Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Alicja Nowaczyk and Version 2 by Jessie Wu.
Dysrhythmia is a term referring to the occurrence of spontaneous and repetitive changes in potentials with parameters deviating from those considered normal. The term refers to heart anomalies but has a broader meaning. Dysrhythmias may concern the heart, neurological system, digestive system, and sensory organs. Ion currents conducted through ion channels are a universal phenomenon. The occurrence of channel abnormalities will therefore result in disorders with clinical manifestations depending on the affected tissue, but phenomena from other tissues and organs may also manifest themselves.
- dysrhythmia
- electrobiological techniques
- pharmacotherapy
- patch clamp
1. Cardio Arrhythmia
The term arrhythmia has been in use for many years. It appears in this form even in the titles of textbooks and manuscripts. However, the strict meaning of it is no rhythm. Primarily, the word was meant to indicate the lack of a “correct” rhythm (Figure 1). Due to doubts, recently the term dysrhythmia is increasingly being used. It should be emphasized, however, that arrhythmia is still a word assigned to cardiology.
An irregular heartbeat indicates that something has interfered with the heart’s natural rhythm. However, a regular rhythm does not necessarily mean that there is no arrhythmia. There are numerous examples of abnormal heart rhythms with regular ventricular activity. They are thus more difficult to detect when relying solely on clinical symptoms. Examples include an atrial flutter with permanent conduction block, atrial fibrillation with third-degree atrioventricular block, or rhythms coming from centers lower than the sinoatrial node. This might be caused by an odd electrical signal that regulates the heartbeat. This signal could be halted by cardiac scar tissue, or it might begin too early and appear as though the heart is skipping beats. Disturbances in the electrical characteristics of the heart are known as arrhythmias. These disturbances can be split into two categories: automatic abnormalities, characterized by an automatically generated electrical impulse, and abnormalities, which occur when an impulse is conducted after it has been generated (transmission abnormality). From a different perspective, arrhythmias can be classified into tachyarrhythmias, corresponding to an accelerated rhythm, and bradyarrhythmias, referring to an abnormally slow heartbeat. (Figure 1). Moreover, heart rate variability (HRV), the fluctuation in the time intervals between adjacent heartbeats, is commonly used to characterize the interplay between sympathetic and parasympathetic systems [1][2][15,16]. It has been demonstrated that HRV parameters are frequently decreased in patients with cardiac, autonomic neuropathy, and other pathological conditions [3][4][5][17,18,19]. Arrhythmias are further divided into groups according to their anatomical origin, such as atrial, atrioventricular (AV), or ventricular (occurring below the bundle bifurcation) [6][7][8][9][20,21,22,23]. Paroxysmal atrial fibrillation, altered HRV, tachycardia, ventricular repolarization disorders manifested by prolonged corrected QT segment (Figure 2), changes in the morphology of the ST segment and T-wave, as well as numerous supraventricular and ventricular extrasystoles, are among the most frequently mentioned electrocardiographic disorders [10][11][24,25]. The frequency of these disorders significantly correlates with an unfavorable final outcome of treatment, not only during hospital treatment but also in the period up to a year after its completion [10][24].
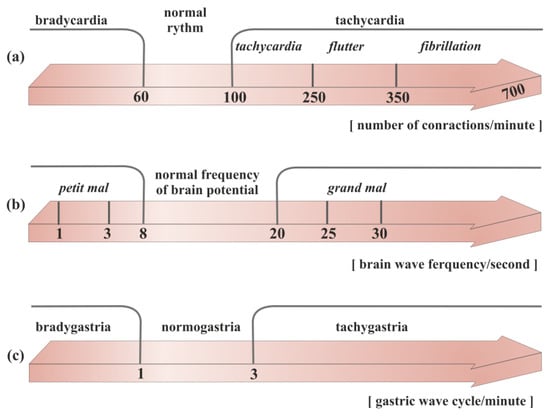
Figure 1.
Division of the rhythm of propagation of electrical waves in different organs of the body: (
a
) heart, (
b
) brain, (
c
) stomach.
