Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Xiqian Tan.
Fermentation is a traditional method used to preserve vegetables. Many regions worldwide have a tradition of consuming fermented vegetables. Numerous fermented vegetables exist according to the raw materials, formula, and fermentation technologies used. Typical fermented vegetables include sauerkraut, paocai, zhacai, and kimchi. The primary constituents for producing fermented vegetables are cruciferous vegetables, such as cabbage, kale, mustard green, or radish. Other prevalent vegetables include chili pepper, lotus root, carrot, ginger, cucumber, eggplant, beetroot, garlic, olive, papaya, and chayote. There are variations in the production procedures used for different fermented vegetables.
- fermented vegetables
- nutritional composition
- function
1. Health Benefits of Fermented Vegetables
The nutritional components generated during the pickling process and the probiotics in fermented vegetables are generally responsible for the health benefits of fermented vegetables. Table 1 summarizes some probiotics isolated from different fermented vegetables with distinct functions.
Table 1.
Probiotics in fermented vegetables and their function.
| Fermented Vegetables | Probiotics | Function | Main Results | Ref. |
|---|---|---|---|---|
| Szechwan-style pickled vegetables | Lactobacillus Plantarum CQPC05 | Inhibits constipation. | Up-regulated the mRNA expression of the stem cell factor receptor (c-Kit and SCF) and glial cell-derived neurotrophic factor genes, down-regulated the transient receptor potential cation channel subfamily V member 1 and inducible nitric oxide synthase. | [79][1] |
| Kimchi | Lactococcus lactis KC24 | Antimicrobial, anti-inflammatory, antioxidant, anti-cancer. | Listeria monocytogenes and Staphylococcus aureus inhibition. Nitric oxide reduction. Inhibited gastric carcinoma (AGS), colon carcinoma (HT-29 and LoVo), breast carcinoma (MCF-7), and lung carcinoma (SK-MES-1) cells. |
[25][2] |
| Kimchi | Lactobacillus plantarum EM | Lower cholesterol. | Cholesterol was removed by the cell wall fraction of the probiotics under the mechanism of enzymatic assimilation and was cell wall concentration-dependent. | [80][3] |
| Mango pickle | Bacillus licheniformis KT921419 | Anti-cancer. | Works against the HT-29 colon cancer cell line | [29][4] |
| Chinese Sauerkraut | Bacillus velezensis T701 | Antitumor. | The lipopeptide iturin A-2 produced by the strain showed good cytotoxic activities against Hela, MCF-7 and BT474 cell lines which related to cervical and breast cancer. | [81][5] |
| Sauerkraut | Enterococcus | Heavy metal elimination. | Eliminated heavy metals such as Cu, Pb, and Cd that are difficult to eliminate through cooking | [82][6] |
The health benefits of fermented vegetables include antibacterial effects, improvements in constipation, anticancer properties, the treatment of chronic diseases, the alleviation of irritable bowel syndrome, and immunity enhancement. Some of their main functions are shown in Figure 31.
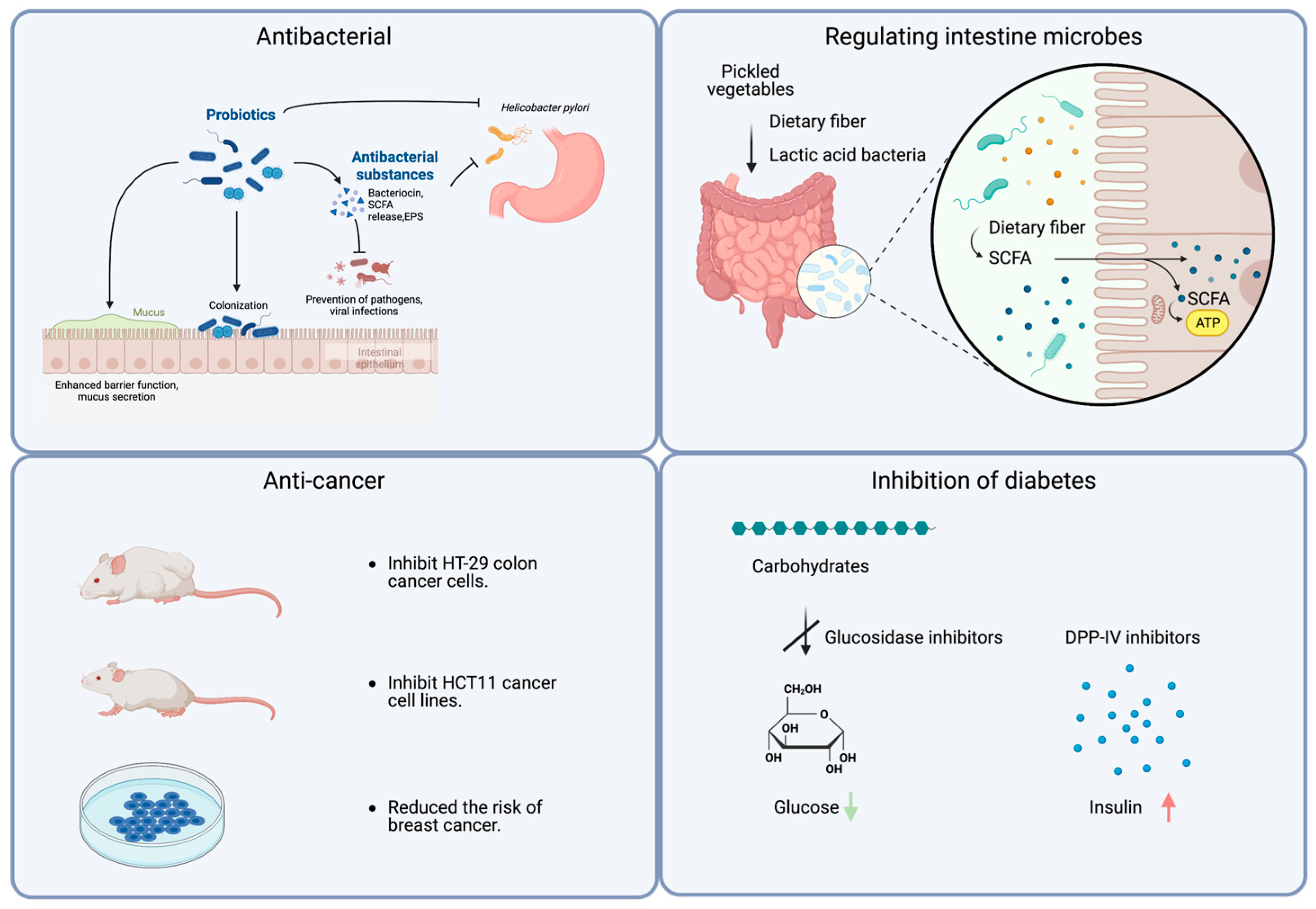
Figure 31. A schematic diagram illustrating the functions and potential mechanisms of pickled vegetables. Created with BioRender.com (https://www.biorender.com, accessed on 18 June 2023).
