1. Introduction
A removable prosthesis residing in the oral cavity exposes the existing planktonic microbiota (bacteria, archaea, viruses, and eukaryotic organisms) to stress
[1][2][3][78,79,80]. These conditions are favorable for the growth of DMP
[4][5][6][81,82,83]. Quantitatively, this biofilm is defined as a community of more than 10
11 microorganisms per gram of dry weight
[7][8][84,85], attached to the extrados and intrados of the surface of the prosthesis and sur-rounded by an extracellular matrix (ECM) produced by the bacteria and
Candida themselves
[9][10][86,87]. This matrix, composed of macromolecules such as exopolysaccharides, proteins, and DNA
[11][88], provides structural integrity to the biofilm and offers a physical barrier that may be impenetrable to drugs.
In contact between the soft tissues, living tissue and the inert polymer provide another favorable environment in the oral cavity for microbial colonization
[12][13][14][89,90,91]. At the level of the intrados, this decreased space leads to a reduction in oxygenation, salivary flow, and pH, which promotes the activity of secreted aspartyl proteinases (SAPs) in the matrix. This environment plays a central role in the pathogenicity of
Candida [15][16][17][92,93,94].
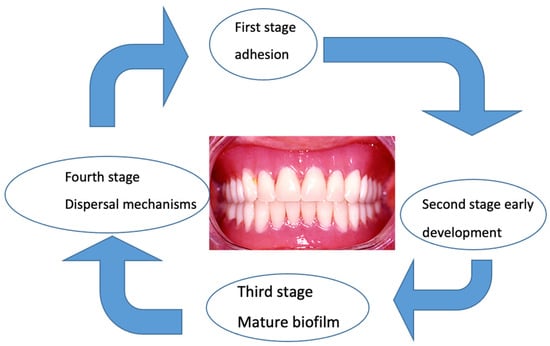
The maturation of the
C. albicans biofilm proceeds according to the same steps but more slowly than the bacterial biofilm. The presence of hyphae and pseudohyphae is the main difference between the two biofilms. Recent targeted studies have explained the initial adhesion to the prosthetic surface, the subsequent development of mature biofilms
[18][95], the formation of the extracellular matrix, and finally, the dispersal mechanism
[19][20][21][96,97,98] (
Figure 1).
Figure 1. Biofilm envelops the denture in distinct stages. In the transition from the planktonic, free-floating state to the sessile state, attached microorganisms begin radically changing their gene and protein expression profiles.
Up to three quarters of patients who wear removable prostheses can develop an inflammation called “denture stomatitis” (DS). This pathology is characterized by an imbalance of the microbial flora or dysbiosis, resulting simultaneously in an abundance of opportunistic pathogens such as
C. albicans [22][23][99,100], the differential proliferation of certain bacterial species determined using culture and next-generation sequencing (NGS)
[24][25][26][27][28][29][30][31][32][101,102,103,104,105,106,107,108,109], and a decrease in microbial diversity
[25][26][27][102,103,104].
Dental surgeons aware of the risk posed by this infectious condition to vulnerable patients should regularly check the oral health of users of removable prostheses
[33][110]. For this, although DMP cannot be totally eradicated, it can be controlled through oral hygiene practices that include a daily regimen of brushing the mucous membrane and the denture, followed by rinsing with an antiseptic mouthwash
[34][35][36][111,112,113]. Maintaining a healthy state helps to avoid the transition from a harmless commensal to a pathogen.
Several current precautions and methods make it possible to limit the drift of the oral microbiota toward dysbiosis in wearers of removable prostheses.
2. Polymers in the Oral Environment
Once it is introduced in the mouth, a denture is rapidly coated by saliva and constitutes the ideal platform for dynamic microbial growth of DMP
[37][38][39][115,116,117]. These biofilms represent a wide range of microorganisms, comprising all three domains of life. Their proximity to the denture polymer offers numerous possibilities for physical and chemical interactions between different species and kingdoms (Delaney, C.; 2019)
[40][118]. On the other hand, the interaction between the prosthetic base and the biofilm on the surface of the oral mucosa can favor the release of potentially toxic substances from the polymer that in turn interact with the host tissues
[41][119].
