Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Betsy C. Herold and Version 2 by Catherine Yang.
The COVID-19 pandemic challenged the medical field to rapidly identify and implement new approaches to the diagnosis, treatment and prevention of SARS-CoV-2 infections. The scientific community also needed to rapidly initiate basic, translational, clinical and epidemiological studies to understand the pathophysiology of this new family of viruses, which continues to evolve with the emergence of new genetic variants. In contrast to most other respiratory viruses, children developed less severe acute and post-acute disease compared to adults.
- pediatric COVID-19
- SARS-CoV-2 variants
- innate immunity
1. Introduction
One of the most striking observations from the beginning of the SARS-CoV-2 pandemic was the marked difference in disease severity in children compared to adults. Al-though infection rates were similar or even higher in children, most children were asymptomatic or had mild illness and rarely required hospitalization. In contrast, the morbidity and mortality in adults was substantial. Approximately, 15.6 million US children were infected with SARS-CoV-2 from March 2020 to May 2023, which accounts for ~17.9% of total cumulated cases; notably, children (<18 years) comprise ~22% of the total US population [1]. Thus, the age-related differences in disease severity are not attributable to reduced susceptibility but more likely reflect differences in host responses. Moreover, the rates of infection and clinical manifestations changed with the emergence of new variants that differed in tissue and cell tropism and the ability to elicit and evade host immune responses. Natural and vaccine-induced immunity, treatment strategies and changes in social distancing policies also impacted the clinical manifestations and host responses to new viral variants.
2. Clinical Manifestations of Acute COVID-19 in Children with Successive SARS-CoV-2 Waves
During the first wave of SARS-CoV-2 infection (March–November 2020), the ancestral virus originating from Wuhan, China was rapidly replaced by the first major variant of concern, the Alpha variant (lineage B.1.1.7), which carried multiple mutations including a major change in the spike protein (D614G) [2]. The most common symptoms reported in infants and children were fever (46.3%), cough (36.9%) and less frequently, dyspnea (6.5%) [3]. Gastrointestinal symptoms including diarrhea, vomiting and abdominal pain were also documented. Hospitalizations among children, however, were uncommon. Clinical manifestations were similar during the second major wave through the fall of 2021, when the Delta variant (B.1.617.2) dominated. However, the Delta variant was associated with greater disease severity and increased hospitalization rates, possibly reflecting increased transmission, enhanced viral replication and immune escape [4]. Older children and adolescents with risk factors similar to those observed in adults including obesity progressed to acute respiratory distress syndrome (ARDS), although the incidence was low relative to what was observed among adults. The disease spectrum changed substantially with the emergence of Omicron variants (primarily B1.1.529) in December 2021 (Figure 1). More upper rather than lower respiratory tract diseases, including bronchiolitis and laryngotracheobronchitis (croup), were described with Omicron. Loss of taste, which was relatively uncommon in children compared to adults in the earlier waves, was almost never reported with Omicron [5][6][7][5,6,7]. A single-center study from New York reported an increase in the proportion of pediatric patients hospitalized with croup from 1.1% pre-Omicron to 6.6% during the Omicron wave [8]. Similar findings were observed using the US National COVID-19 Cohort Collaborative, which found that upper airway infection rates associated with SARS-CoV-2 disease increased from 1.5% to 4.1% from the pre-Omicron to Omicron period [9]. There was also an increase in febrile seizures associated with SARS-CoV-2 infections in the Omicron period, although the underlying reasons for this increase are unclear [10][11][12][10,11,12].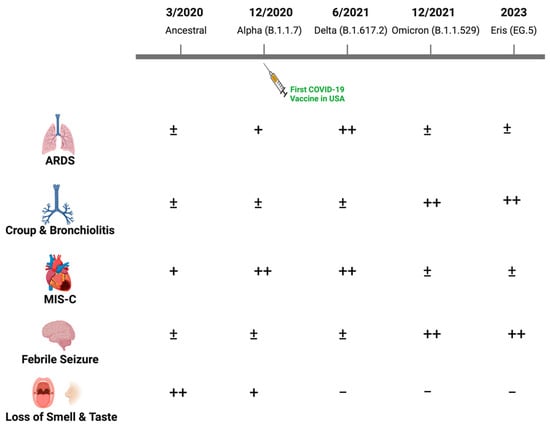
Figure 1. Changes in the clinical manifestations of SARS-CoV-2 in children with evolving viral variants. While most children infected with SARS-CoV-2 have been asymptomatic or developed self-limited fever with mild respiratory or gastrointestinal symptoms throughout the pandemic, the relative frequency of other clinical manifestations changed with emergence of new dominant viral variants as illustrated (−, ±, +, ++).
3. What Makes the Acute and Post-Acute Response to SARS-CoV-2 Different in Children?
The pre-Omicron variants (Alpha and Delta) were associated with more severe acute disease and higher rates of post-acute sequelae compared to Omicron in both adults and children. However, across all waves, children consistently exhibited a milder clinical course than adults. The milder disease course cannot be attributed to differences in rates of infection, expression of ACE2 or TMPRSS2, viral loads or cross-reactivating antibodies to other coronaviruses [22][23][24][50,51,52] (Table 1). Rather, studies suggest that children mount a more vigorous innate response, which protects against severe disease. In a study comparing children and adults hospitalized with SARS-CoV-2 pre-Omicron and prior to the introduction of vaccines, serum levels of IL-17A and IFN-γ were higher in children versus adults and correlated significantly and inversely with age [23][51]. The source of these cytokines was not likely the peripheral blood because adults had more robust spike-specific T cell responses; this led to the speculation that local innate responses may differ [23][51]. This hypothesis was supported by a subsequent study where the transcriptional profile of nasopharyngeal cells (bulk RNA sequencing) and quantification of mucosal cytokines and antibodies in nasopharyngeal swabs obtained from children or adults with SARS-CoV-2 were compared [22][50]. SARS-CoV-2 RNA copies, ACE2 and TMPRSS2 gene expression were similar in children and adults, but higher expression of genes associated with IFN signaling, NLRP3 inflammasome and other innate pathways were detected in the children compared to the adults. Consistent with the RNA data, protein levels of IFN-α2, IFN-γ, IP-10, IL-8 and IL-1β proteins were also higher in the nasal fluid of children versus adults, but anti-spike IgA and IgG were detected at similar levels in the nasal fluid of both groups. The notion that children mount a stronger local innate response was further supported by a study that used single-cell profiling of nasal, airway and blood samples from pediatric and adult patients. Interferon pathways were activated in SARS-CoV-2 uninfected healthy children compared to adults and this was further increased in those with SARS-CoV-2 infection. Conversely, adults with COVID-19 exhibited a greater peripheral blood cytotoxic T cell response.Table 1.
Age-associated host features and immune responses to SARS-CoV-2 that may contribute to disease severity.
| Host Feature | Children versus Adults |
|---|---|
| Rates of infection and initial SARS-CoV-2 RNA copies | No differences |
| ACE-2 and TMPRSS2 expression | No differences |
| Innate responses nasal mucosa ↑expression IFN signaling, NLRP3 inflammasome transcripts |
Increased in children |
| Systemic cytokine inflammatory response | Increased in adults |
| SARS-CoV-2 systemic neutralizing antibodies | No differences |
| Activated T cell responses | Increased in adults |