1.1. Antibacterial
Due to the probiotics and their metabolites in fermented vegetables, such as antibacterial peptides and peroxides, one of the functions of fermented vegetables is the ability to inhibit the pathogen microorganisms. The primary mechanisms include disrupting cell structures, influencing the replication of genetic material, obstructing energy metabolism pathways, interfering with quorum sensing systems, regulating biofilm formation, and competing for essential nutrients. These antimicrobial substances include extracellular polysaccharides, phenolic compounds, and antibacterial peptides. Helicobacter Pylori (H. pylori) is considered the primary cause of stomach cancer, and eradicating this bacterium can be used as one of the therapies in its treatment. The research found that the extracellular polysaccharides produced by Lactobacillus sp. PW-7 isolated from fermented pickles can inhibit H. pylori [83][7]. Studies have shown that consuming kimchi can inhibit the growth of H. pylori in H. pylori-infected C57BL/6 mice [84][8]. Moreover, Pediococcus pentosaceus isolated from kimchi can inhibit Listeria monocytogenes, a Gram-positive bacterium that can readily cause listeria infection, and the antibacterial active site is on the LysM protein structure domain [85][9]. Probiotics in the pickles can generate a variety of antibacterial peptides, including those approved for use as food additives by the US Food and Drug Administration (FDA), such as nisin [86][10], and new antibacterial peptides are still being isolated from fermented vegetables [87][11]. In addition, a strain of Lactiplantibacillus plantarum CXG9 isolated from pickles can produce LD-phenylacetic acid [88][12]. Furthermore, phenolic compounds such as 2,6-dihydroxy acetophenone (DHAP), 4-hydroxybenzaldehyde (HBA), and 4-hydroxyphenyl alcohol (4-HPEA) have been discovered in sauerkraut juice and exhibit antibacterial activity to variable degrees [89][13].
1.2. Regulating the Intestine Microbes and Improving Intestine Health
Consuming fermented vegetables can increase intestinal microbiota diversity [90][14]. In addition to containing many lactic acid bacteria [27][15], most fermented vegetables are an essential source of dietary fiber and various vitamins. Moreover, they contain antioxidant-active compounds such as glutamine and glucosinolate. Black garlic, for instance, is abundant in short-chain fatty acids, which inhibit the development of harmful bacteria and promote the growth of beneficial bacteria. Oligosaccharides, such as fructooligosaccharides, melanoidins, and specific dietary phenolic substances, can also regulate intestinal microbiota [91][16]. The health effects of consuming fermented vegetables may also be long lasting [2][17]. Studies show that ingesting fermented vegetables for six months can ameliorate the imbalance in gut dysbiosis, and irritable bowel syndrome (IBS) symptoms can also be alleviated by consuming fermented vegetables fermented with lactic acid for some time [92][18]. Additionally, they can help to relieve constipation. Constipation diminishes the quality of life, and the accumulation of fecal contaminants in the intestines may increase the risk of intestinal diseases. In most cases, fermented vegetables and the probiotics they contain work together to treat constipation [93][19].
1.3. Anti-Cancer
The anti-cancer properties of fermented cabbage depend on the raw pickling ingredients. Studies have shown that fermented cruciferous vegetables have an anti-cancer effect, possibly related to the anti-cancer value of cruciferous vegetables being preserved after pickling based on in vitro experiments. A series of studies proved that kimchi could inhibit the proliferation of HT-29 colon cancer cells [94,95][20][21]. Studies showed that fermented mustard leaf does not affect normal colon myofibroblast CCD-18Co cells but can terminate the proliferation of HCT116 cancer cell lines and lead to large-scale cell apoptosis. Another study based on a 131 case-control study about breast cancer among Polish-born migrants in Cook County and the Detroit Metropolitan Area found that consuming fermented cruciferous vegetables can reduce the risk of breast cancer. According to their results, consuming raw or short-cooked fermented cabbage can substantially reduce the risk of breast cancer, while consuming long-cooked pickles has no association with it, possibly due to the probiotics or active substances being destructed by heat [96][22].
1.4. Inhibition of Diabetes
A disorder in carbohydrate metabolism primarily causes type 2 diabetes. Glucosidase inhibitors can delay the hydrolysis of carbohydrates into glucose, decreasing blood sugar levels. Inhibitors of dipeptidyl peptidase-IV (DPP-IV) prevent the degradation of glucagon-like peptide one (GLP-1) and gastric inhibitory peptide (GIP), thereby enhancing insulin secretion and lowering blood glucose levels since DPP-IV can substantially reduce GLP-1 and GIP; these two peptides can stimulate pancreatic insulin secretion after meals and significantly decrease blood glucose content. A ten-year prospective cohort study has confirmed that regular consumption of fermented vegetables reduces the risk of diabetes [97][23] because fermented vegetables contain luteolin and isorhamnetin-3-O-glucoside, a natural α-glucosidase and a potent inhibitor of DPP-IV, respectively [98][24].
2. Safety Problems in Fermented Vegetables and Current Solutions
Although there are nutritional and health benefits from fermented vegetables, their safety problems should not be neglected. Three main safety problems occurred in different stages of fermented vegetable production (Figure 42): biogenic amine, nitrite, and microbial safety.
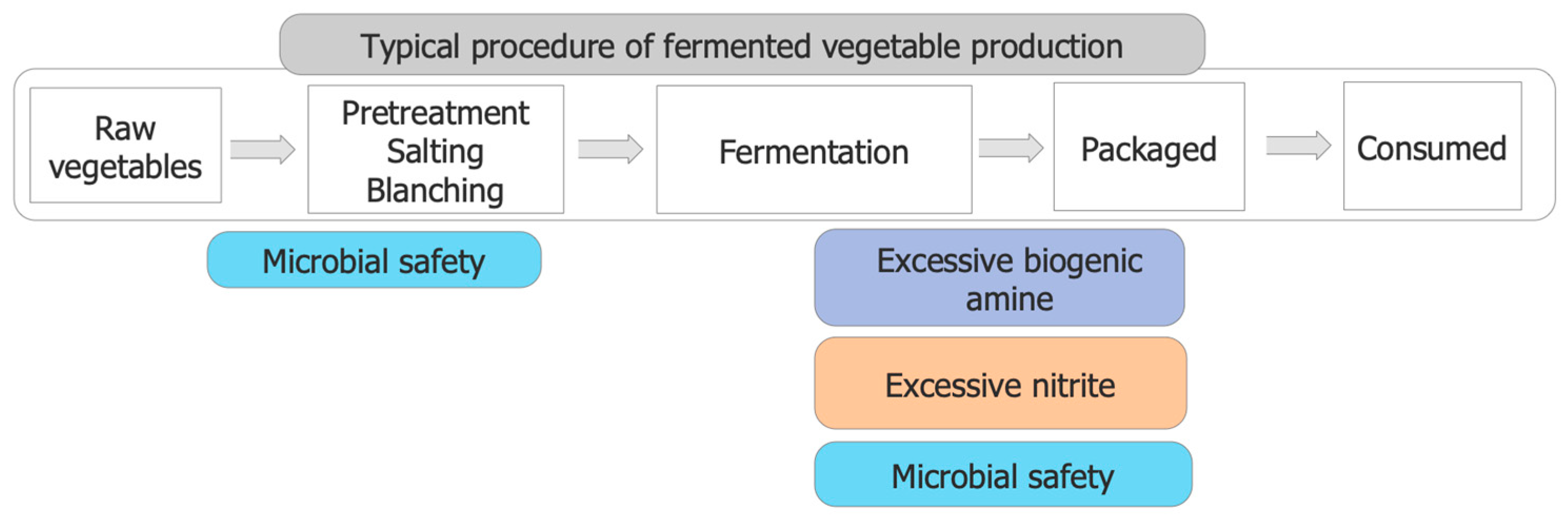
Figure 42.