Biofilm development under an acrylic denture increases the risk of DS fivefold com-pared with a metallic denture
[42][120]. Another drawback associated with poor denture hygiene is bad breath, which can be the cause of patient discomfort
[43][121]. These bad odors are related to the microbial plaque of the denture
[44][122]. Studies using new technologies (next-generation sequencing, NGS) in the field of bacterial identification highlighted the emergence of the phyla Firmicutes and Fusobacteria and the genera Leptotrichia, Atpobium, Megasphaera, Oribacterium, and Campylobacter as being associated with the bad smell of prostheses. Here, good oral hygiene is essential to combat bad odors
[45][123].
In DS, lack of or ineffective brushing in the absence of a cleanser promotes the rapid growth of biofilm on the surface of prostheses
[46][47][9,10]. Clinically, the selection of polymer used for the prosthetic base must consider the adhesion of microorganisms. This colonization promotes the penetration of the microbiota and reduces the fracture resistance of prostheses
[48][124].
2.1. Polymer and Microbial Adhesion
A roughness (Ra) promotes adhesion and bacterio-fungal aggregation on acrylic resins
[49][125]. However, some authors point out that the initial colonization does not differ in accordance with the range of dental materials
[50][51][126,127]. In the same way, research has not highlighted a link between the roughness of the surface, the hydrophobicity/hydrophilicity of the acrylic resin, and the metabolic activity of adherent
C. albicans cells
[52][128]. Aggregation of
C. albicans with other microorganisms and the influence of saliva, through its antimicrobial power, flow, and composition, seem to dominate the conditions of adhesion to the surface of a prosthesis (roughness and SFE)
[53][129]. For other authors,
Candida adhesion was strongly affected by Ra, saliva, and bacteria, but not by SFE
[49][125]. Despite this discrepancy, the results suggest that a reduction in the
C. albicans biofilm may be related to modifications of the surface of the PMMA thanks to the coating. The coating promotes hydrophilicity and in addition to the influence of roughness
[54][130]. In addition, the DMP is subject to various mechanical constraints such as food tenacity, temperature fluctuations, chewing forces, and the load of the prosthetic device
[27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][48][49][50][51][52][53][54][55][56][104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131]. Microbial adhesion has been studied in relation to PMMA, in particular, and much less so in relation to PA and PEEK.
2.2. PMMA and Adhesion
PMMA is naturally hydrophobic
[57][19] Gad MM, 2022, but this material, which is used in the composition of dentures, contains many carboxylate and methyl ester groups. This chemical composition, on the one hand, accounts for the hydrophilic nature of the dentures and, on the other hand, produces a large amount of SFE. In vitro, the adhesion of Pseudomonas fluorescens proved to be favorable to hydrophobic surfaces, with the lowest adhesion threshold for a roughness of 0.4 μm. Although the weakest adhesion of mammalian cells occurred at a roughness of 0.1 μm, the latter was favored in the presence of hydrophilic surfaces (PMMA) Choi SY, 2016
[58][132]. However, the variations in the chemical composition of the material used for the denture base partly explain the disparity in characteristics between the different brands of PMMA on the market Sipahi, 2001
[59][133]. Compared to the traditional fabrication method, acrylic resin injection offers a reduction in the surface roughness of the prosthesis base as well as decreased bacterial adhesion
[60][21] Moslehifard E, 2022
(Table 1).
2.3. Polyamide and Adhesion
Analysis of the adhesion of microorganisms, in particular, yeasts, to PA remains very limited. Nevertheless, an experiment conducted on the effect of a prosthetic cleanser on the formation of a mycofilm on a PA resin (Flexite MP) and a polymethyl methacrylic resin (Acron MC) showed that
C. albicans had a significantly higher growth rate on PA than on PMMA de Freitas Fernandes FS
[61][134].
As a crystalline polymer, PA has better biocompatibility for patients who are allergic to acrylic resins. But over time, PA has significant disadvantages, displaying high water absorption, increased solubility, an overly rough surface, and bacterial contamination. In addition, this material remains difficult to polish and may result in color deterioration in the mouth Vojdani, 2015
[62][135]. Higher microbial adhesion was recently observed on injection-molded PA than PMMA
[63](Table 1) [16] Sultana, 2023.
