You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Marijana Vičić and Version 2 by Jessie Wu.
Lichen planus is a chronic disease affecting the skin, appendages, and mucous membranes. A cutaneous lichen planus is a rare disease occurring in less than 1% of the general population, while oral illness is up to five times more prevalent; still, both forms equally impair the patient’s quality of life.
- antibodies
- dendritic cells
- etiology
- lichen planus
1. Introduction
Lichen planus (LP) is a chronic, immune-mediated, mucocutaneous inflammatory disorder [1]. The classic disease is defined by the so-called “rule six P”, which summarizes the skin lesions’ characteristics, i.e., planar, purple, polygonal, pruritic, papule and plaque [2]. Cutaneous LP is a rare dermatosis whose prevalence ranges from 0.22 to 1% and involves equally people of both sexes and different races. Instead, the more frequent oral disease occurs in 2 to 5% of the general population and is twice as common in women [3][4][5][6][3,4,5,6]. Although LP primarily includes the skin and oral mucosa, other mucous membranes and skin appendages, such as nails and hair, can also be damaged [5]. LP usually appears in middle-aged adults from 30 to 60 years of age and rarely affects other age groups [4]. Although the cutaneous disease has numerous clinical variants, its typical form is the classic LP, presenting with small, sharply demarcated, flattened and polygonally shaped erythematous-livid papules, which may coalesce as the disease progresses [4]. A prominent feature of LP is epidermal hypergranulosis, manifested as whitish reticulate structures or Wickham’s striae on the lesions’ surface [2]. A classic LP usually presents as localized form, with skin changes confined to the extremities, especially the wrists, ankles, dorsal surfaces of the hands and feet, and the lumbar region [2], or less commonly as a generalized condition, involving the entire body, including the oral and anogenital mucosa [4]. Despite the impressive clinical presentation, the latter has a good prognosis, marked by a spontaneous withdrawal within two years of onset [2]. Skin LP is generally followed by severe pruritus, whose intensity corresponds to the affected surface but lacks visible scratches or secondary infections [5].2. Etiology of Lichen Planus
2. Etiology of LP
The cause of LP has not been fully determined; however, genetic and environmental factors are thought to play a significant role in the onset of the disease (Figure 1).
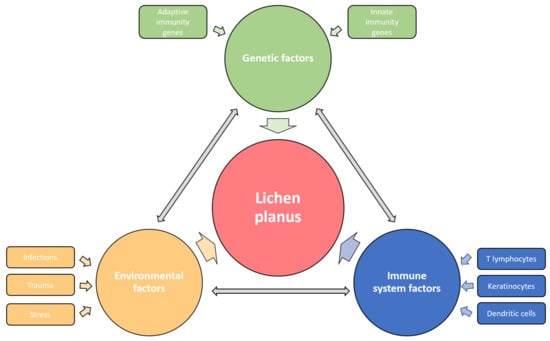
Figure 1. Etiological factors involved in the pathogenesis of LP. The disease affects the carrier of predisposing genes, in whom the various environmental factors trigger the immune response disorder resulting in specific LP phenotypes. The influences of genetic, environmental, and immune factors in LP development are dependent and mutually interconnected.
2.1. Genetic Factors
Genetic predisposition to disease was first suspected after noticing the LP presence in identical twins and 10% of the patients’ first relatives [2]. This observation confirmed the existence of a familial LP form, which constitutes up to 10% of all LP cases and is characterized by earlier onset, commoner relapses, treatment resistance, and oral mucosa involvement [7][24]. Still, genetic proneness has been observed in sporadic cases of LP. Furthermore, HLA-based susceptibility association studies identified a heterogeneous group of risk genotypes, some of which are primarily related to the familial form of the disease, such as HLA-A3, -Aw19, -B7, -B18 and -Cw8, the others such as HLA-DR1, -DRB1, -DQ1 and -Bw35 stand out in sporadic cases of cutaneous LP, while HLA-B8, -B51, -Bw57, -Bw61, are mostly expressed in oral lichen planus (OLP)OLP [2]. More detailed data on LP genetics were provided by a phenome-wide association study (PheWAS) revealing six single nucleotide polymorphisms (SNP), among which rs794275 was identified as the most significant, and HLA-DQB1 * 05:01 as the highly associated with LP [8][25]. Additionally, one genome-wide association study (GWAS) identified two more SNPs relevant for LP, i.e., rs884000 in the NRP2 and rs538399 in the IGFBP4 locus [9][26]. The conducted analyses discovered that the abovementioned polymorphisms principally affect genes with immune functions, such as Th1 response signaling molecules, i.e., TNF-α and IFN-γ, but also IL-4, IL-6, IL-10 and IL-12, IL-18, genes of oxidative stress, synthesis of thyroid hormones, prostaglandin E2, prothrombin, and NF-κB, which may impact the inflammatory mediators’ activity and cause disturbed signaling [1]. Genetic liability determines the reactivity of the patient’s skin and mucous membranes to other etiological factors.