Safety problems occurred in different stages of fermented vegetable production.
2.1. Biogenic Amine
Biogenic amines result from the interaction of pickles’ ingredients and the metabolic activity of microorganisms [99][25]. The biogenic amine content of fermented vegetables is closely related to the biogenic amine content of the primary materials and the fermentation conditions [100][26]. Histamine, putrescine, and cadaverine are the primary biogenic amines found in fermented vegetables. Due to the difficulty of assuring hygienic conditions during fermentation, homemade fermented vegetables may contain more biogenic amines than commercially produced ones [101][27]. Reusing brine may also increase biogenic amine production [102][28]. The biogenic amine content of fermented vegetables from various regions may also vary [103][29].
The predominant beneficial bacterial species in fermented vegetables are Lactobacilli, particularly L. plantarum, and the presence of lactic acid bacteria can inhibit the proliferation of pathogenic bacteria to some extent; however, numerous studies have revealed that lactic acid bacteria can also produce biogenic amines [104][30], and for them, strain is more critical than species or genus in determining the biogenic amines-producing abilities of the lactic acid bacteria. Bacteria strains that can produce biogenic amines include Enterococci, Lactobacilli, Streptococci, Pediococci, and Oenococci [105][31]. Lactobacilli from several naturally fermented pickles can produce putrescine (PUT), cadaverine (CAD), and histamine (HIS) [106][32]. CAD and nitrite are associated with Leuconostoc, while Lactobacillus and Pseudomonas are associated with tyramine (TYR) [107][33]. Lactobacillus brevis primarily produces TYR [108][34]. Most studies, however, indicate that the levels of biogenic amines produced by these bacterial strains do not exceed the threshold for toxicity.
It is possible to reduce the biogenic amine content of fermented vegetables by manipulating the fermentation conditions. Although salt concentration has some effects on the formation of biogenic amines in certain varieties of pickles [109][35], studies have found that altering the salt concentration and temperature has a limited impact on inhibiting the formation of biogenic amines in pickles [110][36]. Changing the formula of pickles with a relatively low precursor of biogenic amines could, then, reduce its final content. For instance, adding fish sauce during the production of fermented cabbage can increase HIS; decreasing the additional amount of Myeolchi-aekjeot, a kind of fish sauce, can reduce the level of HIS and CAD in the final pickled products. Adding less fish sauce and more red pepper has the same effect on the total amount of biogenic amines content in kimchi [99][25]. Introducing onion and coriander can also reduce the concentration of biogenic amines. The addition of onion can inhibit four out of eight biogenic amines, including CAD, spermine (SPE), phenethylamine (PHE), and TYR, during the fermentation of sauerkraut, due to its antibacterial activity, which could inhibit the critical enzyme-producing bacteria in the biosynthesis of biogenic amines [111][37].
Selecting starter cultures of fermented vegetables that do not produce biogenic amines or can decompose biogenic amines is a highly effective strategy for reducing biogenic amines [108][34]. Various fermented foods have been discovered that contain bacteria capable of degrading biogenic amines [112][38], and the strains which are related to fermented vegetables are listed in Table 2. Lactobacillus plantarum [113][39], L. plantarum GZ-2, L. brevis SC-2 [107][33], Levilactobacillus brevis PK08, Lactiplantibacillus pentosus PK05, Leuconostoc mesenteroides YM20, L. plantarum KD15, and Latilactobacillus sakei YM21 [114][40] have all been identified as strains that do not produce or degrade biogenic amines. Different strains can degrade biogenic amines at varying rates [115][41]. Staphylococcus carnosus M43 can decompose HIS and TYR, while Pediococcus acidilactici M28 can decompose eight types of biogenic amines [116][42]. The biogenic degradation of the strains is firmly due to the secretion of biogenic amine-degrading enzymes [117][43]. L. brevis PK08, for instance, has a potent ability to degrade TYR, and this strain primarily degrades TYR by secreting multicopper oxidase (MCO) [114][40]. MCO can oxidize various phenolic and non-phenolic aromatic compounds while reducing dioxygen to water. Multicopper oxidase is superior to other biogenic amine-degrading enzymes since it has more potential applications [118][44]. In Lactobacillus plantarum J16 CECT 8944, another biogenic amine-degrading enzyme—laccase—was found, which has similar spectroscopic properties to blue copper oxidase and could primarily oxidize biogenic amines of the TYR type [119][45]. While another strain, Halomonas shantousis SWA25, can degrade a variety of biogenic amines. It can effectively degrade TRY, PHE, PUT, CAD, HIM, and TYR in fish sauce, and its biogenic amine-degrading action depends primarily on the membrane-distributed amine oxidase [120][46].
Table 2.