In order to remedy this, minimal changes in the injection manufacturing protocol of two PA prosthetic base materials were tested in vitro (Perflex Biosens (BS), Netanya, Israel and VertexTM ThermoSens (TS), Soesterberg, The Netherlands. By slightly modifying the melting temperature (5 °C) and pressure (0.5 bar), no improvement in the surface finish was observed for Biosens, whereas for ThermoSens, the surface roughness was significantly reduced Chuchulska, 2022
[64][136].
2.4. PEEK and Adhesion
As early as 2007, Kurtz et al. emphasized the non-allergenic properties of PEEK and its low affinity for dental plaque. PEEK is considered hydrophobic and has a low SFE. As a result,
C. albicans adhesion is facilitated
[65][66][27,137]. This was compared to the formation of biofilm on the surface of different materials in vitro (zirconia, titanium, PMMA, and PEEK). In their study, PEEK and PMMA yielded the same results but were linked to less biofilm formation than zirconia and titanium. However, the surface condition of PEEK was smoother than that of zirconia and titanium
[67][138]. It has been reported that PEEK has good biocompatibility in vitro and in vivo, causing neither toxic nor mutagenic effects nor clinically significant inflammation. In addition, PEEK lends itself to sufficiently effective polishing so as to delay the fixing of microbial plaque
[68][139]. PEEK without any additives is biologically inert and naturally hydrophobic when in contact with saliva. The 80°–90° contact angle of saliva can be reduced by adding plasma coatings, which are effective methods for modifying surface properties
[69][140] to improve the hydrophilicity
[67][138].
When comparing PEEK with other computer-aided design/computer-aided manufacturing (CAD/CAM) materials, PEEK samples are slightly rougher than PMMA samples. The reason is linked to the ceramic particles that are added to PEEK
[70][25] (Table 1).
2.5. Polymer and Accumulation of DMP
After the adhesion of the first colonizers on the denture surface, to preventively limit the accumulation of microorganisms, and particularly of
Candida and bacteria populations, several parameters can be modified to facilitate the optimization of the manufacture of polymers. The incorporation of antifungal agents into denture base resin may reduce the colonization of
C. albicans [71][141]. There are few data on PAs and PEEKs, whereas PMMAs, in contrast, have been the subject of numerous experiments
(Table 3).
For example, nanoparticles (such as fluoridated apatite-coated titanium dioxide, FAp-TiO
2) in PMMA facilitate the production of reactive oxygen species by promoting the photocatalytic effect after irradiation, which neutralizes the attachment of
C. albicans. This effect is sought to facilitate the maintenance of removable prostheses in geriatric patients
[72][142]. The incorporation of bioactive glass (BAG) in thermopolymerized or polymerized acrylic resins at room temperature significantly lowers the adhesion of
C. albicans. For both types of polymerization, the hardness of acrylic resins is improved by adding BAG
[69][140].
Another parameter can be modified to promote hydrophilicity to limit the adhesion of
C. albicans on an acrylic resin denture with photopolymerized coating
[73][143]: Plasma treatment of PMMA on the surface increases SFE, facilitates wettability, and lowers the contact angle, all of which reduce the adhesion of
C. albicans [74][75][144,145]. In contrast, trimethylsilane coating increases hydrophobicity, reduces wettability of the denture base surface, and inhibits the adhesion of
C. albicans [76][146]. The TiO2 coating creates a super-hydrophilic surface. It thus promotes wettability, which is essential for reducing
Candida adhesion. The implementation of the PMMA surface coating involves only moderate costs while preserving the properties of the original material
[77][78][147,148]. Recently, to assess the effectiveness and the antibacterial properties of a silver nanoparticle (NAg), a solution of NAg mixed with acrylic acid and methyl methacrylate (MMA) monomer was tested (in vitro and in vivo on animals) and compared with a PMMA solution without NAg. The results concerning the state of the prosthetic surface, the mechanical properties, the antimicrobial effect of NAg, the longevity, and the biological and toxic harmlessness of the NAg/PMMA prosthesis base were superior to the PMMA base without NAg. However, clinical confirmation must be provided by studies with humans
[69][79][80][81][82][83][84][85][41,42,43,44,140,149,150,151].