2.2. Environmental Factors
Since the clinical course of the classic cutaneous LP is mainly marked by the acute beginning of one episode of the disease and its self-limiting nature, the microorganisms were often suspected as an LP causative agent. The association of LP with hepatitis C virus (HCV), which is up to thirteen times more common in LP patients, is best studied and verified by the results of several reports and meta-analyses conducted during the last decades [10][11][12][13][14][27,28,29,30,31]. A positive correlation between LP and HCV was observed among the populations of the Mediterranean, Germany, Japan, and the USA [15][32]. Although the exact role of HCV in LP development has still not been explained, viral components probably lead to host immune response dysregulation, as terminally differentiated and virus-specific CD8+ T lymphocytes have been found in the LP lesions [15][16][32,33]. Di Stasio et al. reported the improvement of OLP after direct-acting antiviral therapy, supporting the possible pathogenetic role of HCV infection in LP [16][33]. The increased prevalence and pathogenetic association of human papillomavirus (HPV) with OLP lesions have been proved [17][34], while the other possible causes include human herpesvirus 7 (HHV-7), found in infiltrating plasmacytoid dendritic cells in lesional skin biopsies [18][19][20][35,36,37], hepatitis B virus (HBV) [21][22][38,39], varicella-zoster virus (VZV) [23][40], and Epstein–Barr virus (EBV) [24][41]. In some cases, LP appeared or worsened following vaccination against hepatitis A and B [25][26][27][42,43,44], influenza [28][45], rabies [29][46], tetanus-diphtheria-pertussis [30][47] or SARS-CoV-2 in recent times [31][32][33][48,49,50]. Despite opposing views, Helicobacter pylori could contribute to LP etiopathogenesis, as it is associated with the altered function of salivary microbiome enhancing inflammation, while its eradication alleviates disease symptoms [34][35][36][51,52,53]. New data indicate that microbial infection could also play a role in OLP etiopathogenesis since it has been shown that a large number of bacteria and T lymphocytes were present in the affected tissue [37][54]. Novel fungi-based pathogenesis of OLP considers the role of Candida and Malassezia mycotypes in instigating the LP antigen expression and consequential Th17 lymphocytes’ reaction [38][55]. Furthermore, significant bacterial, fungal, and viral dysbiosis was detected in the saliva of these patients, with higher levels of Solobacterium, Fusobacterium, Porphyromonas, Prevotella, Candida, Aspergillus, Alternarium, tick-borne encephalitis virus, brochotrix bacteriophage virus and bacillus virus SPO1 and lower levels of Streptococci, Actinobacteria and Firmicutes compared to healthy controls [37][39][54,56]. Since the oral cavity is the initial part of the digestive system and forms part of the gut–skin axis, disturbances of the oral and salivary microbiome composition can directly trigger the patient’s immune system dysregulation, causing OLP [37][39][54,56].
However, in addition to microbial factors, it has been shown that OLP can be caused by contact allergens, primarily dental filling metals, such as mercury and gold [40][41][42][57,58,59], while its occurrence is associated with areca nut and betel quid chewing in the Far East as well [43][44][60,61]. Chemicals such as para-phenylenediamine, dimethyl fumarate and methacrylic acid esters can cause LP-like contact dermatitis [2][45][2,62]. Various drugs, especially those from the group of antihypertensives (beta-blockers and diuretics), antimalarials, antibiotics, antidiabetics, phosphodiesterase (PD)-1 inhibitors, and biologicals such as anti-TNF-α and dupilumab, can induce lichenoid reactions [46][47][48][49][50][51][52][63,64,65,66,67,68,69]. Lichen skin changes can occur due to radiation field exposure and radiotherapy [53][70]. Likewise, LP can be caused by mechanical injuries, such as surgical procedures or tattooing, which is confirmed by the appearance of the Köebner phenomenon in the linear form of the disease [54][55][71,72]. It has been proven that severe psychological stress can cause LP in hitherto healthy individuals, and anxiety and depression have the same effect on the onset or worsening of an existing illness [56][57][58][73,74,75].