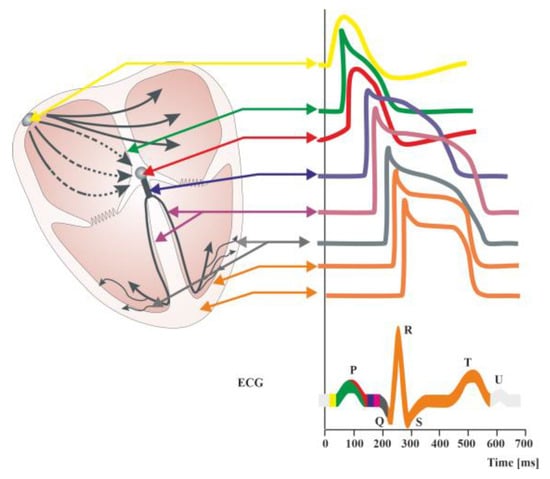
Figure 2. Diagram of the distributions of action potentials in individual cells of the cardiac stimulus–conduction systems. Summary changes in cardiac action potential are recorded in the form of an electrocardiographic curve (ECG). ECG showing the course of electrical phenomena during normal cardiac activity (healthy heart). The ECG obtained from the surface of the skin is the sum of the potentials conducted in the working heart. It is possible to record potentials in individual structures of the conduction system of the heart during electrophysiological examination. The QT interval includes the duration of depolarization (QRS complex) and repolarization (ST segment and T-wave) of heart muscle cells. A normal QT interval is defined as less than 450 [ms] in men and less than 460 [ms] in women [12][9]. Prolongation of the QT interval (LQTS syndrome) is indicative of a slowing of the repolarization process, meaning that the return to the resting membrane potential value after the depolarization of cardiomyocytes is delayed. This favors the occurrence of polymorphic ventricular tachycardia of the torsade de pointes type clinically referred to as torsade de pointes ventricular arrhythmia (TdP), which can cause syncope and sudden cardiac death in healthy, young individuals [12][9].
2. Gastro Dysrhythmia
Tachygastria is characterized by an increase in the frequency of the slow wave; bradygastria occurs when the frequency is lower than normal; and arrhythmia occurs when there is no rhythmic activity, based on the dominant frequency of gastric myoelectric activity (GMA) (Figure 1). Abnormalities of the gastric slow-wave frequency (tachygastria, bradygastria, and arrhythmia) may occur spontaneously, which is associated with disturbances in normal gastric contractile activity.
Numerous human and animal investigations have demonstrated that a variety of neurohumoral variables contribute to the development of stomach dysrhythmias. Slow-wave disturbances may be brought on by antral distension and enhanced lipid supply to the intestines, which may then result in the emergence of nausea. The cholinergic and serotonergic pathways may play a role in mediating this. Similar to estrogen, progesterone can also interfere with a person’s stomach’s slow-wave pattern. Slow-wave dysfunction in diabetics and smokers appears to be mediated by an excess of prostaglandins in the gastric smooth muscle. On the other hand, the development of stomach dysrhythmias related to motion sickness is significantly influenced by the central cholinergic pathways. Vasopressin secreted by the pituitary may play a role in mediating this. Although it is challenging to pinpoint a precise causal role for slow-wave rhythm disruptions in the development of nausea and vomiting, the quest for innovative antiemetic medicines has begun based on their potential to eliminate or stop the development of stomach dysrhythmia. Prostaglandin synthesis inhibitors, central muscarinic receptor antagonists, and dopamine receptor antagonists are examples of this [13][26].
Gastric dysrhythmias are associated with a variety of clinical disorders, some of which may contribute to the production of nausea and vomiting. With diabetic gastroparesis, tachygastria and bradygastria can develop in up to 70% of patients [14][27]. Some diabetics show concurrent loss of the increase in signal amplitude typically seen with meal eating in addition to electrocardiogram rhythm abnormalities. Vomiting frequently occurs in conjunction with the occurrence of stomach dysrhythmic activity.