2.6. Polishing to Limit Microbial Adhesion
The adhesion of early microbial colonizers is closely related to the finish of the denture surface. This adhesion during the initial phase of microbial colonization on flexible prostheses is similar to that of acrylic resin prostheses. This result was confirmed by a laboratory study showing that acrylic resin and PA resin are easily colonized by
Candida species. However, the growth rate of this fungus is significantly higher on PA resin than on PMMA (
p < 0.001)
[86][152].
Different tests of the surface condition of the material (polishing) have shown that the polishing method alone (wood sandpaper: grit 180) is essential in terms of roughness compared with the drying method of self-curing acrylic resin. Moreover, chemical polishing (at 70 °C for 10 s) aggravates the roughness
[87][88][74,153]. Regarding PMMA resin, the residual monomer acts on the SFE by reducing adhesion and
Candida growth
[61][134]. For PA resin (Breflex polyamide, Bredent, GmbH Co. KG, Senden, Germany) fabricated using the injection-molding technique, no significant correlation was observed in contact angles for mechanical polishing versus chemical polishing. This difference was related to the specific physical properties of the materials used
[89][31].
The design and manufacture of CAD/CAM prostheses machined from blocks of polymerized PMMA under high temperature and high pressure led to a smoother surface finish than PMMA-HC based on CAD/CAM prostheses
[90][154]. As a result, for patients at risk of
Candida fungal infection, the surface properties of CAD/CAM PMMA represent a possibility of reduced adhesion of this fungus
(Table 2).
Quezada (2022)
[91][37] and Corsalini (2009)
[92][33], using the same in vitro mechanized and manual polishing methods, attempted to standardize a polishing protocol. However, since contradictory results were reported, with one favoring the manual method and the other the mechanized method, new investigations have to be carried out.
An explanation for the contradictory results is offered by previous research. The structure of PMMA directly after polymerization had a low initial roughness, and subsequent polishing made it easy to reach clinically acceptable values. On the other hand, PAs were more difficult to polish due to their fibrous semi-flexible structure and low surface hardness
[93][155]. Although PEEK and PMMA have similar values of Vickers hardness, the composition and the state of the surface roughness differed between the two materials
[94][156]. Therefore, surface polishing that is specific to the two materials is required.
Thus, regarding the polishing of PEEK, Kurahashi et al. (2020)
[95][39] suggest the use of a soft brush coupled with a cleaning agent for more than 3 min to achieve clinically acceptable surface roughness
(Table 2).
Heimer et al.
[79][41] compared the effects of laboratory and chairside polishing methods on the surface roughness of PEEK and reported that chairside polishing of PEEK yielded lower surface/laboratory roughness values
(Table 2).
Fused deposition modeling of PEEK is one of the most practical additive techniques; compared to other polymers, PEEK remains stable over the long term regarding its wear and color
[96][97][157,158]. The biocompatibility and biostability of PEEK are supported by the U.S. FDA drug and device master files
[98][159]. Another way to limit the initial adhesion of microorganisms and particularly of
C. albicans on the prosthetic surface is to use a coating.
2.7. Denture Base Surface Coating to Limit Adhesion
Among the types of coatings available, cold plasma under heat-polymerized acrylic resin prevents the early adherence of
C. albicans [99][160]. Another goal for coating the polymer (PMMA) with creamers is to enhance the resistance of the denture base surface. Indeed, coating creamers (inorganic–organic hybrid polymeric) enhance the scratch resistance of PMMA denture resin (increasing the flexural strength (FS), flexural modulus (FM), and hardness)
[100][101][161,162]. To date, in view of the diverse results of experiments, no consensus has been reached on this topic. To fight against the adhesion of
Candida, the surface of the denture base must be smooth, hydrophilic, and without roughness. Further investigations are needed to better understand the correlation between factors affecting the hydrophobicity of the denture base and the adhesion of
C. albicans.2.8. Effects of Cleaning on Denture Materials
Currently, the use of a prosthesis cleanliness index makes it possible to assess the hygiene of prostheses by visualizing the quantity of stains on the intrados of the denture. Rinsing beforehand eliminates invisible microbial plaque. The scores, ranging from 0 (best) to 4 (worst), help to adapt the hygiene instructions for the wearers of dental prostheses
[102][163].
