacological purposes is delivering precise amounts of drugs at pre-planned rates to provide the desired level of drugs for treatment. Cellulose-based hydrogels that respond to various stimuli exhibit a remarkable ability to undergo rapid volume changes in reaction to environmental triggers, transitioning between collapsed and swollen states. This unique behavior renders them highly promising for biomedical applications. These environmental stimuli encompass factors like pH, temperature, redox reactions, light exposure, solvent composition, electric and magnetic fields, and even biological or biochemical signals [83][87]. Body temperature and variable pH ranges in different parts of the body function as external stimuli to activate the release of the drug. Cellulose hydrogels can perform as safe transport systems with desired therapeutic effects and with minimum side effects. Moreover, the results of the utilization of hydrogels in target therapy strategies obtained in clinical trials are very encouraging [84][85][31,88]. Usually, pure-cellulose hydrogels have a disadvantage of low mechanical characteristics because of the physical mechanism of formation. Chemically cross-linked hydrogels demonstrate better properties, as a result, they are preferable. Metal and metal oxide nanoparticles can play dual roles as a complexation center to improve the cross-linking of the hydrogel, as well as an antimicrobial and anticancer component. There are a number of examples when the hydrogels for drug delivery are produced from cellulose derivatives or enhanced with a different component, resulting in a hybrid hydrogel. For instance, a Cu-based metal/organic framework was produced for the encapsulation of ibuprofen to reduce the side effect of this drug on the gastrointestinal tract. In this material, a carboxymethyl cellulose hydrogel bead demonstrated better protection of ibuprofen against stomach acid and a high stability of drug release for a long period of time [86][89]. In another study, leptin, a hormone that helps to maintain normal weight, was entrapped within methyl cellulose hydrogels, with the incorporation of gold nanoparticles. The light-triggered degradation of hydrogels was used to improve accuracy and efficiency for sustained and controllable release. The incorporation of gold nanoparticles into methyl cellulose hydrogels led to a tunable light irradiation response. The study revealed that thermosensitive hydrogels are suitable for loading multimodality therapeutic agents to enhance the bioactivity of leptin for obesity therapy [87][90]. One more complex example involved another natural polymer—chitosan—is described in [88][91]. A nanohybrid hydrogel of L-histidine conjugated chitosan, phyto-synthesized zinc oxide nanoparticles, and dialdehyde cellulose was successfully used as a drug delivery carrier for the polyphenolic drugs naringenin, quercetin, and curcumin. The hydrogel demonstrated a peak loading efficiency at 90.55%, 92.84%, and 89.89%, respectively. The maximum drug release was favorably observed at optimal drug loading and at pH 5. Histidine–chitosan conjugation stabilized the hydrogel and enabled sustained drug delivery. Prominent antimicrobial activity against Staphylococcus aureus and Trichophyton rubrum strains was anticipated to develop through a synergistic formulation. Significant biocompatibility with L929 cells demonstrated support for normal cell survival. Anticancer analysis of A431 cells displayed excellent cytotoxicity, with a 15- to 30-fold increase using a hybrid carrier, in contrast to free polyphenol drugs [88][91]. On the other hand, there are examples of a cellulose hydrogel being applied without any enhancements other than metal nanoparticles. Thus, regenerated cellulose obtained from sugarcane bagasse was used for hydrogel preparation with zinc oxide nanoparticles photosynthesized from musk melon seed extract. For a drug delivery study, curcumin was selected as the model drug for its appealing anticancer and antimicrobial activity. The drug release was performed under varying pH and initial drug loading concentrations. Polymer swelling as the drug release mechanism was the best option according to the results [52][56]. Incorporating metal nanoparticles into hydrogels is not the only approach to design drug delivery metallogels; metal–organic compounds can also be integrated into hydrogels for these applications. Belonging to the class of metal–organic frameworks, zeolitic imidazolate frameworks (ZIFs) are characterized by the coordination of metal clusters with organic ligands to create three-dimensional structures. Usually, ZIFs are composed of tetrahedrally coordinated transition metal ions (e.g., Fe, Co, Cu, and Zn) connected by imidazolate linkers. Addressing the challenge of loading hydrophobic drugs into hydrophilic nanocellulose-based hydrogels, a metal–organic approach was employed. Polydopamine was auto-polymerized on cellulose nanofibril (CNF) hydrogel surfaces, forming a PCNF composite hydrogel. Subsequently, zeolitic imidazolate frameworks-8 (ZIF-8) nanoparticles were grown within the PCNF composite hydrogel structure. The resulting material exhibited robust mechanical strength, and efficient drug delivery characteristics were achieved. The ZIF-8/PCNF composite hydrogel demonstrated prolonged drug release times and anticancer effects, highlighting its potential as a promising biomaterial for drug delivery applications [89][92].2.1.4. Tissue Engineering