Several pieces of evidence point to the stomach’s ability to make its own prostaglandins as a major cause of gastric dysrhythmic activity [15][28].
It has long been understood that the stomach and heart are connected. Heberden initially gave a description of it in 1768. It was referred to as Roemheld’s gastro-cardiac syndrome in later years. This syndrome (AF) shows how upper GI disease can lead to chest pain that feels like angina and heart rhythm problems, such as atrial fibrillation [16][17][29,30].
It is generally acknowledged that gastroesophageal reflux disease (GERD) and non-cardiac chest discomfort are related. It is hard to tell the difference between chest pain caused by esophageal and cardiac ischemia because the heart and distal esophagus share an afferent vagal supply. Also, GERD can cause chest pain that does not come from the heart but feels like it does. This results in the misinterpretation of GERD chest discomfort as angina pectoris, and vice versa, in clinical settings. Additionally, the presence of both GERD and myocardial ischemia may make someone more susceptible to developing myocardial ischemia by tipping the sympathovagal balance in favor of the parasympathetic component. This mechanism might set off a cardiac–esophagogastric reflex, which would reduce myocardial perfusion [18][31].
There are at least four different ways to look at the “pro-arrhythmic” interaction between the circulatory system and the digestive tract: anatomical, neurogenic, endocrine, inflammatory, and neurogenic factors. Each of these components has the ability to alter the activity of the three main elements that lead to the development of AF: (a) the triggering stimulus (physiological and pathological automatism, triggered activity, electromechanical coupling), (b) focal conduction disorders (distribution of atrial muscle fiber refractory periods, local slowing of conduction), and (c) the creation of local circulating excitation waves and loops [19][32].
Vagal activity increases when the esophagus environment becomes more acidic. Therefore, acid gastric reflux may be the cause of arrhythmia events, particularly at night, including the so-called vagotonic AF. When the vagus nerve causes an irregular heartbeat, it usually shows up as changes in the heart rate and conduction velocity, as well as the circulating excitation wave becoming shorter and more spread out [20][21][33,34].
3. Neuro
Neurocardiology is a relatively new, extremely interesting, and still developing field of medical knowledge. The effects of stress and sudden emotional states of high intensity on the cardiovascular system, and particularly on heart function, continue to attract great interest [22][35].
The central nervous system (CNS) is a complex structure containing numerous centers that control the functions of many organs, including the heart muscle. The interactions between brain function and the heart muscle are commonly known as the brain–heart axis. Many CNS pathologies induce myocardial dysfunction [23][36]. Cardiac arrhythmias resulting from traumatic brain injury (TBI) can lead to significant hemodynamic failure or cause sudden cardiac death (SCD). Various types of arrhythmias are also observed in people experiencing strong emotions or stress. These relationships clearly prove a significant relationship between brain function and heart muscle function [11][24][25,37].
It is also noteworthy that 88% of individuals with acute cerebral ischemia experience substantial cardiac damage. Cardiovascular arrhythmias can occur more or less frequently in TBI patients, and their incidence varies and is highly correlated with the nature of the damage and any associated fluid and electrolyte problems. Patients with severe intracerebral bleeding are most at risk for different types of electrocardiographic abnormalities (60–70% of patients need cardiac care), whereas isolated cerebral edema increases the risk of different arrhythmias in roughly 40% of patients. It should be underlined that patients with an altered water and electrolyte balance (e.g., due to therapy or intracerebral disease) were found to experience cardiac arrhythmias significantly more often than other patients [25][38].
Heart arrhythmias are mostly caused by an imbalance in the activity of the sympathetic and parasympathetic systems, a rise in the level of stress hormones in the blood, and a general response of inflammation.
Indeed, both experimentally and clinically, it has been demonstrated that neuroanatomic linkages between the brain and the heart can cause cardiac arrhythmias to occur in response to brain stimulation. Additionally, seizures can cause Takotsubo syndrome (TTS) and a range of temporary cardiac consequences, such as alterations in heart rate, heart rate variability, arrhythmias, asystole, and other ECG abnormalities [24][37].