The use of bleach-based cleansers, according to the recommended dosages (containing 1.5% or 2%
w/
v sodium hypochlorite and/or 1.7%
w/
v sodium hydroxide) and duration of use (at least 3 min daily), is associated with sufficient antimicrobial activity against
Streptococcus mutans and
C. albicans, without any changes to acrylic color, surface roughness, or mechanical properties
[103][104][164,165]. However, in the long term, these cleansers corrode and tarnish metal prostheses. Effervescent cleansers have also proven their effectiveness, but they are not recommended in the presence of prosthesis relining materials.
Manual brushing with a toothbrush plus soap and water is the most common method for maintaining removable dentures (Milward P, 2013)
[105][166]. Several adjuvants to increase the effectiveness of manual cleaning in the form of pastes, gels, foams, and powders are on the market
[106][167].
The use of antiseptics to inhibit or eliminate microorganisms and immersion in a chemical solution for 8 h are recommended. Sodium hypochlorite, chlorhexidine diglconate, and alcohol can disinfect or reduce the dental plaque on acrylic resin dentures without being cytotoxic
[33][35][36][110,112,113]. The different methods of cleaning dentures can influence the physical and aesthetic characteristics of the prosthesis materials. Also, in order to ensure the clinical durability of removable prostheses, patients and clinicians should be aware of the manufacturer’s instructions for use
[107][168].
Although there is no consensus regarding how to best maintain prosthetic hygiene compatible with the patient’s state of health
[108][61], the disadvantages of many procedures have been thoroughly evidenced
[109][169].
Hydrogen peroxide-based disinfectants should not be used regularly, as they cause surface roughness of the PMMA. NaOCl is less aggressive and generates slight alterations on the surface of the prosthetic base
[110][170]. In addition, sodium hypochlorite was found to be non-cytotoxic after six months of use
[111][171].
Flexural strength is reduced by immersion cleaning of removable PMMA prostheses modified with nano-ZrO
2. Thus, a significant decrease in this resistance after immersion in different denture cleansers was reported, which was strong for sodium hypochlorite, intermediate for Corega, and low for Renew
[110][112][113][170,172,173]. Several habits should be avoided, such as rinsing with boiling water and prolonged maintenance in a dry atmosphere or water, because these alter the qualities of PMMA and promote microbial colonization. Both bleach and isopropyl alcohol (IPA) are highly antimicrobial, but bleach is incompatible with components of metal dental prostheses and IPA mouthwashes damage PMMA
[114][174].
Concerning denture cleaning tablets, the polarity of the resins, the concentrations of the tablets, and the chemical content of the cleanser may directly affect the formation of
C. albicans biofilm
[115][68]. Thus, the dosage and prescription of disinfecting tablets can vary depending on the resin used to make the prosthetic base. In tablet form, Polident
® has been proven to be effective as a denture cleanser. But after 30 days of immersion in a solution based on Polident
®, the heat-polymerized acrylic resin may undergo alterations to its physical and mechanical properties. This may be related to the accelerated aging of resins caused by chemicals found in denture cleansers
[116][175].
The mechanical properties of PEEK do not change during the sterilization process. An in vitro study showed that the solubility of PEEK in physiological saliva and distilled water is lower than that of PMMA
[94][156]. In the study by Demirci under the same conditions, the solubility values of PEEK in distilled water were found to be similar to those of PMMA (HP: Ivoclar Vivadent AG., Schaan, Liechtenstein). In the presence of a cleanser (Corega tablet, Protefix tablet (PT), 1% sodium hypochlorite (NaOCl)), the solubility values of PEEK were found to be lower than those of PMMA. In this study, higher water sorption and solubility values were observed than those obtained by Lieberman
[94][156]. The explanation proposed mentions the consequences of the effects of cleansers on PEEK and PMMA surfaces for 120 days. Thus, for these authors, the water sorption and solubility values of PEEK can be attributed to the molecular imbalance occurring on the surface of the PEEK
[117][62].