Researchers in the field of tissue engineering and regenerative medicine are addressing the issues of organ scarcity and biocompatibility by exploring scaffolds as a substitute for transplants [90][93]. Tissue scaffolds play an important role in regeneration by mimicking the ECM in natural tissues and providing a suitable 3D space for cell proliferation and differentiation. Therefore, scaffold materials must have reasonable biocompatibility, mechanical properties, swelling behavior, and porosity [91][94]. Cellulose-based hydrogels have found extensive applications in tissue and biomedical engineering, leveraging their impressive properties, such as remarkable swelling capabilities, strong water absorbency, mechanical resilience, and compatibility with biological tissues, all of which facilitate effective binding. These hydrogels contain a large number of hydroxyl groups, which are conducive to the formation of composite hydrogels with other polymers, metals (usually Au and Ag), or small molecules. They enable the regeneration of various tissues, such as bone, cartilage, heart, blood vessel, nerve, and liver, among others [9][91][9,94]. In the context of skin regeneration, cellulose hydrogels offer an added advantage: they possess the ability to promote hydration healing, exhibit suitable oxygen permeability, absorb wound exudates, enhance epithelialization, and create an environment conducive to tissue regeneration. An example is the BC/acrylic acid hydrogel obtained through electron beam irradiation. This material exhibited a porous network structure, remarkable swelling capacity (4000–6000%), and high water vapor transmission rate (2175–2280 g/m2 per day). The hydrogel displayed biocompatibility in L929 cell viability tests. In vivo trials on rats demonstrated that this hydrogel expedited wound healing, facilitated epithelialization, and accelerated fibroblast proliferation in comparison to the control group. These outcomes highlight the potential of BC/acrylic acid hydrogels as promising materials for burn dressings [92][95]. The integration of metals, namely silver and gold, typically enhances the physical and chemical characteristics of cellulose hydrogels [12][90][12,93]. Metal nanoparticles exhibit inherent bioactivity and, as previously described, natural antibacterial, antiviral, and anti-inflammatory capabilities. In the study conducted by Zulkifli et al., an antimicrobial hydroxyethyl cellulose/Ag nanoparticle scaffold promoted the growth and proliferation of human fibroblasts. It was suggested that the surface roughness of the scaffold due to the presence of Ag nanoparticles contributed to enhanced cell adhesion and proliferation [93][96].Table 1.
The biological properties of cellulose-based metallogels.
| Nanoparticle | Hydrogel Matrice/Additives | Biological Effect | References |
|---|---|---|---|
| Ag | Flax, cotton, and hardwood powder cellulose | Antibacterial activity against S. aureus and E. coli. | [24][42][43][24,46,47] |
| Bacterial cellulose/PVA (2:5 wt.) | Antibacterial activity against E. coli and S. aureus. Promotion of the growth of new blood vessels. Anti-inflammatory activity. Accelerating wound healing. | [37][41] | |
| Microcrystalline cellulose | Antibacterial activity against E. coli and S. aureus. | [41][45] | |
| Bacterial cellulose | Antibacterial activity against K. pneumonia and S. aureus. | [47][51] | |
| Bacterial cellulose/aqueous curcumin | Cytocompatibility, wound-healing properties, and antimicrobial activity against S.aureus, P.aeruginosa, and C. auris. | [49][53] | |
| Hydroxypropyl-β-cyclodextrin complex | Tissue repair and wound healing via a decrease in inflammation; increase in angiogenesis, collagen deposition, and rate of neo-epithelialization; and cytocompatibility. Antimicrobial activity against P. aeruginosa, C. freundii, E. cloacae, E. coli, S. aureus, S. epidermidis, B. subtilis, and C. parapsilosis. | [56][60] | |
| Cellulose nanocrystals isolated from Syzygium cumini leaves | Low toxicity to hFB cells. | [93][96] | |
| Hydroxyethyl cellulose | Antibacterial activity against E. coli and L. monocytogenes. The composite did not significantly reduce the viability of Caco-2 and FHC colon cells. | [94][97] | |
| Cu | Cellulose nanofibrils | Effective ibuprofen drug carrier with controlled release in the gastrointestinal tract. Low toxicity against Caco-2 cells. | [86][89] |