The links between heart and brain disease can be divided into three groups:
-
Cardiovascular ischemic strokes (e.g., atrial fibrillation, valvular disease, etc.). Epidemiological data show that AF increases the risk of stroke by about 5-fold. Predisposing factors for AF include structural and electrical remodeling of the atrial structures, which plays a key role in initiating and maintaining AF. Atherial remodeling is caused by many factors, such as stretch-induced fibrosis, hypocontractility, fatty infiltration, inflammation, vascular remodeling, ischemia, ion channel dysfunction, and calcium instability. It is worth noting that these factors are also associated with stroke. Some of the fast atrial impulses are directed to the ventricles; some penetrate only the atrioventricular node, increasing its refraction; ventricular rate and irregularity are the result of implicit conduction of atrial impulses. During increased sympathetic tone, the refraction of the atrioventricular node sharply decreases, which leads to a disproportionate increase in heart rate. Platelet activation and thrombin production go up when atrial fibrillation (AF) or fast atrial rates (FA) happen. This happens more in the left atrium than in the systemic circulation. AF additionally induces endothelial dysfunction and inflammation. Thus, while a fast atrial rate increases thromboembolic risk, AF may increase it even more [26][39]. In addition, over the last two decades, many studies have indicated the relationship between AF and cognitive functions. AF is now a recognized risk factor and predictor of cognitive decline and dementia [27][40].
-
Neurocardiological diseases are a group of genetic abnormalities that affect the nervous system and cardiac muscle. Examples of these diseases include Friedreich’s disease, myotonic dystrophy, and Kearns–Sayre syndrome. These disorders cause abnormal neuromuscular signaling and mitochondrial dysfunction, which can lead to cardiac manifestations such as arrhythmias and cardiomyopathies due to disruptions in neural signaling and mitochondrial energy deficits.
-
Neurogenic heart diseases refer to conditions where brain dysfunctions have a profound impact on cardiac physiology. For instance, heightened stress or anxiety responses caused by sympathetic nervous system activation can trigger cardiac arrhythmias and affect heart rate dynamics. Additionally, neurological disorders such as epilepsy can cause Takotsubo syndrome (TTS) and a range of temporary cardiac effects that include fluctuations in heart rate variability, arrhythmia, and even asystole, as shown in electrocardiographic recordings [22][28][29][30][35,41,42,43].
4. Epilepsy
One of the best-known rhythm abnormalities that can lead to a number of disorders is brain electric dysrhythmia (Figure 1). The normal electrical activity of the brain is disrupted when seizures occur. More precisely, a clinically observed seizure is merely an external manifestation of a disturbed rhythm of brain potentials. The 2017 International League Against Epilepsy (ILAE) revised the classification of seizure types [31][6]. The electroencephalograph (EEG) can clearly classify rhythms into three main groups, which correspond to the three main categories of epileptic seizures. They are all characterized by a large increase in voltage during the peak of the seizure. The feature distinguishing them is the difference in the waves’ frequency. In tonic–clonic (also called grand mal), these waves speed up to 25–30 per second [32][8]. In psychomotor attacks (psychic variants), the pace slows down to 3 or 4 per second. In nonmotor (also called absence or petit mal), there are alternating changes at a rate of 3 per second [12][9]. It can be said simply that in grand mal, cortical activity is abnormally fast; in a psychomotor attack, it is abnormally slow; and in petit mal, it alternates between fast and slow [33][7].