The use of microwave disinfection in combination with denture cleansers and brushing has also been shown to effectively disinfect dentures, although microwaves may also physically distort denture resin
[118][176]. The personalized implementation of the currently available means for disinfection is informed by the general condition of the patient, the material composition of the prosthetic base, and the presence or absence of DS.
3. Denture Base Relining
After some time (following bone resorption), it is necessary to reline the intrados in order to improve the stability, support, and retention of removable dentures. There are several commonly used relining materials, such as cold or hot polymerization, polymerization in visible light, and acrylic resins polymerized in microwaves
[119][120][177,178].
At the interface between the reliner and the prosthetic base, the bond strength depends on the chemical composition of the two materials that come into contact with each other
[121][179]. The bonding strength can be improved by treating the two surfaces that are in contact with each other
[121][122][123][124][179,180,181,182]. The parameter characteristic of relining is the shear bond strength (SBS). This parameter is better for relining using thermosetting resin as well as both CAD/CAM and conventional thermosetting denture resin compared to self-curing relining resin
[125][126][183,184]. An in vitro study showed that reliners with thermopolymerizable acrylic resins had an increased SBS compared to reliners with self-curing acrylic resins. This also applied to bases of conventional dental prostheses and CAD/CAM but without a significant difference. However, there was a significant difference between autopolymerizing acrylic resin bond strength with CAD/CAM and conventional denture bases.
Autopolymerizing reliner material seems to produce a stronger bond with CAD/CAM denture bases. It has been pointed out that self-curing relining material appears to produce a significantly stronger bond with a CAD/CAM denture base compared to a conventional resin base
[126][184]. Recently, various in vitro tests of the adhesion of composite materials on thermosetting resins, on CAD/CAM, and on printed groups yielded the following results: In order of the best performance regarding the adhesion of high-viscosity/low-viscosity composites (SR Nexco, high viscosity (SR); and Kulzer Creactive, low viscosity (K)), the thermosetting resin group was first, followed by the CAD/CAM group, and finally the 3D-printed groups. However, the differences noted between these groups were not significant
[127][185].
To assess the maintenance of rebased resin bases, five disinfectant solutions were tested: sodium hypochlorite, sodium perborate, chlorhexidine gluconate, apple vinegar, and distilled water. A prosthesis base (Vipi Wave) rebased with an acrylic resin (Tokuya-ma Rebase Fast II) after dipping showed alterations in its roughness regardless of the solution used
[113][128][173,186]. Kim et al. tested relining using two hard resins, one of the self-hardening type (Tokuyama rebase II) and the other of the light-activated type (Mild Rebaron LC). They carried out these two relinings on a thermoplastic polyamide resin (Biotone; BT), on a classic thermopolymerizable acrylic resin (Paladent 20; PAL20), and, finally, on a thermoplastic acrylic resin (Acrytone; ACT). The results showed that the thermoplastic polyamide resin (Biotone) had the lowest adhesion strength of the three materials tested
[129][69].
More recently, Vuksic Josip et al. (2023)
[130][70] tested relining (with a soft denture liner and a silicone-based, direct relining method) on several resins: (1) Meliodent heat cure (Kulzer, Hanau, Germany), denture base material, PMMA, heat-cured; (2) Vertex Thermosens (Vetex Dental, Soesterberg, The Netherlands), denture base material, PA, technical injection; (3) three CAD/CAM subtractive materials; and (4) two CAD/CAM additive materials. With the same reliner (GC Reline II Soft), the bond strength of the PA (Vertex) and both additive manufactured denture bases was significantly lower than that of the three materials used for subtractive denture fabrication and heat-cured PMMA
(Table 4). However, the authors expressed their reservations because, to date, there are only a few studies available, mainly on flexible rebasing. The tests differ between these studies, and different materials were used as controls (PMMA from different manufacturers).
The bioinert nature of PEEK can make adhesive bonding difficult. The SBS of PEEK can be increased by roughening the material or by embedding molecules on the surface through sandblasting, acid treatment, laser, or adhesive systems.