| CuO, Cu2O, and Cu | Carboxymethyl cellulose/ibuprofen | Antibacterial activity against E. coli and S. aureus. Biocompatibility with HaCaT cells. | [38][42] |
| CuO | Carboxymethyl cellulose, hydroxypropylmethyl cellulose | Antibacterial activity against K. pneumonia and S. aureus. | [47][51] |
| Au | Bacterial cellulose | Stimuli-responsive thermosensitive carriers for therapeutic agents to enhance the bioactivity of leptin for obesity therapy. | [87][90] |
| Methylcellulose/leptin | Antibacterial activity against E. coli and P. aeruginosa; biocompatibility. Promotion of wound repair. | [39][43] | |
| Bacterial cellulose/4,6-diamino-2-pyrimidinethiol | Antibacterial activity against S. aureus and E. coli. | [42][46] | |
| ZnO | Flax and hardwood powder cellulose | Antibacterial activity against E. coli and S. aureus. | [45][49] |
| Bacterial cellulose | Antifungal activity towards phytopathogen F. oxysporum. Reduced the wilt disease symptom incidence of pepper plant. | [40][44] | |
| Cellulose from watermelon peel waste | Antibacterial activity against K. pneumonia and S. aureus. | [47][51] | |
| Bacterial cellulose | The synergetic antimicrobial effect against E. coli, B. subtilis, and C. albicans. Without propolis extract, BC/ZnO hydrogels had no influence on Gram-negative and eukaryotic cells. | [50][54] | |
| Bacterial cellulose/ethanolic propolis extracts | Effective quercetin drug carrier. Antimicrobial activity against S. aureus and T. rubrum. Biocompatibility and anticancer properties against normal L929 murine fibroblast cells and A431 human skin carcinoma cell lines, respectively. | [51][55] | |
| Chitosan/dialdehyde cellulose derived from Sugarcane bagasse/phyto-derived quercetin | Effective curcumin drug carrier. Antimicrobial activity against S. aureus and T. rubrum. | [52][56] | |
| Sugarcane bagasse cellulose/curcumin | Stimuli-responsive (pH) carriers for therapeutic agents. Antimicrobial activity against S. aureus and T. rubrum. Biocompatibility towards L929 cells. Anticancer activity towards A431 cells. | [88][91] | |
| Chitosan/dialdehyde cellulose derived from Sugarcane bagasse/L-Histidine/Naringenin, quercetin, and curcumin | Antibacterial activity against E. coli, Salmonella, L. monocytogenes, and S. aureus. A decline in the capacity and virulence of microorganisms to pose infections. | [95][98] |
2.2. Food Packaging
Packaging materials serve multiple purposes, with the primary function being to safeguard products against diverse environmental factors: mechanical stress, gases and vapors, moisture, light, temperature, microbes, and contamination. The selection of materials considers their potential to provide protection, as well as facilitate transportation, enhance presentation, and convey consumer information. Packaging may also encompass functional components for extending the shelf-life (active packaging) [96][99]. Conventional plastic food packaging is primarily composed of synthetic polymers and is notoriously difficult to recycle. Challenges like food contamination, the presence of paper stickers, and the use of composite plastics, e.g., C/PP (composite polypropylene), further complicate the recycling process. Moreover, synthetic plastic packaging takes an extensive amount of time to decompose in landfills, eventually breaking down into harmful microplastics. To address these issues, the transition from petroleum-based plastics to biodegradable cellulose films and hydrogels appears to be a promising direction for advancing the packaging industry. Being effective adsorbent materials, non-toxic, and biodegradable [11][12][11,12], cellulose hydrogels can be applied in food packaging to control the humidity and water activity of food, provide antibacterial protection, as well as act as a food quality indicator due to the ability to change color at different pH levels. Such broad possibilities are achieved mainly by filling the porous network of cellulose hydrogels with various additives. The antimicrobial properties of hydrogels are gained by loading natural cytotoxic substances (such as curcumin, quercetin, and grapefruit seed extract) or embedding metal nanoparticles (Ag and ZnO), and can also be achieved by combining these types of antimicrobial agents (Table 1) [11]. For example, biodegradable BC films modified with ZnO nanoparticles and propolis extract with antimicrobial properties were designed for food packaging [47][51]. Not only antimicrobial, but also antioxidant, UV-blocking, oxygen-scavenging, and water vapor permeability effects, as well as a low environmental impact, are among the benefits of cellulose-based composite materials [97][100]. Since packaging usually comes into contact with edible products, metal nanoparticles can pass into food. Therefore, usually, it is important for the content of nanoparticles in hydrogels to be very low. As noted earlier, about 1 wt.