The common neurodegenerative condition, epilepsy, is characterized by paroxysmal cerebral dysrhythmia. Through their influence on the autonomic system, which serves as the last effector that modifies heart activity, cortical regions and subcortical structures are connected to cardiac function (Figure 2). Additionally, feedback from the cardiovascular system can affect the autonomic outflow by activating neurocardiac reflexes. Controlling the flow of calcium, sodium, and potassium through different ion channels on the surface of cardiomyocytes is important for controlling the heart’s electrical and mechanical activity through sympathetic and parasympathetic nerves. The parasympathetic system has the opposite effect, and the sympathetic system improves heart rate (HR), repolarization, contractility, and relaxation by changing these things. Given that reduced arrhythmic thresholds are caused by increased sympathetic activity, genetic susceptibility to enhanced responsiveness to autonomic innervation is demonstrated by a variety of ion channel diseases [24][37].
Since epileptic people have a higher prevalence of cardiac comorbidities than the general population, seizures can cause a variety of transient cardiac effects during the pre-ictal phase, including an increase in blood pressure (BP), changes in HR, arrhythmias, and other ECG abnormalities [24][34][37,44].
The risk of arrhythmias and sudden cardiac death is higher in people with a genetic predisposition to an increased response to autonomic innervation, as evidenced by a reduced arrhythmic threshold caused by increased sympathetic activity (e.g., long QT syndrome [LQTS], Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia, Figure 3).
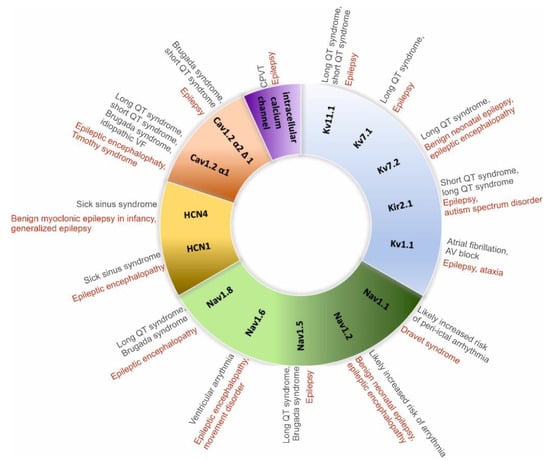
It is possible to see signs of peri-ictal arrhythmias as a result of the autonomic imbalance brought on by seizure activity. The most prevalent clinically important arrhythmia, asystole, which is typically self-limiting and has a greater prevalence in people with TLE, is more common than sinus tachycardia during the ictal phase. There are several factors that can cause asystole, sinus bradycardia, and atrioventricular (AV) block [24][37]. These include sympathetic activation followed by the vagal cardioinhibitory reflex, and stimulation of the limbic cortex leading to parasympathetic outflow.
5. Muscle Dysrhythmia
Neuromuscular diseases (NMDs) are inherited or acquired conditions affecting skeletal muscles, motor nerves, or neuromuscular junctions [35][45]. The neuromuscular junction is the site of impulse transmission between nerve terminals and muscle fibers [36][46]. This process mainly requires the release of presynaptic acetylcholine (ACh) and its subsequent binding to a postsynaptic ACh receptor (AChR). The result is the opening of Na+/K+ channels, which leads to muscle contraction. Synaptic vesicles containing ACh are released from the presynaptic membrane after an action potential activates voltage-gated calcium channels (VGSCas), allowing calcium influx into the nerve terminal [37][47].
In mammals, each skeletal muscle fiber is innervated at a single site by a single myelinated motor axon [38][48]. Between the nerve and muscle cells is a synaptic gap about 50–100 nm wide. The EMG recording shows potential fluctuations of varying amplitude, frequency, and periodicity. Myotonic syndromes (conditions characterized by the inability to diastole immediately after skeletal muscle contraction) are the cause of low-amplitude and high-frequency electrical activity during muscle relaxation after contraction, which gradually disappears. When the muscle begins to contract, the magnitude of these oscillations’ amplitude ranges from 100 to 150 μV, while, in the state of maximum contraction, it is between 100 and 3000 μV. Primary muscle diseases (such as myositis or muscular dystrophy) cause a decrease in the amplitude of oscillations. Depending on the stage of the disease, the indicators will show different results: up to 500 μV in the initial phase, up to 20 μV in the final phase. On the EMG chart, the amplitude of potentials’ signals and their duration decrease. These indicators depend on the patient’s age and physical development. An abnormal graph can be caused by a layer of subcutaneous fat in the test area as well as blood-clotting disorders. A decrease in the amplitude of oscillations during repeated rhythmic stimulation of the muscle is a symptom of myasthenia gravis, a condition that disrupts the transmission of impulses from the motor nerve endings to the muscles [39][49]. Myotonic syndromes (conditions characterized by the inability to diastole immediately after skeletal muscle contraction) are the cause of low-amplitude and high-frequency electrical activity during muscle relaxation after contraction, which gradually disappears [40][50].