Starter cultures used in reducing the content of the biogenic amine in the fermented vegetable.
| Strains | Isolation Origin | Characterization of the Strain and the Main Effects | Ref. |
|---|---|---|---|
| Lactobacillus plantarum GP11 | Homemade pickled samples | Show no biogenic amine production ability. Exhibit antifungal activity against the Aspergillus sp. and Penicillium sp., which always leads to the contamination of the pickled vegetables. | [113][39] |
| L. brevis SC-2 | Fermented mustard | A lower capacity of biogenic amine-producing ability, 13.95 mg/kg total biogenic amine producing ability with corresponding precursors; did not produce tryptamine, putrescine, and cadaverine, and could reduce the content of the biogenic amine in the fermented mustard from 137.16 mg/kg to 39.16 mg/kg | [107][33] |
| L. plantarum GZ-2 | Fermented mustard | A lower capacity of biogenic amine-producing ability, 4.65 mg/kg total biogenic amine-producing ability with corresponding precursors, and could reduce the content of the biogenic amine of the fermented mustard. | [107][33] |
| L. brevis PK08 | Kimchi | Has a multicopper oxidase gene, and showed a high reduction in tyramine content. | [114][40] |
| Limosilactobacillus fermentum G9 | Cantonese pickles (containing mustard, cabbage, and bamboo shoots) | Has no biogenic amine-producing ability and could significantly reduce the biogenic amine content of Cantonese pickles to nearly 25 mg/kg compared to 150 mg/kg in the naturally fermented sample. | [121][47] |
2.2. Nitrite
Nitrite, another unsafe substance in fermented vegetables, can form cancer-causing nitrosamines and induce cancer of the digestive system. The GB 2762-2017 standard (Food safety national standard food pollutant limits of China) specifies a maximum nitrite content of 4 mg/kg for raw vegetables and 20 mg/kg for fermented vegetables. The amount of nitrite in various pickles differs based on the ingredients used. According to recent studies, the maximum nitrite level has been found in fermented cabbage, followed by fermented mustard, bamboo, and radishes [122][48]. The concentration of nitrite is related to the composition of the microbial community of the pickles. The high-throughput sequencing results revealed an inverse relationship between the relative abundance of Lactobacillus and nitrite concentration [123][49], indicating that altering the microbial community’s structure by adding seasoning could reduce the nitrite concentration [123][49]. Studies have proved that adding garlic can substantially increase the number of Lactobacillus and Weissella in fermented vegetables and prevent the growth of undesirable microorganisms during fermentation. However, the nitrite residue was still relatively high in these pickles [124][50]. Another strategy for decomposing nitrite is by adding certain bioactive substances. Polyphenols extracted from apple rind with alkaline can effectively remove nitrite from pickles. Unlike ethanol-extracted polyphenols, non-ethanol-extracted polyphenols effectively eliminate nitrite without affecting the pickles’ flavor [125][51]. Furthermore, the addition of specific microelements, such as selenium, would accelerate the degradation of nitrite. Selenium (Se) can boost the antioxidant activity of lactic acid bacteria. The addition of Se can enhance the elimination ability of hydroxyl and superoxide radicals of the strain, enhancing the reaction rate of lipid peroxidation and ion-chelating and increasing the activity of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), which are involved in the breakdown of nitrite [126][52].
Due to the growth of lactic acid bacteria in fermented vegetables during fermentation, nitrite can be degraded naturally [127][53]. Various strains that break down nitrite have been isolated from different pickles. These bacterial strains positively affect nitrite degradation when used as a starter culture for producing pickles and contribute to the excellent qualities of the fermented vegetables [128][54], including Lactobacillus casei subsp. rhamnosus LCR 6013 [129][55], Lactiplantibacillus plantarum ZJ316 [130][56], Stachys sieboldii Miq. [131][57], and Lactobacillus coryniformis [132][58]. There are currently three potential mechanisms which explain the nitrite-degrading abilities of the strains: acid degradation, enzyme degradation, and metabolic pathway degradation (Table 3).
Generally, nitrite decomposition can occur below a pH of 4 [133][59]. The low pH value results from the accumulation of organic acid produced by lactic acid bacteria, including lactic acid, acetic acid, butyric acid, tartaric acid, succinic acid, citric acid, and malic acid [131][57]. The nitrite content in mixed-strain fermented pickles is lower than in single-strain fermented ones because of the accumulation of organic acids. In addition, accumulating organic compounds can improve the flavor of pickles [134][60]. Hence, naturally fermented or mixed-strain fermented pickles would have great flavors.
Enzyme degradation is another mechanism used to reduce nitrite levels. Numerous lactic acid bacteria contain a nitrite reductase enzyme system that converts nitrite into NO2, NO, and N2 [123][49]. The nitrite reductase system of nitrite-reducing bacteria consists of genes such as nirK, nirS, and nirBD [135][61]. According to genomic research, nitrite-reducing bacteria have a certain tolerance for nitrite, and exposure to nitrite can cause elongation and shrinking in the bacteria, thereby decreasing their surface hydrophobicity. It was found that the genome of the strain contains genes encoding proteins and peptidoglycan proteins involved in regulating osmotic pressure, which can influence the expression of the cell wall in response to nitrite stress. In L. plantarum DMDL 9010, nitrite ions can bind to the active Cd1NiR (pgl) site through two hydrogen bonds [136][62]. There are also identical sequences of microbial nitrite reductase in other foods. For instance, mushroom-isolated nitrite reductase with a molecular weight of 90 kDa is homologous to the peptide sequence of fungi-derived nitrite reductase [137][63]. Consequently, some natural foods containing nitrite reductase have the potential to be used to produce fermented vegetables to reduce nitrite content.
A last nitrite degradation mechanism is metabolic pathway degradation [138][64]. A two-component system is capable of transferring nitrite to the pericytoplasm. The phosphotransferase system, glycolysis, and tricarboxylic acid cycle pathways generate reduced nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide-2 (FADH2). These substances produce electrons via the catalytic action of dehydrogenase catalytic reaction, and these electrons are transferred to nitrite via the electron transfer chain. Nitrite reductase reduces some nitrite molecules to NH3 by receiving electrons; glutamine synthetase then converts NH3 to L-glutamine [139][65]. In Limosilactobacillus fermentum RC4, its three secreted metabolites, mesaconate, 3-methylthiopropionic acid (MTP), and trans-aconitic acid, are effective at degrading nitrite. The particular mechanism is associated with the decarboxylation reaction [140][66], and it was found that nirB is associated with nitrogen metabolism [141][67]. There are also archaea capable of nitrite decomposition in fermented vegetables. Archaea obtain the electrons necessary for truncated denitrification by absorbing exogenous glucose from pickles, then they reduce nitrite to nitrogen with high efficiency, preventing nitrate from converting to nitrite [142][68]. Halomicrobium sp. ZPS, one of the nitrite-degrading archaea, has a mechanism for absorbing potassium and excluding sodium, and multiple varieties of nitrite reductase are involved in its nitrogen metabolism [143][69].
Table 3.