% of silver or gold nanoparticles is sufficient for the metallogel to have antimicrobial activity [24][42][24,46]. One of the recent studies on the cytotoxicity of silver ions released from cellulose carriers confirmed that CNF/Ag composites with an average Ag nanoparticle size of 10 nm did not significantly reduce the viability of Caco-2 and FHC colon cells, although the uptake of Ag nanoparticles through an endosomal mechanism was observed [94][97]. Also, it was proved that Ag and TiO2 nanoparticles releasing metal ions from packaging into its contents was insufficient to cause harm to human cells [97][100]. Moreover, another study suggests sulfated CNF/ZnO bio-nanocomposites are a novel preservative to inhibit microbial growth and repress the synthesis of exotoxins in the food industry [95][98]. These findings indicate that although there are relatively fewer applications of hydrogels in food packaging compared to films, cellulose/metal composites hold potential as antimicrobial materials for use in active food packaging systems.2.3. Wastewater Treatment
Nowadays, a considerable number of water bodies around the globe are significantly polluted and are assessed as environmentally unfavorable. There are complex challenges posed by sites leaking hazardous waste, contaminated sediments, and the atmospheric deposition of acidifying and toxic substances, in addition to agricultural pollution sources, which also contribute to transboundary pollution. More than 80% of sewage generated by human activities is discharged into rivers and oceans without any treatment, which results in environmental pollution and more than 50 diseases [98][101]. A large proportion of the world’s population has to use water that does not meet hygienic requirements; moreover, contaminants pose a vital threat to aquatic ecosystems. The biggest contribution to water pollution is made by such contaminants as heavy metals, nitrogen and organic substances, fertilizers, pesticides, sediment, dyes, and oil, which increase the chemical oxygen demand of water bodies [99][100][101][102,103,104]. Activated carbon is widely utilized in commercial purification systems for wastewater treatment due to its exceptional adsorption capacity. Nevertheless, various unconventional, cost-effective, and renewable cellulose-based adsorbents are being suggested as alternatives for contaminant removal [102][105]. Cellulose gels offer beneficial properties, namely a unique structure, an elevated specific surface area, porosity, and a high density of functional groups, making them well suited for the effective removal of various pollutants from wastewater as well as for the recovery of precious or hazardous metals from wastewater in mining, electroplating, and metal processing [101][103][104,106]. The integration of inorganic compounds, e.g., metal and metal oxide nanoparticles, into cellulose gels enhances their contaminant removal capacity and photocatalytic performance while also imparting specific affinities toward certain pollutants [104][107]. This approach can effectively target the range of aquatic pollutants listed above, including organic dyes [105][108], pharmaceuticals [106][109], and specific anions [44][48]. To enhance sorption capabilities, hydrogels are sometimes transformed into aerogels [107][110] or modified through polymer grafting [108][109][111,112]. Metallogels are characterized by two main methods of water purification. The first method involves chemically transforming a dangerous organic pollutant into a simpler, less toxic substance. In addition to reducing the hazard class of wastewater, the advantage is to reduce the level of chemical oxygen demand in such wastewater. The metal phase in a cellulose metallogel can provide this material with catalytic activity in the reactions of such “chemical” water purification (Figure 3). For example, zinc oxide reveals outstanding photocatalytic performance, making it suitable for degrading complex organic compounds such as dyes and pharmaceuticals [110][113]. Thus, a ZnO/cellulose composite was synthesized by regenerating a cellulose hydrogel from a NaOH/urea/H2O (7:12:81) solution, followed by immersion in an ethanol solution of zinc acetate. The composite Zn2+-loaded hydrogel was then freeze-dried, and the resulting aerogel was calcined to produce flower-like ZnO structures inside the cellulose matrix. Rhodamine B (Rh B) dye served as a model compound to evaluate the photocatalytic degradation rate in this study. Upon exposure to UV light, a photocatalytic reaction was triggered, leading to a significant decrease in Rh B concentration. After 180 min, the degradation rate of Rh B using the ZnO/cellulose composite and ZnO without the hydrogel reached 95.2% and 45.2%, respectively. This suggested that the cellulose hydrogel acted as a microreactor, enhancing the catalyst’s activity twofold [110][113].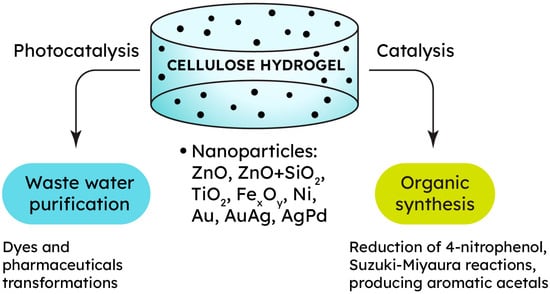
Figure 3.