6. Eye—Optic Neuropathy
The human optic nerve is composed of 1.2 million axons of the retinal ganglion cells (RGCs), whose cell bodies are located in the inner layer of the retina inside the globe [41][51]. RGCs, as the sole connection between the eye and brain, are indispensable for visual function [42][52]. Extraocular optic neuritis is the most common neuropathy in adults (also known as demyelinating neuropathy), which localizes to the distal portion of the nerve, and in the early stages there are no changes to the optic disc. Intraocular optic neuritis is more common in children and localizes to the anterior segment of the nerve. Optic nerve neuropathy (ONN) is characterized by a group of eye diseases that result in damage to the nerve that conducts impulses from the retina to the visual centers in the brain [43][53]. There are two types of optic nerve neuropathy: ischemic and toxic. The disease leads to atrophy of the optic nerve, resulting in complete and irreversible loss of vision. The main cause of the changes occurring in the section of the optic nerve located outside the eyeball is a demyelinating process. Glial tissue replaces damaged myelin, which interferes with normal nerve conduction in the optic fibers [44][54]. It is worth noting that the demyelinating process in extraocular inflammation is analogous to the changes that occur in multiple sclerosis (MS). In both of these pathologies, there is breakdown of the myelin of the white matter of the brain and spinal cord (MS) and breakdown of the myelin sheath surrounding the optic nerve fibers. ONN is among the most devastating disorders in ophthalmology [45][55]. It has also been proven that ONN is epidemiologically associated with the occurrence of MS [46][56]. It has been estimated that, in about 20% of patients, it may be the first manifestation of MS. It has also been observed that 50% of MS patients will develop ONN during the disease. ONN is commonly seen in the early stages of MS, mainly in developed countries, and this form of ONN is usually referred to as “typical ONN” [47][57].
The basic factors causing optic neuropathy include infections and inflammatory processes (most common in MS), as well as ischemic changes such as hypertension, a hypercoagulable state, diabetes, hypercholesterolemia, and ischemic heart disease [48][58]. This toxic neuropathy is most often triggered by the abuse of alcohol (MeOH, EtOH, CH2(OH)CH2(OH)) and tobacco (also known as tobacco–alcohol optic neuropathy) [49][59]. Additionally, it has been proven that B vitamins (mainly B1, B9, B12), together with folate deficiencies and the cyanide in tobacco, may be associated with the demyelination of the optic nerves [50][60]. Another cause of toxic neuropathy is nutritional deficiencies, as well as the use of certain drugs with high doses [51][61]. The drugs responsible for ONN include antimicrobial agents (linezolid, ciprofloxacin, cimetidine, and chloramphenicol), antituberculotic drugs (isoniazid and ethambutol), halogenated hydroquinolones, benzofuran derivatives (amiodarone), antiepileptic agents (vigabatrin), cGMP-specific phosphodiesterase type 5 inhibitors (sildenafil, tadalafil, and vardenafil), methotrexate, cisplatin, carboplatin, 5-fluorouracil, vincristine, cyclosporine, tamoxifen, and disulfiram, as well as tumor necrosis factor-alpha (TNF-α) antagonists (etanercept, infliximab, and adalimumab) [52][62]. The prognosis for drug-induced neuropathy is better than that for alcohol–tobacco neuropathy. Early discontinuation of the drug gives a chance for improvement in vision, although improvement may only occur after a long time (even after a year).