SBS values greater than 10 MPa between PEEK and resin-based composites have been reported to be clinically acceptable. However, the hydrophobic surface and low SFE of PEEK make it difficult to establish a strong and long-lasting bond. Therefore, PEEK material surface treatments and adhesive systems with resin are hot research topics focused on the application of PEEK in the restorative field. Modalities concerning the effectiveness of bonding to the surface of PEEK are not yet sufficiently developed for routine use.
4. General Conditions and Dentures
Whichever material is chosen, after adhesion, inadequate oral hygiene facilitates the accumulation of biofilm, colonizing the surface of the prosthesis. This biofilm can constitute a risk factor for infection, especially for patients who are older or who are immuno-compromised and/or have endocrine deficiency
[131][187]. For these patients, special vigilance is necessary regarding prosthetic oral hygiene in order to avoid infectious complications.
Indeed, this additional microbial load can lead to an imbalance between bacterial species, bacteriophages, and fungi, thus promoting the resistance and virulence of mycofilms to the detriment of the host. The secretions of bacteria and fungi, by participating in the aggression of biotic surfaces (mucous membranes and teeth), promote the production of various inflammatory mediators such as cytokines
[132][188]. The use of removable prostheses in these conditions after a certain period of time promotes bone resorption.
Some older denture wearers have medical conditions such as arthritis and dementia that can impair their ability to carry out oral hygiene procedures effectively, thus requiring assistance from caretakers and some education
[133][189]. Specific treatments are available if a
Candida infection is suspected
[134][190], with accompanying denture disinfection/cleaning or replacement
[135][8]. Other conditions such as Parkinson’s disease can lead to dentures falling out of patients’ hands because of trembling. In these cases, thanks to its flexibility, the PA prosthesis makes it possible to overcome small bone and mucous undercuts. Crossing this undercut promotes retention. The prosthetic base made of PA, due to its high resilience and impact resistance, is less prone to fractures than PMMA
[136][191].
For patients who are hypoallergenic to prosthetic materials, different alternatives exist. Whether PMMA, PEEK, or PA, the polymerization reaction releases more or fewer toxic molecules. By dissolving in the saliva, these molecules can diffuse away from the mouth
[137][138][192,193]. These are essentially, after the polymerization, the residual monomers (MMA, methyl methacrylate; BuMA, butyl methacrylate; EMA, ethyl methacrylate; EGDMA, ethylene glycol dimethacrylate) that are responsible for the toxic and allergenic effects of acrylates
[139][194].
This residual monomer depends both on the method of polymerization (duration, cold or heat) and on the volume of the prosthetic base; it only becomes stable after 2 weeks of wearing the dentures. It is low for thermopolymerized resins and at the palatal level of the thin prosthetic base
[135][8].
Moreover, the acidic environment and the temperature of the oral cavity promote the release of substances contained in resins such as formaldehydes, benzoyl peroxides, benzoic acid, hydroquinone, and phthalates, as well as cobalt, nickel, and beryllium. With respect to the mucous membrane, these products can cause type IV allergic reactions, or an intolerance can appear in the long term
[140][195].
As a remedy, so-called hypoallergenic resins for dental prostheses have appeared on the market. To be suitable for hypoallergenic patients, the denture base resins should contain only a very small amount of MMA
[141][196].
MMA can be replaced by diurethane dimethacrylate, polyurethane, polyethylene terephthalate, polyethylene terephthalate, or polybutylene terephthalate. However, only two of these have similar mechanical characteristics to PMMA resin standards: Polyan Plus
® and TMS Acetal Dental
[140][195].
The in vitro comparison between PMMA and PA regarding cytotoxicity has not revealed any obvious differences. Findings remain disparate about the materials studied and the protocols used. The results vary depending on the duration of the experiments and on the different parameters analyzed, such as temperature and surface condition supplement II. For patients with low stress tolerance and sensitivity to metallic materials, PEEK is indicated for partial removable prostheses. PA bases are also an alternative for patients who are allergic to other denture base materials and for patients with microstomia
[142][26].