Catalytic properties of cellulose-based metallogels for different applications.
2.4. Catalysis
The catalytic potential of cellulose metallogels extends beyond wastewater treatment. These materials hold promise in various catalytic processes, showcasing their versatility in promoting chemical transformations (Figure 3). The unique combination of cellulose’s structural attributes and the catalytic properties of metals creates a platform for tailored and efficient catalysis. Due to the large specific surface area, metal nanoparticles demonstrate fascinating catalytic reactivity in numerous reactions of organic synthesis. Additionally, hydrogel network channels are beneficial to the mass transfer process of liquid phase catalysis, creating relatively stable sites for the catalytic process [116][119]. When noble metals are exploited for catalytic purposes, the question of recovery and recyclability becomes crucial due to the low earth abundance and high cost of noble metals. Lin applied a NaOH/urea regenerated cellulose hydrogel as a matrix for nanoparticles of gold; the method allows controlling the size of the particles by varying the concentration and the reaction temperature [117][120]. During the process of synthesizing nanoparticles within the hydrogel through the chemical reduction method, it was observed that higher concentrations of [AuCl4]− resulted in a significant increase in the average diameter of the formed Au nanoparticles. Additionally, as the reaction temperature was elevated, there was a noticeable decrease in the average diameter of the Au (0) nanoparticles. This underscores the influence of the concentration of a precursor salt and reaction temperature on the size characteristics of the produced nanoparticles within the hydrogel matrix. The Au/cellulose metallogels exhibit potential utility as effective heterogeneous catalysts in the reduction of 4-nitrophenol by NaBH4. Notably, the catalytic activity is enhanced with smaller-sized Au nanoparticles. Furthermore, these Au/cellulose hydrogels can be conveniently isolated following the catalytic reaction [117][120]. Another cellulose-based hydrogel incorporated with bimetallic nanoparticles (AuAg, AuPd, and AgPd) was introduced by Liu as a catalyst for the same reaction of 4-nitrophenol reduction and Suzuki–Miyaura coupling reactions. The bimetallic nanocomposite hydrogels demonstrated the ability to be recycled for more than 10 cycles while maintaining their effectiveness [118][121]. In another study, bacterial cellulose aerogels were selected as the substrate for supporting and dispersing metal nanoparticles, namely Cu and Ni. It was found out that adsorption driven by swelling could effectively govern both the size and distribution of the metal nanoparticles. Cu and Ni nanoparticles were successfully embedded within the bacterial cellulose network, with Cu particles being smaller than Ni particles. The metal-loaded catalysts demonstrated favorable catalytic performance in the reduction of 4-nitrophenol. Notably, the optimal sample prepared with a 0.5 wt.% CuSO4 solution exhibited rapid completion of the reduction process within 8 min. Moreover, this catalyst exhibited remarkable stability and reusability [119][122]. Nanoporous cellulose nano-sponges were synthesized by combining TEMPO-oxidized cellulose nanofibers with branched polyethyleneimine and citric acid in a water solution. These materials were then loaded with Cu (II) or Zn (II) metal ions through the metal chelation properties of the cellulose. The resulting composites exhibited exceptional performance as heterogeneous catalysts in facilitating reactions between aromatic aldehydes and alcohols, producing aromatic acetals. Under optimized conditions, these catalysts achieved conversion rates exceeding 90%, with nearly complete selectivity towards acetal products, minimizing or eliminating the formation of carboxylic acid by-products. Furthermore, these metal-loaded cellulose nano-sponges could be recycled up to five times without losing their catalytic activity [120][123]. A composite hydrogel derived from wheat straw cellulose and feather protein using [Bmim]Cl ionic liquids, which contained copper nanoparticles, was proposed as a catalyst for the reduction of 2-nitrobenzoic acid to 2-aminobenzoic acid. Impressively, the catalytic activity of this composite material remained at 99.02% over five cycles of utilization and 90.60% after 30 days of storage, highlighting its exceptional recyclability and stability [116][119]. As research in this field continues to advance, it is becoming evident that composite cellulose/metal materials have the capacity to develop catalytic processes, offering environmentally friendly and economically viable solutions for catalytic applications in organic synthesis.2.5. Conductive Materials