Potential mechanisms behind the nitrite-degrading abilities of the microorganism.
| Potential Mechanism | Factor That Play a Main Role | Main Results | Ref. |
|---|---|---|---|
| Acid degradation | The organic acid produced by the lactic acid bacteria | A low pH caused by the metabolic products (lactic acid, acetic acid, butyric acid, tartaric acid, succinic acid, citric acid, and malic acid) of the lactic acid bacteria, which cause the degradation of nitrite. Mixed strain fermentation has a more significant degrading effects. | [131,57133,]134][[59][60] |
| Enzyme degradation | The nitrite reductase enzyme system exist in the microorganism | The nitrite reductase system of nitrite-reducing bacteria consists of genes such as nirK, nirS, and nirBD, which could convert nitrite into NO2, NO, and N2. | [123,135][49][61] |
| Metabolic pathway degradation | The particular metabolic pathway of the microorganisms | Received electrons generated by the glycolysis/gluconeogenesis and citrate cycle, which eventually convert nitrite to L-glutamine. Decarboxylation reaction. Denitrification. | [135,141][61][67] |
2.3. Microbial Safety
During the production of fermented pickles, there are potential safety concerns associated with microbiological factors that could threaten consumers. It is necessary to control the pathogens at two time points; The first one is during the pre-treatment procedure, to reduce the miscellaneous bacteria, especially those that have strong biofilm-forming abilities; if these microorganisms are propagated and form a biofilm during the pickling procedure, the flavor of the fermented vegetables would be affected [144][70]. The other timepoint is at the end of the fermentation process. One purpose of this is to terminate the fermentation process, to avoid the adverse effect on the flavor and texture of the fermented vegetables by the excessive fermentation, but the most important purpose is to extend the shelf-life of the fermented vegetables and to maintain its edible safety for consumers; fermented vegetables were always considered to be ready-to-eat products in the market, for most of the homemade fermented vegetables, the sterilization in this step was always missing. Heat treatment is frequently used in the industrial sterilization of fermented vegetables at both time points. However, industrial sterilization alters the volatile compounds and texture of the pickles, hence decreasing consumer acceptability [145][71]. To address these issues, exploring novel non-thermal sterilization technologies to control harmful microorganisms arouses broad interest (Table 4). In the meantime, in conventional fermentation, a high sodium concentration is commonly used for microbial control; as mentioned above, the penetration of the salt to the fermented substrates depends on various factors, and a long period of salting could increase the risk of microbial deterioration; some non-thermal technologies can also accelerate the salting procedure.
Table 4.
Non-thermal technologies used in the sterilization of fermented vegetables.
| Non-Thermal Technology | Sterilization Effects | Effects on the Sensory Quality of Fermented Vegetables | Ref. |
|---|---|---|---|
| HPP | HPP treatment at 550 MPa for 5 min reduces total plate count (TPC) and substantially inactivates yeast and mold in the pickled radish, and maintained microbial safety of pickles in sixty days of storage. | Might have adverse effects on the sensory quality of the pickled radish, and the treatment parameters should be prioritized. | [146][72] |
| Maintained the shelf life of the marinated lotus root slices. | HPP treatment could retain the color and improve the flavor of the marinated lotus root slices. | [10][73] | |
| Cold plasma | Could eliminated 5.00 logCFU/g of microorganisms under the CP treatment (voltage 60 kV, frequency 50 Hz, implementing time 60 s) | Increase the firmness of the radish paocai, could alleviate the softening and browning of radish paocai. | [147][74] |
| Plasma activated water, generated by an AC bi-polar pulsed power supply (driving frequency 14.3 kHz, a peak-to-peak voltage 18 kV) for 120 s, could cause a reduction of 2.0, 2.2, 1.8, 0.9 log CFU/g mesophilic aerobic bacteria, lactic acid bacteria, yeast and moulds of ready-to-use shredded, salted kimchi. | Could reduce the salinity of peroxidase activity of the product. | [148][75] | |
| Photodynamic | Could inhibit the while colony-forming yeast in kimchi seasoning | Maintain the volatile compounds in the kimchi seasoning | [149][76] |
References
- Li, F.; Zhou, H.; Zhou, X.; Yi, R.; Mu, J.; Zhao, X.; Liu, W. Lactobacillus plantarum CQPC05 Isolated from Pickled Vegetables Inhibits Constipation in Mice. Appl. Sci. 2019, 9, 159.
- Lee, N.-K.; Han, K.J.; Son, S.-H.; Eom, S.J.; Lee, S.-K.; Paik, H.-D. Multifunctional Effect of Probiotic Lactococcus Lactis KC24 Isolated from Kimchi. Lwt-Food Sci. Technol. 2015, 64, 1036–1041.
- Choi, E.A.; Chang, H.C. Cholesterol-Lowering Effects of a Putative Probiotic Strain Lactobacillus plantarum EM Isolated from Kimchi. Lwt-Food Sci. Technol. 2015, 62, 210–217.
- Ragul, K.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Evaluation of Functional Properties of Potential Probiotic Isolates from Fermented Brine Pickle. Food Chem. 2020, 311, 126057.
- Jiang, J.; Zhang, H.; Zhang, C.; Han, M.; Du, J.; Yang, X.; Li, W. Production, Purification and Characterization of ‘Iturin A-2′ a Lipopeptide with Antitumor Activity from Chinese Sauerkraut Bacterium Bacillus velezensis T701. Int. J. Pept. Res. Ther. 2021, 27, 2135–2147.
- Yang, Y.; Pei, J. Isolation and Characterization of an Enterococcus Strain from Chinese Sauerkraut with Potential for Lead Removal. Eur. Food Res. Technol. 2020, 246, 2055–2064.
- Hu, J.; Tian, X.; Wei, T.; Wu, H.; Lu, J.; Lyu, M.; Wang, S. Anti-Helicobacter pylori Activity of a Lactobacillus sp. PW-7 Exopolysaccharide. Foods 2021, 10, 2453.
- Jeong, M.; Park, J.-M.; Han, Y.-M.; Park, K.Y.; Lee, D.H.; Yoo, J.-H.; Cho, J.Y.; Hahm, K.-B. Dietary Prevention of Helicobacter Pylori-Associated Gastric Cancer with Kimchi. Oncotarget 2015, 6, 29513–29526.
- Jang, S.; Lee, J.; Jung, U.; Choi, H.-S.; Suh, H.J. Identification of an Anti-Listerial Domain from Pediococcus pentosaceus T1 Derived from Kimchi, a Traditional Fermented Vegetable. Food Control 2014, 43, 42–48.
- Joo, N.E.; Ritchie, K.; Kamarajan, P.; Miao, D.; Kapila, Y.L. Nisin, an Apoptogenic Bacteriocin and Food Preservative, Attenuates HNSCC Tumorigenesis via CHAC1. Cancer Med. 2012, 1, 295–305.
- Song, J.; Peng, S.; Yang, J.; Zhou, F.; Suo, H. Isolation and Identification of Novel Antibacterial Peptides Produced by Lactobacillus fermentum SHY10 in Chinese Pickles. Food Chem. 2021, 348, 129097.
- Zhang, J.; Zhang, C.; Lei, P.; Xin, X.; Liu, D.; Yi, H. Isolation, Purification, Identification, and Discovery of the Antibacterial Mechanism of Ld-Phenyllactic Acid Produced by Lactiplantibacillus plantarum CXG9 Isolated from a Traditional Chinese Fermented Vegetable. Food Control 2022, 132, 108490.