Conductive hydrogels based on natural biopolymers hold immense promise in the realm of wearable and stretchable sensing devices because they combine the reliable long-term healing capabilities of sensors with environmental degradability/recyclability for reducing electronic waste [121][122][123][124,125,126]. These hydrogels leverage the renewable and non-toxic nature of biopolymers, along with their biocompatibility, while also harnessing the exceptional flexibility and conductivity. Unlike conventional flexible substrates originating from petroleum-derived polymers, conductive hydrogels obtained using natural polymers, including cellulose, possess a unique advantage. Their continuous cross-linked polymer networks contribute to mechanical flexibility, while the abundant water content facilitates uninterrupted ionic transport, resulting in an exceptional combination of stretchability and conductivity [122][125]. Conductive hydrogels, derived from cellulose, find practical applications as flexible strain sensors, particularly in the realm of wearable devices aimed at monitoring human movement patterns [123][126]. For instance, a research group led by Fu developed a multifunctional strain sensor for healthcare-monitoring systems by engineering a TEMPO-oxidized nanofibrillated cellulose pre-reinforced gelatin nanocomposite hydrogel infused with Fe (III) ions. Employing a multi-dynamic interaction strategy, they achieved the synchronized modulation of both bulk and interfacial interactions, resulting in impressive properties including high compressive stress (1310 kPa), self-healing capabilities, and electrical conductivity. Leveraging these attributes, a gelatine/NFC/Fe3+ hydrogel was harnessed as a multifunctional strain sensor with a gauge factor as high as 2.24 under 6% strain and a compressive sensitivity of 1.14 kPa−1 under 15 kPa. This sensor exhibited potential applications in manufacturing electronic skin to accurately detect subtle body movements, handwriting, and personal signatures. Notably, the sensor also displayed reliable self-healing properties for long-term usage, environmental degradability, and complete recyclability to reduce electronic waste [121][124]. Several flexible strain sensors with Ag nanoparticles in the matrices of cellulose hydrogels, both antibacterial and conductive, were recently suggested [124][125][127,128]. One of the devices was designed from silver nanoparticles prepared via the solid-state reduction of hydroxyethyl cellulose and compounded into a chemically cross-linked hydrogel with polyacrylamide. The sensor revealed mechanical properties of 0.12 MPa at 704.33% strain, with the highest gauge factor reaching 4.73 in the range of 125–200% strain [124][127]. In the second device, the in situ generation of silver nanoparticles on the cellulose skeleton was easily achieved via a heating process. This process not only offered excellent antibacterial properties to the hydrogels but also improved the mechanical properties of the hydrogels due to the elimination of the negative effect of silver nanoparticle aggregation. The tensile strength and toughness were able to reach as high as 2.0 MPa and 11.95 MJ/m3, respectively. Moreover, a high detection range (up to 1300%) and sensitivity (gauge factor = 4.4) were observed in the strain sensors [125][128]. A fundamentally different approach to the production of sensors using cellulose hydrogels and metals is described in the following example. An inorganic nanotube aerogel that has a framework consisting of hollow nanotubes was reported as a new class of porous material by Korhonen and co-authors. Firstly, nanocellulose hydrogels were prepared; then, they were freeze-dried to obtain a highly porous percolating network of cellulose aerogel. Titanium dioxide, zinc oxide, and aluminum oxide were applied on the aerogel templates using the atomic layer deposition (ALD) technique. Uniform oxide layers were readily deposited via ALD onto the fibrils, leading to organic–inorganic core-shell nanofibers. Once the composite was prepared, it was calcined at 450 °C to remove the organic core, leading to purely inorganic self-supporting aerogels consisting of hollow nanotubular networks. A titanium dioxide nanotube network was successfully applied as a resistive humidity sensor with a fast response [126][129]. The diverse applications of cellulose-based hydrogels integrated with metals and metal oxides illustrate their potential in producing multifunctional strain sensors with superior performance, environmental sustainability, and promising prospects for wearable technologies.2.6. Magnetic Materials
Magnetically responsive cellulose materials represent a class of smart materials characterized by the incorporation of magnetic nanoparticles into a polymer matrix. This combination enables these materials to dynamically alter their physical properties in response to external magnetic fields. Cellulose hydrogels can be made responsive to magnetic stimuli for applications in the areas of biotechnology/biomedicine, healthcare, environmental protection, catalysis, and magnetic resonance imaging [127][128][130,131]. Different iron oxides, namely magnetite (Fe3O4) and hematite/maghemite (Fe2O3), in the form of nanoparticles are commonly employed in the fabrication of magnetic cellulose materials. This is achieved by immersing a pre-formed hydrogel into a precursor salt solution, which is then subjected to a pH shift or a chemical reaction to initiate particle formation [129][132], resulting in the structure presented in Figure 4. For example, the one-step co-precipitation method was applied to produce magnetic cellulose hydrogels by dissolving cellulose in NaOH/thiourea/urea and combining the cellulose solution with pre-formed magnetic particles or via the dropwise addition of a solution of FeCl3 and FeCl2 into the cellulose solution. The NaOH in the cellulose solution acted as the precipitant of iron oxide nanoparticles, and cellulose was used as the template to promote the growth of nanoparticles. The Fe2O3 nanoparticles were dispersed in the polymer due to the synergistic effect. Magnetometric measurements revealed that the resultant cellulose/Fe2O3 composites exhibited sensitive magnetic-induced behavior and could be easily separated from an aqueous solution through the external magnetic field [130][133]. Another instance involved the preparation of a magnetic metallogel with hydroxypropyl cellulose and maghemite nanoparticles (~7 nm in size) at pH 13 without a chemical cross-linker. The metallogel, obtained due to reversible physical gelation and therefore sensitive to variations in pH, exhibited a magnetic moment when subjected to an external magnetic field and displayed superparamagnetic behavior. Significantly, hydroxypropyl cellulose can accommodate up to 100% of its weight in iron (II) oxide, leading to the formation of a complex structure that imparts a substantial magnetic moment to the gels [127][131][130,134]. Lin and collaborators reported a one-pot method for the synthesis of magnetic β-cyclodextrin/cellulose hydrogel beads, which exhibited rapid swelling–deswelling properties under an external magnetic field due to the incorporation of Fe3O4 nanoparticles. Cytotoxicity tests confirmed the excellent biocompatibility of the developed hydrogel [132][135]. In another study, the same research group suggested cellulose and chitosan coatings for Fe3O4 nanoparticles for the removal of heavy metal ions from solutions [133][136].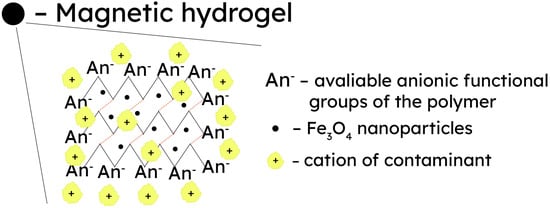
Figure 4.
An overview of treating heavy metal-polluted wastewater by harnessing the magnetic responsiveness of metallogels.
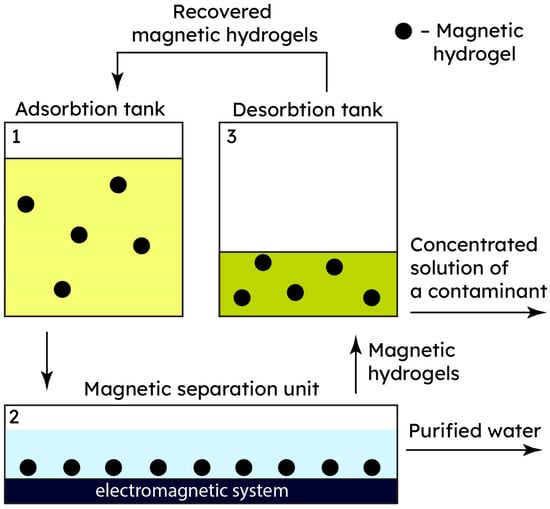
Figure 5.
The flowsheet for treating heavy metal-polluted wastewater using the magnetic responsiveness of metallogels.