- Li, J.; Huang, S.-Y.; Deng, Q.; Li, G.; Su, G.; Liu, J.; Wang, H.-M.D. Extraction and Characterization of Phenolic Compounds with Antioxidant and Antimicrobial Activities from Pickled Radish. Food Chem. Toxicol. 2020, 136, 111050.
- Thriene, K.; Hansen, S.S.; Binder, N.; Michels, K.B. Effects of Fermented Vegetable Consumption on Human Gut Microbiome Diversity—A Pilot Study. Ferment 2022, 8, 118.
- Zhu, K.; Tan, F.; Mu, J.; Yi, R.; Zhou, X.; Zhao, X. Anti-Obesity Effects of Lactobacillus fermentum CQPC05 Isolated from Sichuan Pickle in High-Fat Diet-Induced Obese Mice through PPAR-α Signaling Pathway. Microorganisms 2019, 7, 194.
- Li, J.; Deng, Q.; Zhang, Y.; Wu, D.; Li, G.; Liu, J.; Zhang, L.; Wang, H.D. Three Novel Dietary Phenolic Compounds from Pickled Raphanus sativus L. Inhibit Lipid Accumulation in Obese Mice by Modulating the Gut Microbiota Composition. Mol. Nutr. Food Res. 2021, 65, 2000780.
- Galena, A.E.; Chai, J.; Zhang, J.; Bednarzyk, M.; Perez, D.; Ochrietor, J.D.; Jahan-Mihan, A.; Arikawa, A.Y. The Effects of Fermented Vegetable Consumption on the Composition of the Intestinal Microbiota and Levels of Inflammatory Markers in Women: A Pilot and Feasibility Study. PLoS ONE 2022, 17, e0275275.
- Nielsen, E.S.; Garnås, E.; Jensen, K.J.; Hansen, L.H.; Olsen, P.S.; Ritz, C.; Krych, L.; Nielsen, D.S. Lacto-Fermented Sauerkraut Improves Symptoms in IBS Patients Independent of Product Pasteurisation—A Pilot Study. Food Funct. 2018, 9, 5323–5335.
- Šola, K.F.; Vladimir-Knežević, S.; Hrabač, P.; Mucalo, I.; Saso, L.; Verbanac, D. The Effect of Multistrain Probiotics on Functional Constipation in the Elderly: A Randomized Controlled Trial. Eur. J. Clin. Nutr. 2022, 76, 1675–1681.
- Kim, B.; Song, J.-L.; Ju, J.-H.; Kang, S.-A.; Park, K.-Y. Anticancer Effects of Kimchi Fermented for Different Times and with Added Ingredients in Human HT-29 Colon Cancer Cells. Food Sci. Biotechnol. 2015, 24, 629–633.
- Lee, Y.-J.; Pan, Y.; Kwack, K.-B.; Chung, J.H.; Park, K.-Y. Increased Anticancer Activity of Organic Kimchi with Starters Demonstrated in HT-29 Cancer Cells. Appl. Sci. 2023, 13, 6654.
- Pathak, D.R.; Stein, A.D.; He, J.-P.; Noel, M.M.; Hembroff, L.; Nelson, D.A.; Vigneau, F.; Shen, T.; Scott, L.J.; Charzewska, J.; et al. Cabbage and Sauerkraut Consumption in Adolescence and Adulthood and Breast Cancer Risk among US-Resident Polish Migrant Women. Int. J. Environ. Res. Public Health 2021, 18, 10795.
- Cai, Y.; Yang, X.; Chen, S.; Tian, K.; Xu, S.; Deng, R.; Chen, M.; Yang, Y.; Liu, T. Regular Consumption of Pickled Vegetables and Fermented Bean Curd Reduces the Risk of Diabetes: A Prospective Cohort Study. Front. Public Health 2023, 11, 1155989.
- Li, M.; Bao, X.; Zhang, X.; Ren, H.; Cai, S.; Hu, X.; Yi, J. Exploring the Phytochemicals and Inhibitory Effects against α-Glucosidase and Dipeptidyl Peptidase-IV in Chinese Pickled Chili Pepper: Insights into Mechanisms by Molecular Docking Analysis. Lwt 2022, 162, 113467.
- Kim, S.-Y.; Dang, Y.-M.; Ha, J.-H. Effect of Various Seasoning Ingredients on the Accumulation of Biogenic Amines in Kimchi during Fermentation. Food Chem. 2022, 380, 132214.
- da Silva, M.B.; Rodrigues, L.F.O.S.; Monteiro, G.C.; Monar, G.R.S.; Gomez, H.A.G.; Junior, S.S.; Minatel, I.O.; Lima, G.P.P. Evaluation of Biogenic Amines and Nitrate in Raw and Pickled Jurubeba (Solanum paniculatum L.) Fruit. J. Food Sci. Technol. 2019, 56, 2970–2978.
- Liu, L.; Du, P.; Zhang, G.; Mao, X.; Zhao, Y.; Wang, J.; Duan, C.; Li, C.; Li, X. Residual Nitrite and Biogenic Amines of Traditional Northeast Sauerkraut in China. Int. J. Food Prop. 2016, 20, 2448–2455.
- Zhao, N.; Lai, H.; Wang, Y.; Huang, Y.; Shi, Q.; He, W.; Zhu, S.; Li, Y.; Zhu, Y.; Li, H.; et al. Assessment of Biogenic Amine and Nitrite Production in Low-Salt Paocai during Fermentation as Affected by Reused Brine and Fresh Brine. Food Biosci. 2021, 41, 100958.
- Park, Y.K.; Lee, J.H.; Mah, J.-H. Occurrence and Reduction of Biogenic Amines in Kimchi and Korean Fermented Seafood Products. Foods 2019, 8, 547.
- Özogul, F.; Hamed, I. The Importance of Lactic Acid Bacteria for the Prevention of Bacterial Growth and Their Biogenic Amines Formation: A Review. Crit. Rev. Food Sci. 2017, 58, 1660–1670.
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods 2019, 8, 17.
- Alan, Y.; Topalcengiz, Z.; Dığrak, M. Biogenic Amine and Fermentation Metabolite Production Assessments of Lactobacillus plantarum Isolates for Naturally Fermented Pickles. Lwt 2018, 98, 322–328.
- Yu, Y.; Li, L.; Xu, Y.; An, K.; Shi, Q.; Yu, Y.; Xu, Z. Evaluation of the Relationship among Biogenic Amines, Nitrite and Microbial Diversity in Fermented Mustard. Molecules 2021, 26, 6173.
- Jin, Y.H.; Lee, J.H.; Park, Y.K.; Lee, J.-H.; Mah, J.-H. The Occurrence of Biogenic Amines and Determination of Biogenic Amine-Producing Lactic Acid Bacteria in Kkakdugi and Chonggak Kimchi. Foods 2019, 8, 73.
- Ye, H.; Lang, X.; Ji, Y.; Li, S.; Xin, N.; Meng, X.; Zhang, T.; Shen, X.; Zhao, C. The Interaction between Lactobacillus plantarum SC-5 and Its Biogenic Amine Formation with Different Salt Concentrations in Chinese Dongbei Suancai. Food Res. Int. 2021, 150, 110813.
- Świder, O.; Wójcicki, M.; Bujak, M.; Juszczuk-Kubiak, E.; Szczepańska, M.; Roszko, M.Ł. Time Evolution of Microbial Composition and Metabolic Profile for Biogenic Amines and Free Amino Acids in a Model Cucumber Fermentation System Brined with 0.5% to 5.0% Sodium Chloride. Molecules 2021, 26, 5796.
- Majcherczyk, J.; Surówka, K. Effects of Onion or Caraway on the Formation of Biogenic Amines during Sauerkraut Fermentation and Refrigerated Storage. Food Chem. 2019, 298, 125083.
- Chen, Y.; Wu, C.; Xu, W.; Lu, Z.; Fu, R.; He, X.; Ma, Z.; Zhang, H. Evaluation of Degradation Capability of Nitrite and Biogenic Amines of Lactic Acid Bacteria Isolated from Pickles and Potential in Sausage Fermentation. J. Food Process. Pres. 2022, 46, e16141.
- Priyanka, V.; Ramesha, A.; Gayathri, D.; Vasudha, M. Molecular Characterization of Non-Biogenic Amines Producing Lactobacillus plantarum GP11 Isolated from Traditional Pickles Using HRESI-MS Analysis. J. Food Sci. Technol. 2021, 58, 2216–2226.
- Lee, J.; Jin, Y.H.; Pawluk, A.M.; Mah, J.-H. Reduction in Biogenic Amine Content in Baechu (Napa Cabbage) Kimchi by Biogenic Amine-Degrading Lactic Acid Bacteria. Microorganisms 2021, 9, 2570.
- Qi, Q.; Huang, J.; Zhou, R.; Jin, Y.; Wu, C. Characterising the Mechanism of Abating Biogenic Amines Accumulation by Cocultures of Zygosaccharomyces rouxii and Tetragenococcus halophilus. Lwt 2022, 164, 113672.
- Zhao, J.; Niu, C.; Du, S.; Liu, C.; Zheng, F.; Wang, J.; Li, Q. Reduction of Biogenic Amines Formation during Soybean Paste Fermentation by Using Staphylococcus carnosus M43 and Pediococcus acidilactici M28 as Starter Culture. Lwt 2020, 133, 109917.
- Callejón, S.; Sendra, R.; Ferrer, S.; Pardo, I. Identification of a Novel Enzymatic Activity from Lactic Acid Bacteria Able to Degrade Biogenic Amines in Wine. Appl. Microbiol. Biotechnol. 2014, 98, 185–198.
- Li, B.; Shiling, L. The Importance of Amine Oxidases on the Biogenic Amine Degradation in Fermented Foods: A Review. Process. Biochem. 2020, 99, 331–339.
- Callejón, S.; Sendra, R.; Ferrer, S.; Pardo, I. Cloning and Characterization of a New Laccase from Lactobacillus plantarum J16 CECT 8944 Catalyzing Biogenic Amines Degradation. Appl. Microbiol. Biotechnol. 2016, 100, 3113–3124.
- Xu, Y.; Liu, Y.; Xu, B.; Wang, D.; Jiang, W. Characterisation and Application of Halomonas shantousis SWA25, a Halotolerant bacterium with Multiple Biogenic Amine Degradation Activity. Food Addit. Contam. Part A 2016, 33, 674–682.
- Luo, W.; Wu, W.; Du, X.; Yu, Y.; Wu, J.; Xu, Y.; Li, L. Regulation of the Nitrite, Biogenic Amine and Flavor Quality of Cantonese Pickle by Selected Lactic Acid Bacteria. Food Biosci. 2023, 53, 102554.
- Yu, Y.; Yu, Y.; Xu, Z. Evaluation of Nitrite, Ethyl Carbamate, and Biogenic Amines in Four Types of Fermented Vegetables. Foods 2021, 10, 3150.
- Wang, Z.; Shao, Y. Effects of Microbial Diversity on Nitrite Concentration in Pao Cai, a Naturally Fermented Cabbage Product from China. Food Microbiol. 2018, 72, 185–192.
- Huang, T.-T.; Wu, Z.-Y.; Zhang, W.-X. Effects of Garlic Addition on Bacterial Communities and the Conversions of Nitrate and Nitrite in a Simulated Pickle Fermentation System. Food Control 2020, 113, 107215.
- Niu, P.; Wang, F.; Yuan, K.; Li, X.; Yang, X.; Guo, Y. Alkaline-Extracted Thinned Young Apple Polyphenols as an Effective Scavenger against Nitrite in Pickles: A Comparative Study with Ethanol-Extracted Polyphenols. Food Control 2021, 130, 108387.
- Chen, Y.; Li, Q.; Xia, C.; Yang, F.; Xu, N.; Wu, Q.; Hu, Y.; Xia, L.; Wang, C.; Zhou, M. Effect of Selenium Supplements on the Antioxidant Activity and Nitrite Degradation of Lactic Acid Bacteria. World J. Microbiol. Biotechnol. 2019, 35, 61.
- Ren, D.; Chen, P.; Li, W.; Su, X.; Bao, K.; Wang, Y.; Wang, J.; Liu, H. Screening, Mutagenesis of Nitrite-Degrading Lactobacilli in Chinese Traditional Fermented Sauerkraut and Its Application in the Production of Sauerkraut. J. Food Saf. 2016, 36, 474–481.
- Xia, Y.; Liu, X.; Wang, G.; Zhang, H.; Xiong, Z.; Sun, Y.; Ai, L. Characterization and Selection of Lactobacillus brevis Starter for Nitrite Degradation of Chinese Pickle. Food Control 2017, 78, 126–131.
- Liu, D.; Wang, P.; Zhang, X.; Xu, X.; Wu, H.; Li, L. Characterization of Nitrite Degradation by Lactobacillus casei Subsp. Rhamnosus LCR 6013. PLoS ONE 2014, 9, e93308.
- Zhang, X.; Han, J.; Zheng, X.; Yan, J.; Chen, X.; Zhou, Q.; Zhao, X.; Gu, Q.; Li, P. Use of Lactiplantibacillus plantarum ZJ316 as a Starter Culture for Nitrite Degradation, Foodborne Pathogens Inhibition and Microbial Community Modulation in Pickled Mustard Fermentation. Food Chem. X 2022, 14, 100344.
- Hang, S.; Zeng, L.; Han, J.; Zhang, Z.; Zhou, Q.; Meng, X.; Gu, Q.; Li, P. Lactobacillus plantarum ZJ316 Improves the Quality of Stachys Sieboldii Miq. Pickle by Inhibiting Harmful Bacteria Growth, Degrading Nitrite and Promoting the Gut Microbiota Health in Vitro. Food Funct. 2021, 13, 1551–1562.
- Fang, F.; Feng, T.; Du, G.; Chen, J. Evaluation of the Impact on Food Safety of a Lactobacillus Coryniformis Strain from Pickled Vegetables with Degradation Activity against Nitrite and Other Undesirable Compounds. Food Addit. Contam. Part 2016, 33, 623–630.
- Du, R.; Song, G.; Zhao, D.; Sun, J.; Ping, W.; Ge, J. Lactobacillus casei Starter Culture Improves Vitamin Content, Increases Acidity and Decreases Nitrite Concentration during Sauerkraut Fermentation. Int. J. Food Sci. Technol. 2018, 53, 1925–1931.
- Huang, Y.; Jia, X.; Yu, J.; Chen, Y.; Liu, D.; Liang, M. Effect of Different Lactic Acid Bacteria on Nitrite Degradation, Volatile Profiles, and Sensory Quality in Chinese Traditional Paocai. Lwt 2021, 147, 111597.
- Fei, Y.; Liu, D.; Luo, T.; Chen, G.; Wu, H.; Li, L.; Yu, Y. Molecular Characterization of Lactobacillus plantarum DMDL 9010, a Strain with Efficient Nitrite Degradation Capacity. PLoS ONE 2014, 9, e113792.
- Huang, Y.; Liu, D.; Jia, X.; Liang, M.; Lu, Y.; Liu, J. Whole Genome Sequencing of Lactobacillus plantarum DMDL 9010 and Its Effect on Growth Phenotype under Nitrite Stress. Lwt 2021, 149, 111778.
- Zhang, W.; Tian, G.; Feng, S.; Wong, J.H.; Zhao, Y.; Chen, X.; Wang, H.; Ng, T.B. Boletus Edulis Nitrite Reductase Reduces Nitrite Content of Pickles and Mitigates Intoxication in Nitrite-Intoxicated Mice. Sci. Rep. 2015, 5, 14907.
- Li, Y.; Xiong, D.; Yuan, L.; Fan, P.; Xiao, Y.; Chen, J.; Feng, W. Transcriptome and Protein Networks to Elucidate the Mechanism Underlying Nitrite Degradation by Lactiplantibacillus plantarum. Food Res. Int. 2022, 156, 111319.
- Yao, K.; Liu, D.; Liang, M.; Brennan, C.S.; Brennan, M. Detection of Nitrite Degradation by Lactobacillus plantarum DMDL9010 through the Anaerobic Respiration Electron Transport Chain Using Proteomic Analysis. Int. J. Food Sci. Technol. 2021, 56, 1608–1622.
- Xia, C.; Tian, Q.; Kong, L.; Sun, X.; Shi, J.; Zeng, X.; Pan, D. Metabolomics Analysis for Nitrite Degradation by the Metabolites of Limosilactobacillus fermentum RC4. Foods 2022, 11, 1009.
- Zeng, X.; Pan, Q.; Guo, Y.; Wu, Z.; Sun, Y.; Dang, Y.; Cao, J.; He, J.; Pan, D. Potential Mechanism of Nitrite Degradation by Lactobacillus fermentum RC4 Based on Proteomic Analysis. J. Proteom. 2019, 194, 70–78.
- Wei, W.; Hu, X.; Yang, S.; Wang, K.; Zeng, C.; Hou, Z.; Cui, H.; Liu, S.; Zhu, L. Denitrifying Halophilic Archaea Derived from Salt Dominate the Degradation of Nitrite in Salted Radish during Pickling. Food Res. Int. 2022, 152, 110906.
- Hu, X.; Zeng, C.; Hou, Z.; Wang, Y.; Xu, Q.; Isobe, K.; Senoo, K.; Zhu, L. The Complete Genome Sequence of the Archaeal Isolate Halomicrobium Sp. ZPS1 Reveals the Nitrogen Metabolism Characteristics under Hypersaline Conditions. Ann. Microbiol. 2020, 70, 29.
- Zhang, Q.; Zhang, F.; Gong, C.; Tan, X.; Ren, Y.; Yao, K.; Zhang, Q.; Chi, Y. Physicochemical, Microbial, and Aroma Characteristics of Chinese Pickled Red Peppers (Capsicum annuum) with and without Biofilm. Rsc. Adv. 2020, 10, 6609–6617.
- Ye, Z.; Shang, Z.; Li, M.; Qu, Y.; Long, H.; Yi, J. Evaluation of the Physiochemical and Aromatic Qualities of Pickled Chinese Pepper (Paojiao) and Their Influence on Consumer Acceptability by Using Targeted and Untargeted Multivariate Approaches. Food Res. Int. 2020, 137, 109535.
- Bao, R.; Fan, A.; Hu, X.; Liao, X.; Chen, F. Effects of High Pressure Processing on the Quality of Pickled Radish during Refrigerated Storage. Innov. Food Sci. Emerg. 2016, 38, 206–212.
- Yuan, L.; Xu, F.; Xu, Y.; Wu, J.; Lao, F. Production of Marinated Chinese Lotus Root Slices Using High-Pressure Processing as an Alternative to Traditional Thermal-and-Soaking Procedure. Molecules 2022, 27, 6506.
- Zhao, N.; Ge, L.; Huang, Y.; Wang, Y.; Wang, Y.; Lai, H.; Wang, Y.; Zhu, Y.; Zhang, J. Impact of Cold Plasma Processing on Quality Parameters of Packaged Fermented Vegetable (Radish Paocai) in Comparison with Pasteurization Processing: Insight into Safety and Storage Stability of Products. Innov. Food Sci. Emerg. 2020, 60, 102300.
- Choi, E.J.; Park, H.W.; Kim, S.B.; Ryu, S.; Lim, J.; Hong, E.J.; Byeon, Y.S.; Chun, H.H. Sequential Application of Plasma-Activated Water and Mild Heating Improves Microbiological Quality of Ready-to-Use Shredded Salted Kimchi Cabbage (Brassica pekinensis L.). Food Control 2019, 98, 501–509.
- Song, H.; Dang, Y.-M.; Ha, S.; Ha, J.-H. Effect of Ultraviolet-C Light-Emitting Diode Irradiation on Inactivation of White Colony-Forming Yeast in Kimchi Seasoning. Food Control 2022, 140, 109157.
More
